Abstract
Purpose
This study aimed to investigate the prognostic value of different organs metastases in patients with non-small cell lung cancer (NSCLC) and its most common subtypes.
Methods
We identified 45,423 NSCLC cases (25,129 men and 20,294 women) between 2010 and 2013 with distant metastases, with complete clinical information obtained from the surveillance, epidemiology, and end results (SEER) database.
Results
Bone and liver were the most and the least common metastatic sites with rates of 37.1 and 16.8%, respectively. The mortality rates associated with bone, brain, liver, lung metastases, and multiorgan metastases (MOM) were 73.2, 72.7, 78.3, 65.4, and 77.5%, respectively. Kaplan–Meier analyses demonstrated that patients with MOM and liver metastasis had the worst survival. Compared with NSCLC cases with other organ metastasis, but without the four organs metastasis, hazard ratios (HRs) for lung, bone, brain, and liver metastases, and MOM were 0.906 (95% CI 0.866–0.947), 1.276 (95% CI 1.225–1.330), 1.318 (95% CI 1.260–1.379), 1.481 (95% CI 1.388–1.580), and 1.647 (95% CI 1.587–1.709), respectively. Similar results were obtained for adenocarcinoma (AD) cases.
Conclusions
The mortality risk is highest with MOM and liver metastasis followed by bone, brain, other organ, and lung metastases in NSCLC and AD which is the most common variant for NSCLC. These results will be helpful for pre-treatment evaluation regarding the prognosis of NSCLC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a major public health problem worldwide with a rapid rise in incidence in recent years. Lung cancer is the leading cause of cancer mortality in 94 countries for men and in 17 countries for women (Bray et al. 2012). In 2017, the American Cancer Society estimated the number of deaths from new cases of lung and bronchus cancer to be 222,500 and 155,870, respectively. In addition, lung cancer has become the second most common cancer in both males and females (Siegel et al. 2017).
Lung cancer consists of several histological variants, non-small cell lung cancer (NSCLC) and small cell lung cancer. Adenocarcinoma (AD) and squamous cell carcinoma (SQCC) are the major variants of NSCLC. Approximately 40% of new NSCLC cases have been reported to have distant metastases at diagnosis (Morgensztern et al. 2010), and most patients had a low 5-year survival rate (Detterbeck et al. 2017). The eighth edition of the lung cancer staging manual published by the American Joint Committee on Cancer (AJCC) defines metastases as follows: M1c—multiple extra-thoracic metastases in a single organ or multiple organs; M1b—single extrathoracic metastasis; and M1a—intrathoracic metastasis only (Rami-Porta et al. 2017). Although previous studies have shown that NSCLC patients with single metastatic lesions survive longer than those with multiple metastatic lesions, it is unclear whether metastases to particular organ systems have different prognoses (Eberhardt et al. 2015). Some studies have reported that liver metastasis is associated with poor survival (Chang et al. 2017; Ren et al. 2016), however, most of these studies had small sample sizes and limitations with the statistical analysis performed. There is still a paucity of data pertaining to the prognosis of different organ metastasis in NSCLC.
In the present study, we aimed to determine the prognosis associated with metastasis to different organs in patients with NSCLC and its major subtypes using data from the surveillance, epidemiology, and end results (SEER) database.
Methods
Patients and clinicopathological data
This retrospective study assessed the association between MOM and disease-specific survival in patients with NSCLC and its subtypes, utilizing data on NSCLC patients from the SEER database, maintained by the National Cancer Institute. The SEER project is a United States population-based cancer registry that began in 1973 and includes approximately 10% of the US population.
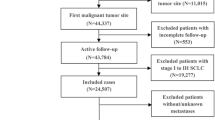
Cases of lung cancer diagnosed from 2010 to 2013 with complete information about distant metastases available in the SEER database were included in this study. Based on imaging studies, distant metastasis refers to the appearance of malignant lesions outside the locoregional thorax and/or mediastinum. Initially, 90,817 cases with lung cancer were identified. A total of 45,423 cases were included for analyses, after excluding the following ineligible cases: 20,576 cases with two or more primary sites, 6918 with incomplete information on organs metastases, 55 cases with no information on survival, and 17,845 cases with small cell lung cancer.
