Abstract
Purpose
The benefit of adding docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy to chemoradiotherapy (CRT) in head and neck squamous cell carcinoma (HNSCC) remains uncertain. We aimed to investigate whether ICT is well tolerated when given with prophylactic treatment against predicted adverse effects and which patients benefit most.
Methods
A single-centre audit identified 132 HNSCC patients with stage IVa/b neck node-positive disease, prescribed TPF followed by CRT. TPF involved three cycles of docetaxel (75 mg/m2 IV) and cisplatin (75 mg/m2 IV) on day 1 plus 5-FU (750 mg/m2 IV) on days 2–5. Planned CRT was 66 Gy in 30 fractions of intensity-modulated radiotherapy with concurrent cisplatin (100 mg/m2 IV) at the beginning of week 1 and 4 (days 1 and 22). All patients received prophylactic antibiotics and granulocyte colony-stimulating factor.
Results
Median follow-up was 39.5 months. 92.4% of patients completed three cycles of TPF; 95.5% of patients started chemoradiotherapy. Grade 3/4 adverse events were low (febrile neutropenia 3.0%), with no toxicity-related deaths. 3-year overall survival was 67.2%; disease-specific survival was 78.7%; locoregional control was 78.3%. Distant metastases rate was 9.8% (3.0% in those without locoregional recurrence). Good performance status (p = 0.002) and poor tumour differentiation (p = 0.018) were associated with improved overall survival on multivariate analysis.
Conclusion
With prophylactic antibiotics and granulocyte colony-stimulating factor TPF was well tolerated with good survival outcomes. TPF should remain a treatment option for stage IV neck node-positive patients with a good performance status. The use of tumour grade to aid patient selection for TPF warrants investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately, two-thirds of patients with head and neck squamous cell carcinoma (HNSCC) present with high-stage disease and have a poor prognosis (Argiris et al. 2008). Patients are treated with (chemo)radiotherapy (CRT) with or without surgery (Blanchard et al. 2011; Bourhis et al. 2012; Nguyen-Tan et al. 2014). The standard of care is to give chemotherapy concurrently with radiotherapy, which acts primarily as a radiosensitiser to improve loco-regional control rather than treat potential microscopic metastases and reduce distant failure (Pignon et al. 2009). However, randomised trials and meta-analyses showed induction chemotherapy (ICT) prior to CRT reduces the risk of distant metastases compared to CRT alone underpinning exploration of its use in high-stage HNSCC (Cohen et al. 2012, 2014; Ma et al. 2012; Pignon et al. 2009). These studies involved different ICT regimens but did not include many patients treated with the current preferred regimen of taxane (T; docetaxel), platinum (P; cisplatin) and 5-fluorouracil (F) (TPF), which has superior survival outcomes compared with PF (up to 27% reduced risk of death) (Hitt et al. 2014; Lorch et al. 2011; Posner et al. 2007; Vermorken et al. 2007). The overall benefit of adding ICT to CRT alone, however, is controversial as there are concerns for increased toxicity, treatment-related deaths and the potential to delay and compromise definitive CRT (Takacsi-Nagy et al. 2015). Lack of definitive trial evidence showing a survival benefit may well lead to a move away from the use of ICT (Stokes et al. 2017).
It can be argued, however, that it is premature to dismiss adding ICT prior to CRT in selected patients with a high risk of metastases (Ghi et al. 2017; Vidal et al. 2017) as recent trials demonstrating no overall survival benefit using ICT, can be criticised (Stokes et al. 2017). For example, the large mixed-treatment comparison suggesting ICT with CRT is inferior to CRT alone (Blanchard et al. 2011) used PF and not TPF (Vermorken et al. 2007). Additionally, meta-analyses incorporating TPF trials (Budach et al. 2016; Zhang et al. 2015) included trials which had: poor design, compliance and power; long delays between ICT and CRT; and recruited patients with a low risk of distant metastases (i.e., stage III, N0/ N1 disease). A large study by Stokes et al. relied on a retrospective review of the National Cancer Database with no access to patient notes or key details such as regimens used, undermining the applicability of the results (Stokes et al. 2017).
