Abstract
Introduction
Patients (pts) with locally advanced (LAPC) or metastatic pancreatic ductal adenocarcinoma (mPDAC) have a dismal prognosis. Recently, new combination chemotherapies such as FOLFIRINOX and nab-paclitaxel/gemcitabine have demonstrated superiority over gemcitabine monotherapy. However, a substantial proportion of pts cannot tolerate these intensive front-line protocols. Moreover, the long-term superiority of multiagent protocols over less intensive strategies remains to be shown. To provide a benchmark for future studies, we analyzed the outcome of patients with LAPC or mPDAC treated at the West German Cancer Center before the FOLFIRINOX/nab-paclitaxel + gemcitabine era.
Methods
This retrospective analysis included 201 consecutive pts with LAPC and mPDAC treated between 2007 and 2011. Efficacy parameters were correlated with type of chemotherapy, number of treatment lines and clinicopathological parameters.
Results
Gemcitabine monotherapy was given as first-line therapy in 51.1%, whereas 48.9% received combination chemotherapies such as gemcitabine/oxaliplatin or FOLFOX. Patients received a median of two lines of treatment, with 54.8% receiving second-line and 37.9% receiving third- and further-line therapies. There was no significant difference between gemcitabine monotherapy and combination therapies. Despite moderate activity of first-line treatment, median overall survival for LAPC was 11.3 months and 8.7 months for mPDAC. Multivariate analysis identified age and number of treatment lines as prognostic markers.
Conclusion
The long-term outcome of unselected pts with LAPC and mPDAC treated before the introduction of aggressive multiagent chemotherapy protocols compares favorably with the results of contemporary benchmark trials. This suggests a multifactorial benefit from interdisciplinary care provided over sequential treatment lines at high volume expert centers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with pancreatic ductal adenocarcinoma (PDAC) have a poor prognosis with 5-year survival rates of 5–20% in patients with resectable tumors and < 5% in patients with locally advanced or metastatic disease. In Western countries, PDAC is the fourth leading cause of cancer-related death with a rising incidence over the last 10 years (Jemal et al. 2010; Saif 2013). No effective screening programs have been implemented in the population. In line only 20% of patients are diagnosed with early stage disease and can be resected in curative intent. In spite of curative resection and adjuvant or additive chemotherapy with dose-intense modern regimes (Neoptolemos et al. 2017), the relapse rate remains extremely high in the range of 70%. The majority of patients are diagnosed with locally advanced (LAPC) non-resectable or metastatic disease (mPDAC). Treatment of those patients is challenging due to the aggressive tumor biology with intensive tumor stroma interaction, early metastasis and primary resistance to multiple cytotoxic drugs. In 1997, a pivotal clinical trial defined gemcitabine as a standard of care for pts with advanced PDAC (Burris et al. 1997). Over the past two decades, efforts to improve treatment efficacy by combining gemcitabine with cytotoxic agents were unsuccessful (Brodowicz et al. 2000; Heinemann et al. 2000, 2006; Xiong et al. 2004; Louvet et al. 2005; Oettle et al. 2005; Xiros et al. 2005; Okusaka et al. 2006; Ghosn et al. 2007). Also, combinations of gemcitabine with molecularly targeted agents failed to provide relevant improvements (Xiong et al. 2004). Only erlotinib lead to a statistically significant prolongation of median OS when added to gemcitabine in a large phase III study (Dragovich et al. 2007; Moore et al. 2007). It appeared that only a subgroup of patients, which is not precisely defined by predictive biomarkers, benefits from erlotinib. In the overall population, a clinically meaningful advancement was not observed (Dragovich et al. 2007; Moore et al. 2007).