NSCLC cases were classified according to histologic type: AD (histologic codes 8244, 8245, 8250–8255, 8260, 8290, 8310, 8323, 8333, 8480, 8481, 8490, 8507, 8550, 8570, 8571, 8574, and 8576), SQCC (histologic codes 8052, 8070–8075, 8083, 8084, 8123), large cell carcinoma (histologic codes 8012–8014), and code (8046, 8050, 8003, 8004, 8022, 8031–8035, 8082, 8200, 8240, 8249, 8430, 8560, 8562, 8980). A total of 45,423 NSCLC cases, including 28,329 ADs and 9081 SQCCs with complete information were divided into six groups and analyzed for association between disease-specific survival and organ metastasis. The six groups included patients with (1) distant metastases, but none to bone, liver, lung, and brain (defined as other metastasis group), (2) bone metastasis without brain, liver, or lung metastasis, (3) brain metastasis without bone, liver, or lung metastasis, (4) liver metastasis without bone, brain, or lung metastasis, (5) lung metastasis without bone, brain, or liver metastases, and (6) two or more metastatic organs among lung, liver, brain and bone (multiorgan metastases, MOM).
Statistical analyses
The Pearson Chi square test was used for comparisons of categorical variables. NSCLC-specific survival probability was calculated using Kaplan–Meier analysis with follow-up time censored. Cox regression was performed to examine the effect of organ metastasis on disease-specific survival and presented in terms of HRs and 95% CI. A two-tailed P value less than 0.05 was considered to be significant. Data were analyzed using the Statistical Package for Social Science version 18.0 (SPSS, Inc., New York, NY, USA).
Results
Patient characteristics
Data for a total of 45,423 NSCLC patients (25,129 men and 20,294 women) with distant metastasis were investigated. The median age was 68 years (IQR 59–76) and the median follow-up time was 4 months (IQR 1–10). Bone was the most common site with a metastatic rate of 37.1% (16,850/45,423), and liver was the least common metastatic site with a rate of 16.8% (7653/45,423). The rates of brain and lung metastasis were 25.6% (11,647/45,423) and 30.6% (13,907/45,423), respectively. Similar results were obtained for AD cases, with a metastatic rate of 39.2% (11,102/28,329) in bone, 27.5% (7797/28,329) in brain, 16.0% (4525/28,329) in liver, and 31.4% (8907/28,329) in lung. The metastatic rates associated with SQCC cases were 31.7% (2879/9081), 16.7% (1520/9081), 17.4% (1583/9081) and 31.4% (2848/9081) in bone, brain, liver, and lung, respectively (Supplementary Table 1).
Table 1 shows the general characteristics of all patients. Among the most common organ metastasis, 7615 (16.8%) cases had bone metastasis alone, 6615 (14.6%) cases had lung metastasis alone, 5572 (12.3%) cases had brain metastasis alone and 2033 (4.5%) cases had liver metastasis alone. In addition, 12,167 (26.8%) cases showed multiorgan metastases (MOM; M1c). As for the location of the primary tumors, 54.3% (24,656/45,423) occurred in the right upper lobe (Table 2).
