Abstract
Objective
The impact of angiogenesis on long-term survival of high-grade serous ovarian cancer (HGSOC) patients remains unclear. This study investigated whether angiogenic markers correlated with 5-year progression-free survival (PFS) in a large cohort of matched advanced HGSOC tissue samples.
Methods
Tumor samples from 124 primary HGSOC patients were retrospectively collected within the Tumor Bank Ovarian Cancer (http://www.toc-network.de). All patients were in advanced stages (FIGO stage III–IV). No patient had received anti-angiogenesis therapy. The cohort contains 62 long-term survivors and 62 controls matched by age and post-surgical tumor residuals. Long-term survivors were defined as patients with no relapse within 5 years after the end of first-line chemotherapy. Controls were patients who suffered from first relapse within 6–36 months after primary treatment. Samples were assessed for immunohistochemical expression of vascular endothelial growth factor (VEGF) A and VEGF receptor 2 (VEGFR2). Expression profiles of VEGFA and VEGFR2 were compared between the two groups.
Results
Significant correlation between VEGFA and VEGFR2 expression was observed (p < 0.0001, Spearman coefficient 0.347). A high expression of VEGFR2 (VEGFR2high) was found more frequently in long-term survivors (77.4%, 48/62) than in controls (51.6%, 30/62, p = 0.001), independent of FIGO stage and VEGFA expression in multivariate analysis (p = 0.005). Also, VEGFR2high was found the most frequently in women with PFS ≥ 10 years (p = 0.001) among all 124 patients. However, no significant association was detected between VEGFA expression and 5-year PFS (p = 0.075).
Conclusions
VEGFR2 overexpression significantly correlated with long-term PFS in HGSOC patients, independent of age, FIGO stage, tumor residual and VEGFA expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Almost 80% of ovarian cancer deaths result from high-grade serous ovarian cancer (HGSOC), a distinct subtype that has shown little improvement in overall survival during the past 30 years (Holmes 2015; Bowtell et al. 2015; Seidman et al. 2004). The development of treatment resistance has been identified as a crucial factor for patients’ poor prognosis. Eighty percent of the patients will benefit from initial cytoreduction and respond to platinum-based chemotherapy. However, almost all of them will experience disease recurrence and ultimately develop platinum resistance (Holmes 2015). In this context, identification of biomarkers to characterize the long-term survivors after completion of platinum-based chemotherapy is urgently needed. Furthermore, including long-term survivors into standard treatment regimens may result in patients’ overtreatment, whereas into clinical trials may lead to selection biases and subsequently unreliable results. A recent large-scale clinical analysis demonstrated that long-term survival was “not unusual” in patients with advanced epithelial ovarian cancer (EOC). Among 3582 women with EOC who survived longer than 10 years, nearly one-third was initially diagnosed at advanced stages (FIGO-International Federation of Gynecology and Obstetrics-stage III-IV), including high-grade serous cancer (Cress et al. 2015). It is still unclear which factors may contribute to long platinum-free intervals for HGSOC patients. Thus, investigating such biomarkers is important to set up more tailored approaches for cancer surveillance, to improve the poor prognosis.
Angiogenesis has been highlighted as a central event in tumor growth and progression of ovarian cancer (Dvorak 2002; Monk et al. 2013; Eskander and Tewari 2014). This process is considered to be mainly regulated through the vascular endothelial growth factor (VEGF) A and VEGF receptor 2 (VEGFA/VEGFR2) signaling pathway (Ferrara 2005). However, in ovarian cancer, the prognostic value of VEGFA/VEGFR2 pathway still remains controversial (Williams et al. 2012; Siddiqui et al. 2010, 2011; Yamamoto et al. 1997; Sallinen et al. 2014; Hartenbach et al. 1997; Engels et al. 2009; Raspollini et al. 2004; Nishida et al. 2004; O’Toole et al. 2007; Wimberger et al. 2014; Klasa-Mazurkiewicz et al. 2011). VEGFA or VEGFR2 often loses prognostic significance when established prognostic factors are included into multivariate analyses, such as age, tumor stage, grade, histologic subtypes and tumor residual mass after surgery (Yamamoto et al. 1997; Engels et al. 2009; Raspollini et al. 2004; Nishida et al. 2004). Besides, the follow-up time in these studies were mostly shorter than 5 years, whereas patients with a PFS ≥ 5 years were rarely included. Herein, better patient selection and long-term follow-up are imperatively needed, to clarify the role of VEGFA/VEGFR2 pathway in HGSOC patients’ prognosis.
This study aims to investigate whether intratumoral VEGFA/VEGFR2 expressions correlate with long-term PFS, analyzing the homogeneous samples from 124 advanced primary HGSOC patients.