The Takacsi-Nagy et al. trial reinforced concerns about toxicity as it closed early due to three patient deaths from febrile neutropenia after ICT (Takacsi-Nagy et al. 2015). In addition, 31% of patients did not progress past ICT because of toxicity in the three-arm phase III randomized trial by Hitt et al. (2014). Towards the end of the trial, however, the addition of prophylactic granulocyte colony-stimulating factor (GCSF) to the study protocol decreased dramatically the number of adverse events (AEs) and treatment-related deaths and increased the number of patients receiving CRT. In per-protocol patients receiving at least one cycle of ICT and CRT, the addition of TPF ICT to CRT significantly improved progression-free survival, time to treatment failure and locoregional control. The trial, therefore, showed a significant benefit from TPF ICT compared to CRT alone when given with prophylactic GCSF.
The Christie NHS Foundation Trust uses TPF prior to CRT in patients with stage IVa/b (Edge et al. 2009) neck node-positive HNSCC, i.e., in those with a high risk of distant metastases. All patients receive prophylactic antibiotics and GCSF to reduce toxicity and minimize delays between ICT and CRT. As there is uncertainty about the benefit and safety of ICT prior to CRT and lack of published studies involving modern prophylactic treatment and including patients most likely to benefit from this approach; the aim of this single-centre case note review was to investigate whether ICT is well tolerated when given with prophylactic treatment against predicted adverse effects and which patients benefit most.
Materials and methods
Patients
A retrospective case note review was carried out (reference 14/1223) in patients treated between 1st Jan 2009 and Dec 31st 2013. Inclusion criteria were: stage IV neck node-positive primary HNSCC of the larynx, oropharynx or hypopharynx in patients considered inoperable (technically too difficult or low surgical curability) or suitable for organ preservation (final diagnosis made by consensus at head and neck multidisciplinary team meeting and clinic using available information from clinical history and examination, imaging (CT, MRI and/or PET) and endoscopy); use of prophylactic antibiotics and GCSF; and an intention-to-treat regimen of three cycles of TPF ICT followed by CRT using IMRT. Exclusion criteria were: nasopharynx, sinonasal, salivary gland, unknown primary or upper oesophageal primary sites; and any previous treatment for head and neck carcinoma.
Treatment
TAX 323 ICT doses were used (Vermorken et al. 2007), (modified to three cycles of TPF as per Pointreau et al. (2009) and four rather than five days of 5-FU based on clinical experience (Posner et al. 2007; Prestwich et al. (2011); Vermorken et al. 2007): three cycles of docetaxel (75 mg/m2 IV on day 1), cisplatin (75 mg/m2 IV on day 1) and 5-FU (750 mg/m2 IV on days 2–5) followed by 2 weeks rest. Carboplatin was substituted for cisplatin if not tolerated, but recorded as a deviation from the planned regimen. Patients then received 66 Gy in 30 fractions of intensity-modulated radiotherapy (IMRT) over 6 weeks with concurrent cisplatin (100 mg/m2 IV) at the beginning of week 1 and week 4 (day 1 and day 22). If cisplatin was not tolerated, cetuximab or carboplatin was considered and recorded as a deviation from the planned CRT regimen. During ICT, all patients were prescribed a prophylactic regimen of oral ciprofloxacin (or equivalent if allergic/intolerant) 500 mg twice daily for 7 days from day 3 and Filgrastim (GCSF) 300 mcg (< 70 kg) or 480 mcg (≥ 70 kg) once daily, subcutaneous injection for 7 days from day 3. Patients were also given anti-emetics (ondansetron, aprepitant, dexamethasone and as required metoclopramide) as recommended by the Multinational Association of Supportive Care in Cancer (MASCC) guidelines (Feyer et al. 2011), laxatives (senna and laxido), and mouthwash (difflam) that was adjusted as required to suit the patient’s needs. Patients were supervised closely in a specialist nurse-led clinic throughout.