Comparison of disease-specific mortality between different organ metastasis groups
As shown in Table 3, the overall lung cancer specific mortality was 72.3% (32,822/45,423). The mortality rates were 73.2% (5575/7615), 72.7% (4052/5572), 78.3% (1591/2033) and 65.4% (4323/6615) for bone, brain, liver, and lung metastasis groups, respectively. MOM group had a mortality rate of 77.5% (9426/12,167), while it was 68.8% (7855/11,421) for other metastasis group. Compared to other metastasis group, the crude hazard ratios (HRs) (95% confidence intervals [CIs]) were 1.157 (1.117–1.197) for bone metastasis, 1.070 (1.030–1.111) for brain metastasis, 1.391 (1.318–1.468) for liver metastasis, 0.901 (0.868–0.935) for lung metastasis and 1.415 (1.373–1.458) for MOM (all P < 0.001). After adjustment for age, gender, and tumor size, the HRs and 95% CIs were 1.276 (1.225–1.330) for bone metastasis, 1.318 (1.260–1.379) for brain metastasis, 1.481 (1.388–1.580) for liver metastasis, 0.906 (0.866–0.947) for lung metastasis and 1.647 (1.587–1.709) for MOM (all P < 0.001). Liver metastasis and MOM had higher mortality rates and HRs than the other groups. In addition, compared with the liver metastasis group, the MOM group had a crude HR (95% CI) of 1.013 (0.961–1.068, P = 0.633), which became significant after adjustment for age, gender, and tumor size [1.116 (1.048–1.189, P = 0.001)]. Interestingly, when cases with liver metastasis were excluded from the MOM group, the crude HR of MOM was 0.878 (0.829–0.929, P < 0.001) compared to liver metastasis group but became insignificant after adjustment for age, gender, and tumor size. These data suggested that MOM and liver metastasis had comparable HRs. Similar results were obtained when we used lung metastasis as control (Supplementary Table 2). As showed in Supplementary Table 2, compared with lung metastasis group, the crude HRs (95% CIs) were 1.29 (1.24–1.34) for bone metastasis, 1.19 (1.14–1.25) for brain metastasis, 1.56 (1.47–1.65) for liver metastasis and 1.58 (1.53–1.64) for MOM. After age, gender, and tumor size adjustment, the HR remained significant. Our data demonstrated a robust effect of MOM and liver metastasis on NSCLC-specific survival.
Comparison of disease-specific mortality between the liver metastasis and MOM groups for AD and SQCC cases
The analyses for AD showed similar results as seen in all NSCLC patients. As shown in Table 4, the overall disease-specific mortality rate was 69.4% (19,650/28,329). The mortality rates were 69.8% (3364/4820), 69.3% (2467/3561), 75.7% (773/1021), and 62.5% (2481/3967) in the bone, the brain, the liver, and the lung metastasis groups, respectively. The MOM group had a mortality rate of 74.2% (6032/8127), while it was 66.3% (4533/6833) in other metastasis group. Compared to other metastasis group, the crude HRs (95% CIs) were 1.116 (1.067–1.167, P < 0.001) for bone metastasis, 1.025 (0.976–1.076, P = 0.329) for brain metastasis, 1.362 (1.262–1.471, P < 0.001) for liver metastasis, 0.879 (0.837–0.924, P < 0.001) for lung metastasis and 1.367 (1.315–1.422, P < 0.001) for MOM. After adjustment for age, gender and tumor size, the HRs and 95% CIs were 1.251 (1.184–1.322, P < 0.001) for bone metastasis, 1.288 (1.213–1.367, P < 0.001) for brain metastasis, 1.445 (1.314–1.589, P < 0.001) for liver metastasis, 0.881 (0.828–0.937, P < 0.001) for lung metastasis and 1.605 (1.528–1.685) for MOM (P < 0.001). Liver metastasis and MOM had higher mortality rates and HRs than the other groups. In addition, compared with the liver metastasis group, MOM group had a crude HR (95% CI) of 0.995 (0.923–1.073, P = 0.898) which became significant after adjustment for age, gender, and tumor size [1.109 (1.011–1.215, P = 0.028)]. When the cases with liver metastasis were excluded from the MOM group, the crude HR of MOM was 0.869 (0.804–0.940, P < 0.001) compared with the liver metastasis group, but lost significance after adjustment for age, gender, and tumor size. In summary, MOM and liver metastasis alone had comparable HRs in AD.