Methods and materials
Sample collection
We retrospectively included 124 matched tumor tissue samples collected from primary advanced HGSOC patients, who were treated from 1985 to 2013 and were included in the Tumor Bank Ovarian Cancer Consortium (TOC Consortium, http://www.toc-network.de). Patients underwent surgery in five European high-volume Gynecologic Oncology Centers: Charité Medical University of Berlin, Germany; University-Medical-Center Hamburg-Eppendorf, Germany; Medical University of Innsbruck, Austria; University Hospital Leuven, Belgium; University of Medicine and Pharmacy Iuliu Hatieganu Cluj-Napoca, Romania. Patients’ inclusion criteria were: (1) primary HGSOC diagnosis; (2) FIGO stage III–IV; (3) no relapse within 6 months after completion of primary platinum-based chemotherapy; (4) no anti-angiogenic therapy was performed from the date of first diagnosis till the first relapse or the last follow-up; (5) availability of patients’ follow-up and clinico-pathological data; (6) availability of formalin-fixed, paraffin-embedded ovarian cancer tissue samples. Exclusion criterion was no chemotherapy naïve tumor tissue available for immunohistochemistry. The 124 samples include 62 “long-term survivors” and 62 controls, which were matched 1:1 by age at first diagnosis (older or younger within 5 years) and macroscopic tumor residual status after primary surgery (no residual vs. with residual). “Long-term survivors” were defined as primary HGSOC patients without recurrence 5 years after primary chemotherapy (PFS ≥ 5 years). Controls were defined as primary HGSOC patients experiencing recurrence within 6 months to 3 years after the completion of adjuvant chemotherapy (PFS = 6 months-3 years). Samples from the long-term group were collected from all five centers of TOC Consortium, whereas control group samples were retrieved only from Charité Medical University of Berlin.
All patients underwent cytoreduction and platinum-based chemotherapy following the standard procedure as described previously (Braicu et al. 2012). Ovarian cancer tissue samples were prospectively collected during primary cytoreduction within TOC consortium. All samples underwent central histopathological review to confirm the diagnosis of HGSOC and to evaluate tissue quality and tumor content. Tumor grading was re-evaluated according to World Health Organization (WHO) pathological classification (2014). Staging was performed and defined in accordance with the FIGO criteria for ovarian cancer (22). Clinical data and follow-up information were also collected prospectively in TOC Consortium Centers.
PFS was defined as time interval from the end of first chemotherapy to first recurrence of disease. Overall survival (OS) was defined as time interval between diagnosis and patients’ death or loss of follow up. Response to treatment and diagnosis of recurrence was determined according to the “Response Evaluation Criteria in Solid Tumors” (RECIST) criteria (Eisenhauer et al. 2009).
The study protocol was approved by each local Ethics Committees (Charité 2004-000034, Innsbruck AN2015-0237 354/4.7, Hamburg EK200313, Leuven MML1022, Cluj 39). Prior to surgery and sample collection, all patients signed written informed consent for using their bio-specimens and clinico-pathological data for research purposes.
Immunohistochemical staining
For tissue microarrays (TMAs), representative tumor areas of chemotherapy naïve ovarian carcinoma tissue were selected in hematoxylin and eosin-stained sections by an experienced pathologist (SD). Consecutively tissue microarrays (TMAs) were constructed as previously described (Buckendahl et al. 2011). For detection of VEGFA and VEGFR2 on tissue samples, two rabbit polyclonal antibodies directed against the VEGFA or VEGFR2 (Abcam, Cambridge, United Kingdom) were used. Slides were first deparaffinated and rehydrated in a series of descending alcoholic concentration. For antigen retrieval, slides were boiled for 5 min in 0.01 M sodium citrate buffer at pH 9.0 in a pressure cooker and afterwards put in TBS buffer for the same time. After blocking the endogenous peroxidase, slides were incubated with the primary antibody, diluted 1:250 for VEGFA and 1:500 for VEGFR2 in antibody diluents solution (Zytomed Systems, Berlin, Germany) for 30 min at room temperature. For visualization, Dako Real Detection System (Dako, Glostrup, Denmark) was applied according to a standard protocol, as provided by the manufacturer using DAB + Chromogen. Counterstaining was carried out with Haemalaun (Dr Hollborn, Leipzig, Germany). Afterwards, the tissue was dehydrated and cover-slipped with Vitroclud (Medizintechnik Langenbrinck, Emmerdingen, Germany).