Recently, two new chemotherapy combinations were introduced based on superiority over gemcitabine as demonstrated in large randomized trials. A phase II–III trial (ACCORD-11) by the French consortium study group evaluated the efficacy of oxaliplatin, 5-fluorouracil, leucovorin and irinotecan (FOLFIRINOX) compared to gemcitabine monotherapy in pts with metastastic disease (Conroy et al. 2011). The FOLFIRINOX regimen significantly increased the overall response rate (ORR) (31% vs. 9.4%), median PFS (6.4 vs. 3.3 months) and median OS (11.1 vs. 6.8 months) (Conroy et al. 2011). However, hematological and gastrointestinal toxicities of FOLFIRINOX are substantial. Only younger patients (< 75 years) with good performance status without high bilirubin levels (< 1.5 upper limit of normal-ULN) were eligible. A large randomized phase III study (MPACT) compared nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in combination with gemcitabine monotherapy in patients with metastatic PDAC (Von Hoff et al. 2014). Combination therapy significantly improved the ORR (23% vs 7%), median PFS (5.5 vs. 3.7 months) and median OS (8.5 vs. 6.7 months). In this trial, also elderly patients with good performance status (KPS ≥ 70%) and bilirubin levels within normal range (< ULN) were included (Von Hoff et al. 2014).
In clinical practice, a large proportion of patients with LAPC and mPDAC failed to meet the inclusion criteria of the ACCORD-11 and MPACT trials, and are not eligible for intensive upfront combination chemotherapy. It remains unclear, whether such patients might benefit from sequential administration of active agents over several treatment lines embedded in multidisciplinary supportive care that can be provided at expert centers.
Against this background, we analyzed the outcome of unselected pts with LAPC and mPDAC treated at the West German Cancer Center (WTZ) in the era before the broad clinical implementation of nab-paclitaxel and FOLFIRINOX.
Methods
Study design
We retrospectively evaluated the outcome of patients with histological confirmed LAPC or mPDAC treated with palliative chemotherapy at the West German Cancer Center between January 2007 and September 2011. Patients were evaluable, if they have received at least one dose of chemotherapy. If patients were resected and had a relapse or developed metastasis, patients were included at the time point of first palliative chemotherapy. The chemotherapy regime was selected based on the performance status, comorbidities and patient’s wish by the oncologist after the patient was routinely discussed in the multidisciplinary tumor board of the West German Cancer Center (WTZ) consisting of oncologists, pathologists, surgeons, gastroenterologists, radiation oncologists and radiologists. Some patients received therapy within a prospective clinical trial conducted at the WTZ. Follow-up and toxicities were routinely assessed and documented in the electronic health record (EHR) of the WTZ. Clinicopathological parameters, applied chemotherapy protocols, number of chemotherapy courses, and efficacy data as well as laboratory values were also retrieved from the EHR. All data were pseudonymized for further analysis. Statistical and correlative analyses were performed by a blinded independent researcher using SPSS Statistics (V19, IBM, Armonk, NY, USA). The study was approved by the local Ethics Committee of the Medical Faculty of the University Duisburg-Essen (No. 15-6497).
Assessments
All patients except one with an undifferentiated large cell carcinoma had a histological or cytological confirmed adenocarcinoma. The majority of patients (64.7%) were diagnosed by histology according to the current WHO criteria; the other patients (35.3%) were only diagnosed by cytology (Bosman and Carneiro 2010). The American Joint Committee on Cancer (AJCC)/International Union Against Cancer (UICC) TNM classification (7th Edition) was used for staging. Clinical staging was routinely based on computed tomography (CT) or magnetic resonance imaging (MRI). Overall response rate (ORR) was evaluated according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) (Therasse et al. 2000; Eisenhauer et al. 2009). Patients were eligible for response assessment if at least one baseline CT or MRI and one follow-up imaging upon chemotherapy at our center was available. Progression-free survival (PFS) was defined as time from start of chemotherapy to date of radiologic or clinical progression or death. Radiological examination was performed routinely every 8–12 weeks depending on the respective chemotherapy protocol. Overall survival was defined as time from start of palliative therapy to death. Patients were censored at the time of last follow-up, if time of death was not evaluable.
Statistical analysis
All analyses were conducted using SPSS Statistics (V19, IBM, Armonk, NY, USA). Correlation analyses were performed using Pearson’s Chi-square test. Kaplan–Meier calculations with the log rank test were used for analysis of OS and PFS. Univariate and multivariate analyses were performed by a Cox proportional-hazard model. Hazard ratio (HR) and 95% confidence intervals (CI) were indicated. Overall, p values ≤ 0.05 were regarded statistically significant. A trend was assumed in case of p values ≤ 0.1.