In SQCC, the overall disease-specific mortality rate was 76.0% (6902/9081). The mortality rates were 80.0% (1201/1501), 80.3% (644/802), 80.3% (454/566), and 68.4% (1188/1738) in the bone, the brain, the liver, and the lung metastasis groups, respectively. While the MOM group had a mortality rate of 85.0% (1578/1857), the mortality rate was 70.2% (1837/2617) in the group without metastasis to the four organs. Compared to the group without metastases to the four organs, the crude HRs (95% CIs) were 1.404 (1.305–1.511, P < 0.001) for bone metastasis, 1.346 (1.230–1.473, P < 0.001) for brain metastasis, 1.410 (1.272–1.564, P < 0.001) for liver metastasis, 0.949 (0.882–1.021, P = 0.163) for lung metastasis, and 1.790 (1.672–1.918, P < 0.001) for MOM. After adjustment for age, gender and tumor size, the HRs and 95% CIs were 1.478 (1.360–1.607, P < 0.001) for bone metastasis, 1.543 (1.392–1.710, P < 0.001) for brain metastasis, 1.544 (1.373–1.737, P < 0.001) for liver metastasis, 0.970 (0.893–1.054, P = 0.473) for lung metastasis, and 1.991 (1.839–2.155, P < 0.001) for MOM. Similar to the cohort of NSCLC and its most common subtype AD, MOM group had highest mortality and HR in SQCC. However, liver metastasis did not show significantly worse survival (Table 5). Similar results were obtained when compared with lung metastasis group (Supplementary Table 2).
Kaplan–Meier analyses of disease-specific survival of patients with NSCLC and its subtypes
The effects of metastases to different organs on disease-specific survival in NSCLC and its variants are shown in Figs. 1, 2, and 3. As shown in Fig. 1, survival for all six groups decreased sharply (log rank P < 0.001). The best survival was noted for patients without metastases to the four organs and intrathoracic, intermediate survival was occurred in patients with bone and brain metastases, and the worst survival occurred in patients with liver metastases and MOM. Similar results were obtained in AD (Log rank P < 0.001) (Fig. 2). In SQCC, as shown in Fig. 3, MOM had the worst survival, bone, brain, and liver metastases had intermediate survival, and intrathoracic and other metastasis group had better survival (log rank P < 0.001).
Kaplan–Meier analysis of the impact of distant metastases on disease-specific survival of NSCLC. Each P value < 0.001 was compared group by group, liver metastasis versus MOM, P = 0.609. The ‘other’ group was defined as cases with metastasis at diagnosis but without bone, brain, liver, and lung metastases
Kaplan–Meier analysis of the impacts of distant metastases on disease-specific survival of NSCLC-adenocarcinoma (other versus bone, liver, lung, MOM P < 0.001, vs. brain = 0.309; bone versus any group P < 0.001; brain versus liver, lung, M1c P < 0.001; liver versus MOM P = 0.892, versus other, bone, brain, and lung P < 0.001; lung versus any group P < 0.001)
Kaplan–Meier analysis of the impacts of distant metastasis organ on disease-specific survival of NSCLC-squamous cell carcinoma (other versus bone, brain, liver, MOM P < 0.001, vs. lung P = 0.143; bone versus brain P = 0.319, versus liver P = 0.910, versus lung and MOM P < 0.001; brain versus liver P = 0.391, versus lung and MOM P < 0.001; liver versus lung and MOM P < 0.001; lung versus MOM P < 0.001)
Discussion
In this study, we analyzed the prognostic role of organ metastases and its relationship with disease-specific survival and HRs in NSCLC patients from the SEER database. Our data demonstrated that bone and liver were the most and least common metastatic sites, respectively. The groups with metastasis to the lung alone had a better prognosis, while those with metastases to liver and MOM had the worst prognosis. The different clinical outcomes among metastatic sites may be useful in determination of prognosis, which could serve as an important reference for clinicians and patients.
Our data show that NSCLC metastasize predominantly to the bone, with a rate of 37.1% (16,850/45,423) in NSCLC and 39.2% (11,102/28,329) in AD. Although the rates of bone metastasis in lung cancer vary (Brodowicz et al. 2012; D’Antonio et al. 2014; Sugiura et al. 2008), a previous study reported that 30–40% of patients with lung cancer develop bone metastases which is consistent with the present study (D’Antonio et al. 2014). Although previous studies (Morgensztern et al. 2009; Nakazawa et al. 2012; Waqar et al. 2015) have delineated the prognostic effects on survival of NSCLC with distant metastasis, it is now widely accepted that multiple metastatic sites confer worse survival compared with isolated metastasis (Eberhardt et al. 2015; Sanchez de Cos Escuin et al. 2014). Our data are in agreement with these studies.