The immunohistochemical expression of VEGFA and VEGFR2 were independently evaluated by two co-authors (JG and ETT), who were blinded to the patient characteristics and outcome. In case of disagreement, results were discussed in a multi-headed microscope till a consensus was reached. The immunoreactivity of VEGFA and VEGFR2 was calculated applying a semi-quantitative immunoreactivity score (IRS), as published before (Yamamoto et al. 1997; Raspollini et al. 2004; Nishida et al. 2004; Horikawa et al. 2017). The scores of staining proportion (0, no cells stained; 1, < 10% of cells stained; 2, 11–50% of cells stained; 3, 51–80% of cells stained; 4, > 80% of cells stained) and intensity (0, negative; 1, weak; 2, moderate; 3, strong) were multiplied to obtain the final IRS (range 0–12). As no established cutoffs for VEGFA/VEGFR2 expression exist, we performed a logistic regression model with different cutoffs to conclude IRS of 0–6 as “low expression” and IRS of 7–12 as “high expression”, to dichotomize the expression of the two biomarkers.
Statistical analyses
Statistical analysis was performed by IBM SPSS Statistics 22.0 (SPSS, Inc., Chicago, IL) The Shapiro–Wilk test was used to ascertain whether continuous variables had a normal distribution. Continuous variables were summarized by means and standard deviations, or median and inter quartile range (IQR) where appropriate. Categorical variables were presented as frequency with percentage. The intra-group differences of continuous variables were investigated by Student t test when normally distributed, or Mann–Whitney U test when non-normally distributed. Chi square test or Fisher’s exact test was used to analyze the difference between categorical variables. Correlation between expressions of two biomarkers was analyzed by correlation test (Spearman coefficient, two-tailed). Multivariable logistic regression model was performed to evaluate the independent value of biomarkers. A two-tailed p value of < 0.05 was considered as statistically significant.
Results
The clinico-pathological characteristics of matched long-term and control groups are summarized in Table 1. Between two groups, no difference in age, tumor residual status and FIGO stage was observed (Table 1; Fig. 1).
VEGFA and VEGFR2 expression
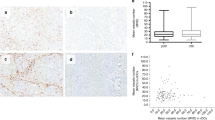
Immunohistochemical staining showed that VEGFA and VEGFR2 proteins were localized in the cytoplasm (Fig. 2a, b, e and f). The IRS distributions of VEGFA and VEGFR2 in long-term and control groups have been presented in Fig. 2c, d, g and h. A high expression of VEGFA (VEGFAhigh) was found in 79.0% (49/62) and 64.5% (40/62) of long-term and control samples, respectively (p = 0.073, Chi square test, Fig. 3a; Table 2). A high expression of VEGFR2 (VEGFR2high), however, was significantly more frequent in the long-term (77.4%, 48/62) compared with the control group (51.6%, 30/62, p = 0.001, Chi square test, Fig. 3b; Table 2). Significant correlation between VEGFA and VEGFR2 expression was observed (p > 0.0001, Spearman coefficient 0.347). After adjusting for FIGO stage and VEGFA expression in multivariate analysis, VEGFR2 expression still remained significantly different between long-term survivors and controls (p = 0.005, logistic regression model, Table 2).
VEGFA and VEGFR2 expression by immunohistochemistry: weak expressions of VEGFA (a) and VEGFR2 (e) in tumor cells; strong expressions of VEGFA (b) and VEGFR2 (f) in tumor cells with weak background staining in stroma cells; and IRS distribution of VEGFA (c, d) and VEGFR2 (g, h) expression in long-term and control groups
Difference in VEGFA and VEGFR2 expressions between long-term and the control groups: VEGFA (a) and VEGFR2 (b) expression and their co-expression (c) between two groups in all patients (n = 124); difference in VEGFA (d) and VEGFR2 (e) expressions between two groups in no-residual patients (n = 96); and difference in VEGFA (f) and VEGFR2 (g) expressions between two groups in with-residual patients (n = 28)
Co-expression of VEGFA and VEGFR2
A combined expression of VEGFAhigh and VEGFR2high was more common in the long term (62.9% 39/62) compared with the control group (42.0%, 26/62, p = 0.003, Chi square test, Fig. 3c).
VEGFA and VEGFR2 expression in subgroups (with or without residual)
We investigated VEGFA and VEGFR2 expression in HGSOC patients according to their residual tumor status (no residual, n = 96, and with residual, n = 28). Among no-residual patients, VEGFR2high was more frequently observed in the long-term group (75.0%, 36/48) than in controls (54.2%, 26/48, p = 0.033, Fig. 3d, e). This difference was even more pronounced among with-residual patients, as 85.7% of (12/14) long-term survivors showed VEGFR2high compared with 28.6% (4/14) of controls (p = 0.006, Fisher’s exact test, Fig. 3f, g). On the other hand, VEGFAhigh was also more frequent among long-term survivors in both no-residual (79.2%, 38/48) and with-residual subgroups (78.6%, 11/14) than in control (66.7%, 32/48, and 57.1%, 8/14, Fig. 3a, b). Nevertheless, these differences were not statistically significant (P = 0.168, and P = 0.221).