Results
Patients’ characteristics
A total of 201 patients (108 males, 93 females) with LAPC or mPDAC treated between 2007 and 2011 were identified and included in this analysis. The median follow-up time was 68 months (range 0.5–135 months).
Patients’ baseline characteristics are summarized in Table 1. The majority of patients presented with metastatic disease (79.6%, mPDAC), 20.4% patients had locally advanced pancreatic cancer (LAPC). In 55.7%, 19.0% and 9.2% of cases, tumors were localized in the pancreatic head, body and tail, respectively. The most prevalent metastatic sites were liver (57.4%), lymph nodes (24.6%), lung (11.2%) and the peritoneum (24.0%) (Table 1). At base line serum bilirubin was elevated (> 1.5 ULN) in 14.2% of patients. Elevation of the tumor marker carbohydrate antigen 19-9 (CA 19-9), defined as increase over at least 1.5 times the upper limit of normal (ULN), was found in 75.4% of patients (median 514 U/ml, range 1–463,600 U/ml). CA19-9 levels were significantly higher in patients with mPDAC (median 637 U/ml) than in patients with LAPC (median 117 U/ml, p = 0.003, t test).
The primary tumor was resected in 21% of patients either in curative or palliative intent, and additive/adjuvant chemotherapy was administered in 87.5% of those patients. Median disease-free survival in patients with complete resection (defined as R0 resection at postoperative histopathology) was 12 months (range 1–82 months). Placement of a stent for management of biliary obstruction was required in 11.6% of patients.
Palliative chemotherapy
Several first-line therapy protocols were administered, which are summarized in Table 2. The majority of patients (53.9%) received first-line treatment with gemcitabine, whereas 16.2% received gemcitabine in combination with oxaliplatin or cisplatin. Additional combination therapies such as FOLFOX, FOLFIRI or gemcitabine in combination with erlotinib were administered in 29.8% of patients. In total, 56.3% of patients received second-line and further-line therapies (third-line therapy 30.6%, fourth-line therapy 11.9%). Those therapies were largely fluoropyrimidine-based combination chemotherapies such as FOLFOX or FOLFIRI. The median number of therapy lines was 2 (range 1–4).
Efficacy of palliative chemotherapy
In total, 102 patients (50.6%) were evaluable for response analysis according to RECIST 1.1. The ORR of first-line therapy was 18.9%, and the DCR (defined as partial remission or sustained disease stabilization) was 72.3% (Table 3). Two-thirds of patients showed some extent of tumor shrinkage at follow-up CT scanning with a mean tumor reduction of − 8% per RECIST 1.1 (Fig. 1).
As expected ORR and DCR of second- and further-line therapy were lower than of first-line treatment (Table 4).
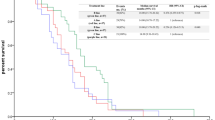
Overall survival and PFS analysis also included patients with incomplete CT or MRI datasets, which were not suitable for RECIST1.1 evaluation. The median PFS with first-line therapy was 2.7 months. There was no significant difference between gemcitabine monotherapy and combination therapies (p = 0.116, log rank) (Fig. 2; Table 5). The median PFS times of second-, third-, and further-line therapies were 2.9, 2.0 and 1.8 months, respectively. The median OS from start of palliative treatment was 10.5 months for the entire population, and 8.5 months for patients with metastatic disease (Table 5). Interestingly, patients receiving first-line treatment with gemcitabine had a significantly longer median OS than patients treated with gemcitabine combination therapies (13.2 vs. 8.1 months, p = 0.006, log rank) (Fig. 3). Patients with higher tumor burden or tumor-related symptoms often receive more intensive cytotoxic combinations, which could introduce bias on OS. As surrogate marker for tumor burden, we correlated the sum of the longest target lesions’ diameters according to RECIST1.1 with the chemotherapy protocol (monotherapy vs. combination therapy). The mean tumor burden for all evaluable patients was 79.3 mm (range 13.6–205.8 mm). Patients who received gemcitabine monotherapy had a baseline mean tumor burden of 82.0 mm (range 13.6–205.8 mm), whereas patients who received combination therapies had a baseline mean tumor burden of 78.4 mm (range 25.0–148.2 mm) (r = 0.038; p = 0.754 pearson). Hence, baseline tumor burden per RECIST 1.1 did not correlate with type of first-line therapy. However, baseline tumor burden significantly correlated with the OS. Patients with a tumor burden above the mean exhibited a median OS of 7.9 months, which was dramatically reduced as compared to patients with a tumor burden below the mean (median OS 15.8 months; p < 0.001, log rank test) (Fig. 4). Next, we correlated the type of first-line therapy with patient-related parameters including age, gender, and serum levels of CA19-9 and bilirubin to exclude further potential sources of bias. None of the analyzed parameters correlated with the type of first-line therapy (all p > 0.05) (Table 6).