The effects of intrathoracic metastasis only (M1a) on prognosis of NSCLC is still controversial. M1a in NSCLC patients with malignant pleural effusion has been predictive of poor survival (Goldstraw et al. 2007; Jett et al. 2003; Sugiura et al. 1997). In the present study, NSCLC patients with lung metastasis had a mortality of 65.4% (4323/6615), which was significantly lower than patients with other organ metastasis (P < 0.001). Similar results were observed for both AD and SQCC subtypes. Our results support previous studies indicating that the prognosis of NSCLC patients with M1a at diagnosis might be relatively favorable (Ichinose et al. 2000; Iida et al. 2015; Ohta et al. 2005).
It has been reported that NSCLC patients with distant metastases outside the chest cavity (M1b), and tumors with MOM (M1c) have significantly worse prognosis than those with single organ metastasis (Eberhardt et al. 2015). Our data demonstrated that the MOM group had the worst outcome. Both in the whole cohort and for each tumor histology, the MOM group had a higher rate of mortality than the groups with bone or brain metastases. In the TNM classification of 7th staging manual of AJCC, MOM was defined as M1b, while the AJCC 8th TNM classifies MOM as M1c due to poor survival. Since our study used the TNM classification of 7th staging manual of AJCC, cases with multiple metastases in one organ were not classified as MOM, which could have weakened the prognostic power of M1c.
Previous studies have shown that liver metastasis is associated with worse survival in NSCLC (Tamura et al. 2015), as well as in small cell lung cancer (Nakazawa et al. 2012). Riihimäki M et al. investigated 17,431 cases of lung cancer and found liver was one of the most common metastatic sites (35%) in small cell lung cancer, while bone was the most common site for metastasis in NSCLC. In addition, liver metastasis has been shown to portend poor survival (Riihimaki et al. 2014). In our study, liver metastasis also had a significant effect on prognosis. The mortality rate for this group was as high as 78.3% (1591/2033). The liver and MOM groups had similar HRs before and after adjustment for age, gender, and tumor size. When the patients with liver metastasis were excluded from the MOM group, the two groups showed no significant differences. Liver metastasis showed a prognosis similar to that of M1c, which is the worst stage of lung cancer. Similar results were obtained in the most common subtype, AD. Interestingly, in SQCC, liver metastasis had similar prognosis as bone and brain metastases, which was better than M1c.
This study has several limitations, primarily due to its retrospective design. Importantly, the SEER database only provides data between 2010 and 2013. Moreover, some important information, such as number of metastatic lesions, histology subtypes, gene mutations, and types of therapy were not provided in the SEER database. This information will be needed in future prospective studies.
In conclusion, in NSCLC patients from the SEER database, lung metastasis had the best chance of survival, while liver metastasis was the worst prognostic factor for NSCLC and AD, but not SQCC. Based on our results, lung cancer specific mortality risk for both NSCLC and AD is as follows: MOM = liver metastasis > bone metastasis = brain metastasis > other organ metastasis > lung metastasis. While for SQCC: MOM > liver = bone = brain metastasis > other organ metastasis > lung metastasis. This study provides evidence for the prognostic effects of metastases to different organs, and will be useful in counseling and treating NSCLC patients.