VEGFA and VEGFR2 expression according to PFS duration
To investigate whether the expression of VEGFA or VEGFR2 may change in relation to PFS, we finally analyzed the two biomarkers in four subgroups (Fig. 4): PFS ≥ 10 years (120 months, n = 23), PFS = 5–10 years (60–119 months, n = 39), PFS = 1–3 years (13–36 months, n = 40), and PFS = 6–12 months (n = 22). All subgroups were well balanced regarding age, FIGO stage and tumor residual. VEGFA expression was not associated with PFS (p = 0.298, Chi square test, Fig. 4c) at the median IRS of 8 in all subgroups (Fig. 4a). On the contrary, the IRS of VEGFR2 was the highest in patients with PFS ≥ 10 years (median IRS = 9, VEGFR2high 91.3%, 21/23), whereas the lowest in patients with PFS = 1–3 years (median IRS = 6, VEGFR2high 48.5%, 17/40, p = 0.001, Chi square test). Interestingly, VEGFR2 expression increased with PFS duration among the three subgroups: PFS = 1–3 years, PFS = 5–10 years and PFS ≥ 10 years (p < 0.001, Chi square test). However, between subgroups of PFS = 6–12 months and PFS = 1–3 years, VEGFR2 expression remained stable (P = 0.211, Chi square test).
Discussion
In the present study, we investigated whether the VEGFA/VEGFR2 pathway plays a role in long-term survival for primary HGSOC patients. VEGFR2high was found to be significantly associated with long-term PFS, independent of age, FIGO stage and residual disease. VEGFA expression was not significantly correlated with long-term survival, although it was positively associated with VEGFR2 expression. This result indicated that other mechanisms might be also involved in VEGFR2 activation.
Although anti-VEGFA and anti-VEGFR treatment have been implemented into first-line treatment of ovarian cancer (Wimberger et al. 2010, 2014; Burger et al. 2011a, b; du Bois et al. 2009), their effects on long-term survival still remain unclear. A median improvement of 4–6 months in PFS has been shown by three phase 3 randomized trials using anti-VEGFA (bevacizumab) and anti-VEGFR treatments (pazopanib) in primary ovarian cancer (Burger et al. 2011a, b; du Bois et al. 2014). Nevertheless, no overall survival differences were found between patients receiving bevacizumab plus chemotherapy compared to chemotherapy alone in GOG 0218 (ClinicalTrials.gov Identifier: NCT00262847). Since grade 3–4 adverse events were significantly higher in patients using bevacizumab and pazopanib compared with placebo control (Burger et al. 2011a, b; du Bois et al. 2014), it is imperative to precisely identify patients who may or may not benefit from anti-VEGFA/VEGFR treatment. However, patients with PFS ≥ 5 years were hardly included in these trials, where median PFS was around 15 months and the follow-up duration was less than 3 years. Thus, our findings are clinically important showing that HGSOC patients with VEGFR2high may be more likely to achieve long-term survival. The anti-VEGFR therapy for these patients might need further consideration.
Our study shows for the first time that VEGFR2 might contribute to long-term PFS of patients with advanced primary HGSOC. Previous findings remained controversial regarding prognostic values of VEGFA/VEGFR2 in ovarian cancer. High expression of VEGFA/VEGFR2 have been found to promote tumor metastases and thus correlate with poor outcome in ovarian cancer (Williams et al. 2012; Siddiqui et al. 2011; Yamamoto et al. 1997; Raspollini et al. 2004; Nishida et al. 2004; Wimberger et al. 2014; Horikawa et al. 2017; Avril et al. 2017). An inverse association has also been reported (Sallinen et al. 2014; Engels et al. 2009; Raspollini et al. 2004; O’Toole et al. 2007). In particular, Nishida N et al. analyzed tumor specimens of 80 patients with ovarian cancer and showed that a high VEGFA expression independently predicted longer disease-free survival in multivariate analyses (Nishida et al. 2004). However, the follow-up time in these studies was mostly around 3 years. Whether the VEGFA/VEGFR2 pathway contributes to long-term PFS in this context has not been clearly elucidated. The association between VEGFR2 overexpression and long-term survival of HGSOC patients was for the first time reported in this study.