Kaplan–Meier plot for progression-free survival (PFS) upon first-line chemotherapy for patients receiving gemcitabine or combination therapies. Patients with gemcitabine monotherapy had a median PFS of 3.6 months and patients receiving combination therapies had a median PFS of 2.3 months. The difference was not significantly different (p = 0.116, log rank)
Kaplan–Meier plot for overall survival (OS) since start of palliative chemotherapy with gemcitabine or combination therapies. Patients with gemcitabine monotherapy had a median OS of 13.2 months and patients receiving combination therapies had a median OS of 8.1 months. The difference between the groups was statistically significant (p = 0.006, log rank)
Kaplan–Meier plot for overall survival (OS) since start of palliative therapy for patients with baseline tumor burden above vs. below the mean tumor target sum (TTS). Patients with a TTS above the mean (TTS > mean) had a median OS of 7.5 months and patients with a TTS below the mean (TTS < mean) had a median OS of 15 months. The difference between the groups was statistically significant (p < 0.001, log rank)
Median PFS of patients achieving an objective response (RECIST 1.1) with first-line therapy was significantly prolonged as compared to patients with progressive disease as best response (median PFS 5.2 vs. 1.4 months, p < 0.001). In contrast, there was no significant difference in median PFS between responders and those patients achieving stable disease with first-line therapy (median PFS 5.2 vs. 3.9 months, p = 0.203) (Fig. 5). Also, there was no difference in median OS between those two groups of responders (p > 0.05) (Fig. 6). As expected, patients with progressive disease under first-line therapy had a dramatically reduced OS (median 4 months).
Kaplan–Meier plot for progression-free survival (PFS) upon first-line chemotherapy for patients achieving a complete or partial response (CR/PR), stable disease (SD/NC) or progressive disease (PD) according to RECIST1.1. Median PFS for CR/PR: 5.2 months, for SD/NC 3.9 months and for PD 1.4 months. In patients with CR/PR and NC/SD median PFS was prolonged compared to those with PD (p < 0.001, log rank)
Kaplan–Meier plot for overall survival (OS) since start of first-line chemotherapy for patients achieving a complete or partial response (CR/PR), stable disease (SD/NC) or progressive disease (PD) according to RECIST1.1. Median OS for CR/PR: 12.3 months, median OS for SD/NC: 11.7 months, median OS for PD 4.0 months. There was no statistically significant difference between the groups (p = 0.139, log rank)
Evaluation of explorative prognostic markers
In an exploratory multivariate analysis, we studied the potential impact of clinicopathological parameters on treatment outcomes. Parameters included age (median), tumor target sum (> vs < mean), stage, response/disease stabilization upon first-line therapy vs. progressive disease, first-line monotherapy vs. combination therapy, number of chemotherapy lines (> 2 vs ≤ 2), CA19-9 (below vs. above median) and bilirubin (< 1.5 ULN vs. >1.5 ULN). Only age, tumor target sum and number of chemotherapy lines significantly correlated with OS (p < 0.05) (Fig. 7). Next, we focused on patients who received sequential treatment lines. In agreement with the results from multivariate analysis, OS significantly correlated with the number of chemotherapy lines in the Kaplan–Meier analysis. Patients with only one therapy line had a median OS of 4.0 months, whereas patients with two lines had a median OS of 11.2 months and patients with three or more lines had a median OS of 18.5 months (p = 0.001 one line vs. two lines, p < 0.001 one line vs. three lines, p = 0.032 two lines vs. three lines) (Fig. 8). Next, we stratified the patients by tumor burden (> vs < mean tumor target sum). Interestingly, median OS significantly correlated with the number of chemotherapy lines in both patient populations. Patients with low tumor burden, which received only one therapy line had a median OS of 8.0 months, whereas patients with two lines had a median OS of 14.4 months and patients with three or more lines had a median OS of 18.5 months (p = 0.035) (suppl. Figure 1A).Patients with high tumor burden, which received one therapy line had a median OS of 4.0 months, whereas patients with two lines had a median OS of 9.6 months and patients with three or more lines had a median OS of 12.8 months (p = 0.005) (suppl. Figure 1B).