Data collection
AEs ≥ grade 3 were recorded in patient records according to the common terminology criteria for adverse events (CTCAE) version 4 (National Cancer Institute NCI, NIH, DHHS. May 29, 2009), except for mucositis which was graded according to the Radiation Therapy Oncology Group Guidelines version 2 (Trotti et al. 2000). AEs were recorded separately depending on whether they occurred during the ICT treatment (from day 1 of ICT to the day prior to starting CRT) or during CRT (from day 1 of IMRT or chemotherapy to the last day of CRT treatment). Other factors of interest included effect of age, tumour grade (reported by referring hospital at time of diagnosis), T stage, N stage, overall tumour stage (Edge et al. 2009), smoking status (assessed at clinic), World Health Organization (WHO) PS (Oken et al. 1982) and p16 status. The methods used for p16 staining and scoring are described elsewhere (Bernstein et al. 2015).
Statistical analysis
The primary end points were overall survival (OS), disease-specific survival (DSS) and locoregional control (LRC) calculated from first day of treatment for all patients. The secondary end point was tolerability of the regimen as assessed by the numbers of AEs and patients completing treatment. Data were analysed using IBM SPSS Statistics 22 (IBM, Armonk, USA). Patient outcomes were calculated using the Kaplan–Meier method. Cox regression analysis was used to identify differences in outcome between groups of interest for univariable analysis including age, tumour grade, nodal grade, stage of tumour, smoking status, WHO performance status and p16 status. Multivariate analysis was performed using the Cox proportional hazards model. A p value of < 0.05 was considered significant.
Results
All head and neck patients prescribed TPF from 2009 to 2013 were reviewed (n = 241) of whom 132 met the inclusion criteria. Patients excluded from the study had: a primary subsite from the exclusion criteria list; staging other than stage IV and neck node positive; not been prescribed IMRT (due to technical problems); previous treatment for head and neck carcinoma; or metastatic disease on diagnosis. To prevent selection bias, any other factors such as poor performance status did not allow exclusion from this cohort. Table 1 summarizes the characteristics of the 132 patients who met the eligibility criteria.
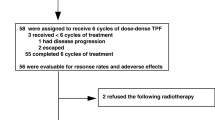
Most patients (n = 122; 92.4%) completed all three cycles of ICT with 90.2% (n = 110) having three cycles of full-dose TPF. Only three patients (2.3%) had no further treatment and three patients (2.3%) had IMRT alone post ICT. 95.5% (n = 126) proceeded to CRT post ICT. All started IMRT with 94.7% (of all patients) completing the full course. 67.4% (n = 89) had full dose IMRT and completed all cycles of prescribed concurrent cisplatin, carboplatin or cetuximab. Online Resource Figure 1 summarizes the ICT and CRT regimens given to patients.
Bar charts of adverse events contributing to deviation from or cessation of planned a induction chemotherapy and b radiotherapy/synchronous chemotherapy. Deviation from or cessation of planned treatment is described as any alteration from the intention to treat regimen including dose reduction, change or cessation of any therapeutic agent at any point in the treatment plan. Patients may have had one or more adverse event
A full course of this ICT takes 9 weeks (63 days) to complete. In our cohort, the median time from the start of ICT to the start of IMRT was 63 days [range 17–94 days, interquartile range (IQR) 2]. A full course of ICT followed by this CRT takes approximately 15 weeks (105 days) to complete. The median time from the start of ICT to the end of IMRT was 105 days (range 56–137 days, IQR 5).
One patient died within 30 days of starting ICT, but a postmortem ascribed death due to myocardial infarction with coronary atherosclerosis being a contributing factor. The coroner did not consider ICT as contributing to death. No patients died within 30 days of finishing ICT or within 30 days of finishing CRT. Grade 3 and 4 AEs are shown in Online Resource Table 1. No grade 5 AEs occurred during ICT or CRT. Most AEs that occurred and contributed to either stopping ICT, or swapping or decreasing the dose of a prescribed drug were grade 1 or 2 only (Fig. 1a). Neutropenia was the most common reason to stop/alter ICT (n = 5) although 60% of these were only grade 1. A reduction in glomerular filtration rate was the most common reason for stopping/altering CRT (n = 10) (Fig. 1b), however, reductions were grade 2 or less. Two patients had reductions in GFR with a CTCAE classification < 1 but this still prompted a precautionary early alteration to the CRT regimen.