The strength of our study is the homogeneous patient selection. On one hand, earlier analyses included patients with all tumor stages, histological subtypes and tumor residual status (Engels et al. 2009; Raspollini et al. 2004; Klasa-Mazurkiewicz et al. 2011), which are the most important prognostic factors for ovarian cancer (du Bois et al. 2009; Wimberger et al. 2007, 2010). These factors were also strongly associated with VEGFA/VEGFR2 expression (Williams et al. 2012; Sallinen et al. 2014; Raspollini et al. 2004; Wimberger et al. 2014; Klasa-Mazurkiewicz et al. 2011). Including established prognostic factors into multivariate analyses, VEGFA and VEGFR2 often lost their prognostic significance (Yamamoto et al. 1997; Engels et al. 2009; Raspollini et al. 2004; Nishida et al. 2004). On the other hand, previous studies mainly recruited both platinum-resistant and platinum-sensitive patients (Williams et al. 2012; Siddiqui et al. 2011; Yamamoto et al. 1997; Raspollini et al. 2004; Nishida et al. 2004; Wimberger et al. 2014; Horikawa et al. 2017; Avril et al. 2017). Since PFS is much shorter in platinum-resistant patients, it might conceal the independent impact of angiogenesis markers on long-term survival. Considering these factors above, we only included platinum-sensitive patients with advanced HGSOC and matched them according to established prognostic factors (age and residual disease). Based on the well-selected samples, we revealed an independent correlation between VEGFR2 overexpression and good prognosis in HGSOC patients.
Defining “long-term survivors” as patients with PFS ≥ 5 years is also a strength of our patient selection. Since the follow-up period in earlier studies was mostly around 3 years, the long-term impacts of VEGFA/VEGFR2 expression in HGSOC remained unclear (Siddiqui et al. 2011; Hartenbach et al. 1997; Raspollini et al. 2004; Wimberger et al. 2014). Furthermore, extended follow-up duration is highly important as the prognostic values of VEGF might change over time. One study showed that VEGFA expression was associated with treatment response within 6 months but not within 12 months after chemotherapy (O’Toole et al. 2007). A similar trend was observed where VEGFA inversely impacted on PFS according to the length of tamoxifen treatment (Sanchez et al. 2010). In this randomized clinical trial, including 402 breast cancer patients with a median follow-up of 9.8 years, VEGFA overexpression predicted shorter PFS in the group of 2-year tamoxifen treatment but correlated with longer PFS in patients treated for 5 years. In our study, VEGFR2 expression remained stable in the group of PFS = 6 months–3 years, whereas it increased significantly among patients with PFS ≥ 5 years. Thus, long-term follow-up and appropriate patient selection should be considered when assessing prognostic values of VEGFA/VEGFR2.
A high expression of VEGFR2 was found more frequently among long-term survivors in our study. Similarly, Zhang et al. found VEGFR2 protein levels were higher in control tissue compared with human squamous cell carcinomas (Zhang et al. 2010). Using anti-VEGFA monoclonal antibody bevacizumab (Avastin) can upregulate VEGFR2 expression in tumor-associated endothelial cells, through the JNK/c-Jun pathway and activity of the ubiquitin–proteasome system (Zhang et al. 2010). In this scenario, improved PFS by bevacizumab in clinical trials might partially attribute to upregulated VEGFR2 induced by the blockage of VEGFA. This may help to explain the association between VEGFR2high and long-term PFS in our study. Anti-VEGFR or anti-VEGFR2 treatment for HGSOC patients may need reconsideration.
The VEGFR2 overexpression in our “long-term” cohort might also be due to the patient selection, as the long-term group might include more patients with BRCA1/2 mutations or other homologous recombination repair (HRR) defects that are strongly related to improved prognosis (Yang et al. 2011; Bolton et al. 2012; Norquist et al. 2018). Our previous study of HGSOC found that VEGF overexpression was most common among patients with somatic BRCA1/2 mutations (Ruscito et al. 2018). Confirming what was already observed in a study on breast cancer, which reported that BRCA mutation carriers were found with higher levels of VEGF mRNA (p = 0.04) compared to patients without BRCA mutations (Danza et al. 2013). Another study on ovarian cancer revealed, that a VEGF-dependent gene signature (VDGs) was overexpressed in BRCA mutation carriers (Yin et al. 2016). The proposed mechanism of correlation between BRCA1 mutation and VEGF overexpression involves Caveolin-1 expression, which down-regulates VEGF and regulates endothelial function; in HGSOC, dysfunctional BRCA1 leads to Caveolin-1 expression inhibition, thus resulting in increased VEGF levels (Desai et al. 2015). However, information on BRCA1/2 mutation was not available for the patients in our study. Further investigations should consider BRCA1/2 mutation status when accessing effect of VEGF expression on long-term survival of HGSOC.