Forest plot of hazard rations for the multivariate analysis of overall survival including age, tumor target sum (TTS), stage (M1 vs. M0), response to first-line chemotherapy [disease control (DCR) vs. progressive disease (PD)], type of first-line chemotherapy (gemcitabine monotherapy vs. doublet), number of therapy lines (> 2 vs ≤ 2), CA19.9 level, bilirubin level. The HR with (95% confidence intervals—CI) for age > vs < median: 0.419 (0.215–0.816, p = 0.011); for TTS > vs < mean: 2.210 (1.026–4.762, p = 0.043); for stage M1 vs. M0: 1.448 (0.563–3.727, p = 0.443); for DCR vs. PD: 0.528 (0.244–1.139, p = 0.104); for first-line mono vs. doublet: 1.496 (0.709–3.154, p = 0.290); for CTX lines > 2 vs < 2: 0.408 (0.196–0.849, p = 0.016); for CA19.9 > vs < median: 1.281 (0.648–2.532, p = 0.476) and for bilirubin < vs > 1.5 x upper limit norm (ULN): 0.356 (0.098–1.290, p = 0.114)
Kaplan–Meier plot for overall survival (OS) since start of palliative therapy in patients, which received different number of chemotherapy lines. Median OS of patients with one therapy line: 4.0 months, patients with two therapy lines: 11.2 months and patients with three or more therapy lines: 18.5 months (p = 0.001 one line vs. two lines, p < 0.001 one line vs. three lines, p = 0.032 two lines vs. three lines, log rank)
Discussion
Here, we describe the clinical outcome of unselected patients with advanced PDAC treated with systemic palliative chemotherapy at a large comprehensive cancer center before the approval and routine implementation of modern chemotherapy protocols such as nab-paclitaxel, FOLFIRINOX and nanoliposomal irinotecan. In contrast to large randomized, multicenter phase III trials enrolling highly selected patients without significant comorbidities, we included all patients with LAPC or mPDAC, which received at least one dose of cytotoxic therapy at our center, irrespectively of performance status, age, laboratory abnormalities or comorbidities. In line, a substantial number of elderly patients and patients with elevated bilirubin levels were included. This is in contrast to the published Prodige4/ACCORD11 and the MPACT phase III trial with FOLFIRINOX or gemcitabine/nab-paclitaxel in first-line for mPDAC or the NAOPLI-1 trial with nanoliposomal irinotecan/5-FU in second-line (Conroy et al. 2011; Von Hoff et al. 2014; Wang-Gillam et al. 2015). In these randomized trials, patients older than 75 years, with poor performance status and/or with elevated bilirubin levels were excluded. In our retrospective study, 7.5% of patients were older than 75 years and 14.2% had elevated bilirubin levels > 1.5xULN. As expected, 20% of our patients had LAPC and 80% had mPDAC. Thus, our cohort is representative of real-world patient populations commonly treated at Medical Oncology Departments in large comprehensive cancer centers.