References
Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 13:790–801. https://doi.org/10.1016/S1470-2045(12)70211-5
Brodowicz T, O’Byrne K, Manegold C (2012) Bone matters in lung cancer. Ann Oncol 23:2215–2222. https://doi.org/10.1093/annonc/mds009
Chang YP et al (2017) The impact of de novo liver metastasis on clinical outcome in patients with advanced non-small-cell lung cancer. PLoS ONE 12:e0178676. https://doi.org/10.1371/journal.pone.0178676
D’Antonio C et al (2014) Bone and brain metastasis in lung cancer: recent advances in therapeutic strategies. Ther Adv Med Oncol 6:101–114. https://doi.org/10.1177/1758834014521110
Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT (2017) The eighth edition lung cancer stage classification. Chest 151:193–203. https://doi.org/10.1016/j.chest.2016.10.010
Eberhardt WE et al (2015) The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 10:1515–1522. https://doi.org/10.1097/JTO.0000000000000673
Goldstraw P et al (2007) The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2:706–714. https://doi.org/10.1097/JTO.0b013e31812f3c1a
Ichinose Y et al (2000) The prognosis of patients with non-small cell lung cancer found to have carcinomatous pleuritis at thoracotomy. Surg Today 30:1062–1066
Iida T et al (2015) Surgical intervention for non-small-cell lung cancer patients with pleural carcinomatosis: results from the Japanese Lung Cancer Registry in 2004. J Thorac Oncol 10:1076–1082. https://doi.org/10.1097/JTO.0000000000000554
Jett JR, Scott WJ, Rivera MP, Sause WT, American College of Chest P (2003) Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest 123:221S–225S
Morgensztern D, Waqar S, Subramanian J, Gao F, Govindan R (2009) Improving survival for stage IV non-small cell lung cancer: a surveillance, epidemiology, and end results survey from 1990 to 2005. J Thorac Oncol 4:1524–1529. https://doi.org/10.1097/JTO.0b013e3181ba3634
Morgensztern D, Ng SH, Gao F, Govindan R (2010) Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 5:29–33. https://doi.org/10.1097/JTO.0b013e3181c5920c
Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, Hizawa N (2012) Specific organ metastases and survival in small cell lung cancer. Oncol Lett 4:617–620. https://doi.org/10.3892/ol.2012.792
Ohta Y, Shimizu Y, Matsumoto I, Tamura M, Oda M, Watanabe G (2005) Retrospective review of lung cancer patients with pleural dissemination after limited operations combined with parietal pleurectomy. J Surg Oncol 91:237–242. https://doi.org/10.1002/jso.20333
Rami-Porta R, Asamura H, Travis WD, Rusch VW (2017) Lung cancer—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:138–155. https://doi.org/10.3322/caac.21390
Ren Y et al (2016) Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget 7:53245–53253. https://doi.org/10.18632/oncotarget.10644
Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K (2014) Metastatic sites and survival in lung cancer. Lung Cancer 86:78–84. https://doi.org/10.1016/j.lungcan.2014.07.020
Sanchez de Cos Escuin J et al (2014) Tumor, node and metastasis classification of lung cancer—M1a versus M1b—analysis of M descriptors and other prognostic factors. Lung Cancer 84:182–189. https://doi.org/10.1016/j.lungcan.2014.02.006
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67:7–30. https://doi.org/10.3322/caac.21387
Sugiura S, Ando Y, Minami H, Ando M, Sakai S, Shimokata K (1997) Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 3:47–50
Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T (2008) Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res 466:729–736. https://doi.org/10.1007/s11999-007-0051-0
Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N (2015) Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol 3:217–221. https://doi.org/10.3892/mco.2014.410
Waqar SN et al (2015) Brain metastases at presentation in patients with non-small cell lung cancer. Am J Clin Oncol 41(1):36–40. https://doi.org/10.1097/COC.0000000000000230
Acknowledgements
We would like to thank all the staff of National Cancer Institute for their efforts toward the SEER program.
Funding
This work was supported by National Natural Science Foundation of China, No. 81600052 (J.Y.) and 81500052 (Y.Z.). This work was also supported by Shanghai Tenth Hospital’s improvement plan for National Natural Science Foundation of China, No. SYGZRPY (Y.H.).
Author information
Authors and Affiliations
Contributions
Conception and design: Y.H., J.Y., and G.J.; acquisition, statistical analysis or interpretation of the data: all authors; drafting of the manuscript: Y.H., J.Y., Y.Z. and X.S.; all authors reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors disclose no potential conflicts of interest related to this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is waived.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, J., Zhang, Y., Sun, X. et al. The prognostic value of multiorgan metastases in patients with non-small cell lung cancer and its variants: a SEER-based study. J Cancer Res Clin Oncol 144, 1835–1842 (2018). https://doi.org/10.1007/s00432-018-2702-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-018-2702-9