The median follow-up time in surviving patients was 39.5 months (range 21–78 months). Median survival was not calculated because a 50% event rate was not reached for any group. 3-year OS, DSS and LRC rates were 67.2, 78.7 and 78.3%. The 1–5-year outcome rates are listed in Online Resource Table 2 and illustrated in Fig. 2. Seventeen patients required surgery during follow-up (see Online Resource Table 3). Fourteen deaths were considered unrelated to HNSCC: 4-s primaries (9.5% of all deaths); 8 inter-current (19% of all deaths) and two of unknown causes (4.8% of all deaths). Thirteen patients (9.8%) developed distant metastases. Four patients (3.0%) developed distant metastases only with no locoregional recurrence. No patient developed their first distant metastases after 29 months from the start of treatment. Most patients with distant metastases developed metastases at multiple sites (n = 6, 46.2%) including lung, liver, bone, sub-carinal nodes, kidney, abdominal nodes and adrenal gland. Only two patient deaths were attributed to distant metastases when no primary or local recurrence was reported. Online Resource Table 3 reports the patterns of recurrence within the cohort.
Table 2 shows the results from the univariable Cox regression analyses for OS, DSS and LRC. All results that were statistically significant for OS on univariable analysis remained statistically significant on multivariate analyses except nodal status (Online Resource Table 4). Figure 3 shows that patients with a performance status of 0 versus ≥ 1 (p < 0.005) and poor versus moderate/well tumour differentiation (p = 0.009) had better overall survival.
Discussion
This study showed TPF ICT with prophylactic antibiotics and GCSF prior to CRT is safe, well tolerated and has good survival outcomes. While we accept the limitations of cross-trial comparisons, our survival outcomes were similar or better than those published (Table 3). Our cohort received IMRT which may account for some of our improved outcomes. As shown in Table 4, our study found distant metastasis rates were similar to other studies involving ICT and favourable when compared to trials using CRT alone. Difficulties can arise when comparing different patient groups and regimens, however, in the absence of locoregional failure, our distant metastases rate of only 3% at 3–5 years is good in patients with stage IVa/b node-positive disease.
The good survival outcomes reported here for high-risk patients are likely due to the use of: modern radiotherapy, CRT rather than radiotherapy alone, prophylactic GCSF and antibiotics, an optimum number of cycles of TPF, low frequency of treatment delays and high numbers completing both ICT and CRT. Our cohort also had a high percentage of p16-positive tumours which is known to confer a good prognosis. However, several studies suggest that patients with p16-positive versus -negative disease have similar distant recurrence rates despite the better LRC (Ang et al. 2010; Huang et al. 2012; O’Sullivan et al. 2012; Sinha et al. 2014), and are more likely to recur later (12–24 months) (Guo et al. 2015; Sinha et al. 2014; Trosman et al. 2015). Offsetting the potential favourable bias of including a high proportion of HPV-positive patients is auditing only stage IVa/b node-positive disease—comparative studies also included stage III and N0 disease.
A concern over using ICT has been its toxicity, which can reduce the ability to progress to (chemo)radiotherapy. However, this study showed a good tolerability to TPF with no death attributed to the regimen during or within 30 days of completing treatment. A similar proportion of patients completed radiotherapy in our study (94.7%) as reported in RTOG 0129 that involved CRT alone (96.4%) (Nguyen-Tan et al. 2014). The lower doses and frequencies of TPF in our audit compared to the TAX trials are likely to have contributed to lowered AE rates and reduced delays. However, the good safety of TPF is most likely due to the prophylactic regimen of GCSF and ciprofloxacin (or equivalent); a regimen not used consistently in other trials. Per protocol univariable analysis highlights the benefit of patients completing three cycles of TPF and a complete course of concurrent cisplatin with IMRT as DSS was significantly better in per protocol patients (p = 0.0.21) (Table 2).