In this retrospective analysis, we included only patients, who were diagnosed before 2011, when the positive results of the Prodige4/ACCORD11 trial were published (Conroy et al. 2011). This explains why the majority of our patients received first-line monotherapy with gemcitabine. However, some patients received combination therapies of gemcitabine with platinum agents (cisplatin or oxaliplatin) or erlotinib. In addition, a substantial number of patients were treated with fluoropyrimidine-based combinations with irinotecan or oxaliplatin. These patients mainly had been pretreated with gemcitabine in the adjuvant setting and had experienced early relapse. In the subgroup of RECIST-evaluable patients, the ORR was 18.9%, which is slightly higher than the ORR reported in the pivotal trial of gemcitabine (Burris et al. 1997) or in the gemcitabine control arms of the Prodige4/ACCORD11 and MPACT studies (Conroy et al. 2011; Von Hoff et al. 2014) (suppl. Table 4). In part, this could result from including patients with LAPC in our analysis. Interestingly, we found no significant difference in ORR between gemcitabine monotherapy and combination therapies in our patient population. In contrast, ORR in patients treated with gemcitabine/platinum combinations was numerically lower than in patients treated with gemcitabine monotherapy or fluoropyrimidin-based therapy. However, the RECIST-evaluable patient population treated with gemcitabine/platinum was of limited size. In line with the lower ORR, patients treated with chemotherapy doublets had lower median PFS and OS.
The median OS of our entire patient population was 10.5 months, and was 8.5 months in patients with metastatic disease. This is higher than expected in this unselected patient population. Thus, the median OS of our patients with mPDAC was identical to the median OS observed in the experimental arm of the MPACT study, in which first-line nab-paclitaxel/gemcitabine was administered in a highly selected patient population (Von Hoff et al. 2014) (Table 7). However, median PFS of first-line treatment in our patient population was rather short with only 2.7 months and comparable with results from other clinical trials of gemcitabine monotherapy. Thus, additional factors must have contributed to this unexpectedly favorable median OS. Of note, two-thirds of our patients received second-line treatment, and one-third even received third- and further-line therapies. This compares favorably with the Prodige4/ACCORD11 trial, where a total of 46.8% of patients received second-line treatment, mostly gemcitabine monotherapy or gemcitabine combinations (Conroy et al. 2011). In contrast, in the MPACT study only 38% of patients received second-line chemotherapies (Von Hoff et al. 2014). Second-line chemotherapy of mPDAC was shown to be effective and prolonged OS vs. best supportive care (BSC) in randomized phase III studies (Pelzer et al. 2011; Rahma et al. 2013). However, there is no established standard, and the choice of second-line therapy is mostly guided by the type of first-line therapy that was chosen. In patients with progressive disease after gemcitabine-based first-line therapy promising results were demonstrated for a combination of oxaliplatin, fluorouracil and folinic acid (OFF) as compared to fluorouracil and folinic acid (FF) in a randomized phase III trial (CONKO-003) (Oettle et al. 2014). Median OS with OFF was 5.9 months as compared to 3.3 months with FF(Oettle et al. 2014). Interestingly, the randomized phase III PANCREOX trial demonstrated no benefit from the addition of oxaliplatin, administered as mFOLFOX6, versus infusional FF in patients with advanced pancreatic cancer after failure of gemcitabine (Gill et al. 2016). In contrast to the OFF protocol the dose intensity of oxaliplatin in the mFOLFOX6 protocol was markedly higher and a substantially higher number of patients were withdrawn due to adverse events in the mFOLFOX6 arm than in the FF arm. In addition, significantly more patients in the FF arm (23%) received subsequent therapies than patients randomized to mFOLFOX6 (7%), which could have impacted on the post-progression OS (Gill et al. 2016). In conclusion, treatment tolerance seems to be one of the important factors in patients with advanced pancreatic cancer. Recently, the NAPOLI-1 trial led to the approval of nanoliposomal irinotecan (nal-iri) in combination with FF in patients with advanced pancreatic cancer after gemcitabine pretreatment (Wang-Gillam et al. 2015). In particular, the implementation of sequential therapeutic strategies is now possible with more effective agents becoming available in this challenging disease. In line, in our patient cohort multivariate analysis confirmed the number of applied therapy lines as an independent prognostic factor for improved OS. Thus, a “continuum of care” with the sequential application of all effective cytotoxic agents should be considered as alternative therapeutic strategy for mPDAC patients. This may be particularly appropriate for patients, which are ineligible for intensive first-line combinations such as FOLFIRINOX.