As the major blood vessel endothelial cell receptor for VEGFA, VEGFR2 can also be activated by VEGFC and VEGFD to collect lymphatic vessels and capillaries undergoing active lymph-angiogenesis (Saharinen et al. 2004), VEGFR2 signaling affects not only primary tumor growth but also lymphatic vessel functionality and tumor cell metastatic spread (Joukov et al. 1997; Alam et al. 2004). In our study, the correlation between VEGFR2 expression and long-term PFS was independent from VEGFA expression, although VEGFA and VEGFR2 expression were significantly correlated. Our findings suggest that VEGFR2 might be activated by other factors such as VEGFC and VEGFD to affect development of HGSOC. Nevertheless, in our study other members of the VEGF family have not been evaluated, which is our major limitation. Further investigation and validation of this signaling pathway is therefore needed.
Another strength of this study, besides the homogeneous population, is the large sample size of matched long-term survivors and controls. As very few patients with advanced HGSOC could achieve 5-year PFS, our 124 matched HGSOC patients is the largest homogeneous population regarding VEGFR2 in long-term survivors. In addition, centers in our tumor bank consortium are all high-volume centers of ovarian cancer, with long-lasting experience in surgical treatment and standardized protocols of sample collection. Most centers have also been approved and allowed to participate in the LION (ClinicalTrials.gov Identifier: NCT00712218), DESKTOP III (Clinical-Trials.gov Identifier: NCT01166737) and TRUST (ClinicalTrials.gov Identifier: NCT02828618) studies, based on the high quality of the tumor debulking.
In conclusion, we found VEGFR2high was strongly and independently associated with long-term PFS in primary advanced HGSOC. VEGFR2high patients may be less likely to experience disease recurrence within 5 years after primary chemotherapy. Our findings may provide a new insight into understanding tumor development and a relevant cornerstone for further studies on HGSOC patients’ prognosis.
References
Alam A, Herault JP, Barron P, Favier B, Fons P, Delesque-Touchard N, Senegas I, Laboudie P, Bonnin J, Cassan C et al (2004) Heterodimerization with vascular endothelial growth factor receptor-2 (VEGFR-2) is necessary for VEGFR-3 activity. Biochem Biophys Res Commun 324(2):909–915
Avril S, Dincer Y, Malinowsky K, Wolff C, Gundisch S, Hapfelmeier A, Boxberg M, Bronger H, Becker KF, Schmalfeldt B (2017) Increased PDGFR-beta and VEGFR-2 protein levels are associated with resistance to platinum-based chemotherapy and adverse outcome of ovarian cancer patients. Oncotarget 8(58):97851–97861
Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, Lambrechts D, Despierre E, Barrowdale D, McGuffog L et al (2012) Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 307(4):382–390
Bowtell DD, Böhm S, Ahmed AA, Aspuria P-J, Bast Jr RC, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA et al (2015) Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 15:668
Braicu EI, Sehouli J, Richter R, Pietzner K, Lichtenegger W, Fotopoulou C (2012) Primary versus secondary cytoreduction for epithelial ovarian cancer: a paired analysis of tumour pattern and surgical outcome. Eur J Cancer 48(5):687–694
Buckendahl AC, Budczies J, Fiehn O, Darb-Esfahani S, Kind T, Noske A, Weichert W, Sehouli J, Braicu E, Dietel M et al (2011) Prognostic impact of AMP-activated protein kinase expression in ovarian carcinoma: correlation of protein expression and GC/TOF-MS-based metabolomics. Oncol Rep 25(4):1005–1012
Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE (2011a) Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 365(26):2473–2483
Burger R, Brady M, Bookman M, Monk B, Walker J, Homesley H, Fowler J, Greer B, Boente M, Liang S (2011b) Prospective investigation of risk factors for gastrointestinal adverse events in a phase III randomized trial of bevacizumab in first-line therapy of advanced epithelial ovarian cancer, primary peritoneal cancer or fallopian tube cancer: a Gynecologic Oncology Group study. Gynecol Oncol 120:S5
Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS (2015) Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol 126(3):491–497
Danza K, Pilato B, Lacalamita R, Addati T, Giotta F, Bruno A, Paradiso A, Tommasi S (2013) Angiogenetic axis angiopoietins/Tie2 and VEGF in familial breast cancer. Eur J Hum Genet: EJHG 21(8):824–830
Desai A, Xu J, Aysola K, Akinbobuyi O, White M, Reddy VE, Okoli J, Clark C, Partridge EE, Childs E et al (2015) Molecular mechanism linking BRCA1 dysfunction to high grade serous epithelial ovarian cancers with peritoneal permeability and ascites. J Gynecol Res 1(1):103
du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J (2009) Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer 115(6):1234–1244
du Bois A, Floquet A, Kim J-W, Rau J, del Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo N et al (2014) Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol 32(30):3374–3382
Dvorak HF (2002) Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol 20(21):4368–4380
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Engels K, du Bois A, Harter P, Fisseler-Eckhoff A, Kommoss F, Stauber R, Kaufmann M, Nekljudova V, Loibl S (2009) VEGF-A and i-NOS expression are prognostic factors in serous epithelial ovarian carcinomas after complete surgical resection. J Clin Pathol 62(5):448–454
Eskander RN, Tewari KS (2014) Incorporation of anti-angiogenesis therapy in the management of advanced ovarian carcinoma–mechanistics, review of phase III randomized clinical trials, and regulatory implications. Gynecol Oncol 132(2):496–505
Ferrara N (2005) VEGF as a therapeutic target in cancer. Oncology 69(Suppl 3):11–16
Hartenbach EM, Olson TA, Goswitz JJ, Mohanraj D, Twiggs LB, Carson LF, Ramakrishnan S (1997) Vascular endothelial growth factor (VEGF) expression and survival in human epithelial ovarian carcinomas. Cancer Lett 121(2):169–175
Holmes D (2015) Ovarian cancer: beyond resistance. Nature 527(7579):S217-S217
Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, Yoshioka Y, Koshiyama M, Konishi I (2017) Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res 23(2):587–599
International Federation of Gynecology and Obstetrics (1987) Changing in definitions of clinical staging for carcinoma of the cervix and ovary. Am J Obst Gynecol 156(1):263–264
Joukov V, Sorsa T, Kumar V, Jeltsch M, Claesson-Welsh L, Cao Y, Saksela O, Kalkkinen N, Alitalo K (1997) Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J 16(13):3898–3911
Klasa-Mazurkiewicz D, Jarzab M, Milczek T, Lipinska B, Emerich J (2011) Clinical significance of VEGFR-2 and VEGFR-3 expression in ovarian cancer patients. Pol J Pathol 62(1):31–40
Monk BJ, Dalton H, Farley JH, Chase DM, Benjamin I (2013) Antiangiogenic agents as a maintenance strategy for advanced epithelial ovarian cancer. Crit Rev Oncol Hematol 86(2):161–175
Nishida N, Yano H, Komai K, Nishida T, Kamura T, Kojiro M (2004) Vascular endothelial growth factor C and vascular endothelial growth factor receptor 2 are related closely to the prognosis of patients with ovarian carcinoma. Cancer 101(6):1364–1374
Norquist BM, Brady MF, Harrell MI, Walsh T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Burger RA, Tewari KS et al (2018) Mutations in homologous recombination genes and outcomes in ovarian carcinoma patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin Cancer Res 24(4):777–783
O’Toole SA, Sheppard BL, Laios A, O’Leary JJ, McGuinness EPJ, D’Arcy T, Bonnar J (2007) Potential predictors of chemotherapy response in ovarian cancer—how do we define chemosensitivity? Gynecol Oncol 104(2):345–351
Raspollini MR, Amunni G, Villanucci A, Baroni G, Boddi V, Taddei GL (2004) Prognostic significance of microvessel density and vascular endothelial growth factor expression in advanced ovarian serous carcinoma. Int J Gynecol Cancer 14(5):815–823
Ruscito I, Cacsire Castillo-Tong D, Vergote I, Ignat I, Stanske M, Vanderstichele A, Glajzer J, Kulbe H, Trillsch F, Mustea A et al (2018) Characterisation of tumour microvessel density during progression of high-grade serous ovarian cancer: clinico-pathological impact (an OCTIPS Consortium study). Br J Cancer 119:330
Saharinen P, Tammela T, Karkkainen MJ, Alitalo K (2004) Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol 25(7):387–395
Sallinen H, Heikura T, Koponen J, Kosma VM, Heinonen S, Yla-Herttuala S, Anttila M (2014) Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer. BMC Cancer 14:696.