References
Bosman FT, Carneiro F (2010) WHO Classification of Tumours of the Digestive System (World Health Organization Classification of Tumours, Volume 3) WHO Classification of Tumours
Brodowicz T, Wolfram RM, Kostler WJ et al (2000) Phase II study of gemcitabine in combination with cisplatin in patients with locally advanced and/or metastatic pancreatic cancer. Anticancer Drugs 11:623–628
Burris HA, Moore III, Andersen MJ J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15:2403–2413
Conroy T, Desseigne F, Ychou M et al (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825
Dragovich T, Huberman M, Von Hoff DD et al (2007) Erlotinib plus gemcitabine in patients with unresectable pancreatic cancer and other solid tumors: phase IB trial. Cancer Chemother Pharmacol 60:295–303
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Ghosn M, Farhat F, Kattan J et al (2007) FOLFOX-6 combination as the first-line treatment of locally advanced and/or metastatic pancreatic cancer. Am J Clin Oncol 30:15–20
Gill S, Ko YJ, Cripps C, Beaudoin A et al (2016) PANCREOX: a randomized phase iii study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 34(32):3914–3920
Heinemann V, Wilke H, Mergenthaler HG et al (2000) Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol 11:1399–1403
Heinemann V, Quietzsch D, Gieseler F et al (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24:3946–3952
Jemal A, Siegel R, Xu J et al (2010) Cancer statistics. CA Cancer J Clin 60:277–300
Louvet C, Labianca R, Hammel P et al (2005) Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 23:3509–3516
Moore MJ, Goldstein D, Hamm J et al (2007) Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 25:1960–1966
Neoptolemos JP, Palmer DH, Ghaneh P et al (2017) Comparison of adjuvan gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-lable, randomised, phase 3 trial. Lancet 389(10073):1011–1024
Oettle H, Richards D, Ramanathan RK et al (2005) A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol 16:1639–1645
Oettle H, Riess H, Stieler JM et al (2014) Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 32:2423–2429
Okusaka T, Ishii H, Funakoshi A et al (2006) A phase I/II study of combination chemotherapy with gemcitabine and 5-fluorouracil for advanced pancreatic cancer. Jpn J Clin Oncol 36:557–563
Pelzer U, Schwaner I, Stieler J et al (2011) Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer 47:1676–1681
Rahma OE, Duffy A, Liewehr DJ et al (2013) Second-line treatment in advanced pancreatic cancer: a comprehensive analysis of published clinical trials. Ann Oncol 24:1972–1979
Saif MW (2013) Advancements in the management of pancreatic cancer. JOP 14:112–118
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Von Hoff DD, Ervin T, Arena FP et al (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703
Von Hoff DD, Goldstein D, Renschler MF (2014) Albumin-bound paclitaxel plus gemcitabine in pancreatic cancer. N Engl J Med 370:479–480
Wang-Gillam A, Li CP, Bodoky G et al (2015) Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387:545–557
Xiong HQ, Rosenberg A, LoBuglio A et al (2004) Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol 22:2610–2616
Xiros N, Papacostas P, Economopoulos T et al (2005) Carboplatin plus gemcitabine in patients with inoperable or metastatic pancreatic cancer: a phase II multicenter study by the Hellenic Cooperative Oncology Group. Ann Oncol 16:773–779
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest in relation to this study.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
A retrospective study does not allow the retrospective obtaining of patient´s informed consent (ICF) from deceased patients. Against this background, the study was approved by the local Ethics Committee of the Medical Faculty of the University Duisburg-Essen (No. 15-6497).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl. Fig. 1
Kaplan–Meier plot for overall survival (OS) since start of palliative therapy in patients with low (A) or high (B) tumor burden (tumor target sum – TTS <mean or >mean), which received different number of chemotherapy lines. Median OS of patients with TTS<mean and one therapy line: 8.0 months, patients with two therapy lines: 14.4 months and patients with three or more therapy lines 18.5 months (p=0.035 log rank). Median OS of patients with TTS>mean and one therapy line: 4.0 months, patients with two therapy lines: 9.6 months and patients with three or more therapy lines 12.8 months (p=0.005 log rank). (TIF 63 KB)
Rights and permissions
About this article
Cite this article
Abendroth, A., Noureddine, R., Abramczyk, M. et al. Long-term outcome of patients with advanced pancreatic cancer treated with sequential chemotherapies before the era of modern combination therapy protocols. J Cancer Res Clin Oncol 145, 445–455 (2019). https://doi.org/10.1007/s00432-018-2789-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2789-z