Sanchez BC, Sundqvist M, Fohlin H, Spyratos F, Nordenskjold B, Stal O, Linderholm BK (2010) Prolonged tamoxifen treatment increases relapse-free survival for patients with primary breast cancer expressing high levels of VEGF. Eur J Cancer 46(9):1580–1587
Seidman JD, Horkayne-Szakaly I, Haiba M, Boice CR, Kurman RJ, Ronnett BM (2004) The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol 23(1):41–44
Siddiqui GK, Elmasry K, Wong Te Fong AC, Perrett C, Morris R, Crow JC, Maclean AB (2010) Prognostic significance of intratumoral vascular endothelial growth factor as a marker of tumour angiogenesis in epithelial ovarian cancer. Eur J Gynaecol Oncol 31(2):156–159
Siddiqui GK, Maclean AB, Elmasry K, Wong te Fong A, Morris RW, Rashid M, Begent RH, Boxer GM (2011) Immunohistochemical expression of VEGF predicts response to platinum based chemotherapy in patients with epithelial ovarian cancer. Angiogenesis 14(2):155–161
Williams E, Martin S, Moss R, Durrant L, Deen S (2012) Co-expression of VEGF and CA9 in ovarian high-grade serous carcinoma and relationship to survival. Virchows Arch 461(1):33–39
Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A (2007) Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO-OVAR). Gynecol Oncol 106(1):69–74
Wimberger P, Wehling M, Lehmann N, Kimmig R, Schmalfeldt B, Burges A, Harter P, Pfisterer J, du Bois A (2010) Influence of residual tumor on outcome in ovarian cancer patients with FIGO stage IV disease: an exploratory analysis of the AGO-OVAR (Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group). Ann Surg Oncol 17(6):1642–1648
Wimberger P, Chebouti I, Kasimir-Bauer S, Lachmann R, Kuhlisch E, Kimmig R, Suleyman E, Kuhlmann JD (2014) Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol Oncol 133(3):467–472
Yamamoto S, Konishi I, Mandai M, Kuroda H, Komatsu T, Nanbu K, Sakahara H, Mori T (1997) Expression of vascular endothelial growth factor (VEGF) in epithelial ovarian neoplasms: correlation with clinicopathology and patient survival, and analysis of serum VEGF levels. Br J Cancer 76(9):1221–1227
Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, Zhang W (2011) Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA 306(14):1557–1565
Yin X, Wang X, Shen B, Jing Y, Li Q, Cai MC, Gu Z, Yang Q, Zhang Z, Liu J et al (2016) A VEGF-dependent gene signature enriched in mesenchymal ovarian cancer predicts patient prognosis. Sci Rep 6:31079
Zhang Z, Neiva KG, Lingen MW, Ellis LM, Nör JE (2010) VEGF-dependent tumor angiogenesis requires inverse and reciprocal regulation of VEGFR1 and VEGFR2. Cell Death Differ 17(3):499
Author information
Authors and Affiliations
Contributions
This study was designed by Jalid Sehouli and Elena Braicu. The clinical data were collected by Sven Mahner, Linn Woelber, Katharina Prieske, Nicole Concin, Ignace Vergote, Els Van Nieuwenhuysen, Patriciu Achimas-Cadariu, Joanna Glajzer, Hannah Woopen, Mandy Stanske, Hagen Kulbe, Silvia Darb-Esfahani and Carsten Denkert. The immunohistochemistry staining was evaluated by Jun Guan, Eliane Taube and Silvia Darb-Esfahani. The results were analyzed by Jun Guan and Rolf Richter. The tables and figures were drawn by Jun Guan. And Jun Guan summarized all the findings and wrote the manuscript. Elena Braicu, Ilary Ruscito, Rolf Richter, Hagen Kulbe, Sven Mahner, Linn Woelber and Katharina Prieske revised the article.
Corresponding author
Ethics declarations
Conflict of interest
Dr. Elena Ioana reports grants, personal fees and other from Roche Pharma, personal fees from Clovis, personal fees and other from TesaroBio, personal fees and other from AstraZeneca, personal fees from Amgen, personal fees from Incyte, grants from Molecular Health, outside the submitted work; Dr. Sven Mahner reports research support, advisory board, honoraria and travel expenses from AstraZeneca, Bayer, Boehringer Ingelheim, Clovis, Eisai, GlaxoSmithKline, Jenapharm, Janssen-Cilag, Medac, MSD, Novartis, PharmaMar, Roche, Sensor Kinesis, Teva, Tesaro; Dr. Linn Woelber reports grants from medac oncology, during the conduct of the study; grants, personal fees and non-financial support from medac oncology, personal fees and non-financial support from Tesaro, personal fees from Roche, Pharmamar, Tewa, Astra Zeneca, Jenapharm and Janssen-Cilag, outside the submitted work; Dr. Carsten Denkert is the cofounder and shareholder of Sividon Diagnostics and in advisory board of Teva, Roche, AstraZeneca, Celgene, Pfizer, Novartis, Daiichi, and MSD. Other authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guan, J., Darb-Esfahani, S., Richter, R. et al. Vascular endothelial growth factor receptor 2 (VEGFR2) correlates with long-term survival in patients with advanced high-grade serous ovarian cancer (HGSOC): a study from the Tumor Bank Ovarian Cancer (TOC) Consortium. J Cancer Res Clin Oncol 145, 1063–1073 (2019). https://doi.org/10.1007/s00432-019-02877-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-019-02877-4