Univariable and multivariate analyses showed that smoking, poor WHO PS, stage and p16-negative tumours were associated with poor prognoses, which are all widely recognized adverse prognostic factors for HNSCC (Ang et al. 2010, 2014; Chang et al. 2013; Dayyani et al. 2010). Our data showed patients with poorly differentiated tumours had a favourable OS compared to well/moderately differentiated tumours treated with TPF ICT and CRT (Fig. 3). This seems counterintuitive as poor differentiation increases the risk of distant metastases and can confer a poor prognosis (Fortin et al. 2001; Garavello et al. 2006). The result may be due to the high number of p16-positive tumours which confers a better prognosis post TPF (Kies et al. 2010; Miah et al. 2015; Posner et al. 2011; Won et al. 2014) and HNSCC in general (Ang et al. 2014; Dayyani et al. 2010; Petrelli et al. 2014). HPV/p16-positive tumours are often thought to be predominantly poorly differentiated (Dahlstrom et al. 2003; Gillison et al. 2000), although there are conflicting reports (Byrd et al. 2012). However, the percentage of p16-positive patients with poorly versus moderately differentiated tumours was the same in our cohort (46.7 versus 46.7%). In addition, this result remained significant on multivariate analysis. An alternative explanation is that the well/moderately differentiated tumours have a higher propensity for accelerated repopulation and that extension of overall treatment time with ICT is detrimental.
Accelerated tumour cell repopulation is widely acknowledged as one of the main risk factors for radiation failure hence the multiple HNSCC trials attempting to improve outcomes with accelerated fractionation schedules (Bentzen 2003). It has been suggested that better-differentiated tumours have a higher potential for accelerated repopulation once radiotherapy has started and hence do better with accelerated regimens (Slevin et al. 1999). Evidence supporting this suggestion also comes from trials showing well/moderately differentiated tumours do less well with protracted treatment regimens (Begg et al. 1999; Hansen et al. 1997). For example, the CHART trial comparing continuous hyperfractionated accelerated radiotherapy to conventional radiotherapy showed well/moderately differentiated tumours benefitted most from the accelerated regimen yet poorly differentiated tumours fared significantly worse (Dische et al. 1997), an effect which has since been verified in more recent trials (Bentzen et al. 2005; Eriksen et al. 2005). Biomarker studies assessing cell cycle regulating genes also suggest that tumours which maintain their ability to continue to proliferate even after injury benefit from accelerated radiotherapy (Bentzen et al. 2005; Wilson et al. 2006). Together, these observations suggest that the long-protracted treatment course that comes with ICT and CRT may be detrimental in patients with well/moderately differentiated tumours but not those with poorly differentiated tumours with less potential for accelerated repopulation. Further studies would need to verify whether histological grade either alone or in combination with other features could be used as a stratification tool for deciding treatment. For example, when grouped with the favourable characteristic of PS = 0, this group had excellent 3-year OS, DSS and LRC rates of 88.2, 95.5 and 90.6%, respectively (p16 positive rate of 75.0%).
Limitations of the study are it is retrospective, single cohort, single-centre and non-randomized. High numbers of p16-positive tumours may contribute to some of the good outcomes although this does not explain the low distant metastases rate in this cohort. Multivariate analysis was performed to provide extra information about factors affecting outcomes. However event rates (deaths) were low (n = 29) for a multivariate analysis containing four variables and hence a larger sample size would have overcome this. The multivariate model was found to be statically stable, however, and so conclusions drawn are still relevant to the discussion. The strengths of the study are its large size, the inclusion of patients prescribed prophylactic medication such as antibiotics and GCSF and the use of modern CRT involving IMRT. In addition, the study focused on patients most likely to benefit from TPF ICT, i.e., only those with high-stage Iva/b, neck node-positive disease that have a higher risk of distant failure.
Conclusion
Incorrectly interpreting lack of definite evidence as definite lack of benefit means that ICT is at risk of falling out of use. Trials demonstrating no benefit for ICT should be interpreted with caution due to poor design, inclusion of patients with a low risk of metastases and lack of use of prophylactic medication to minimize adverse events. Our case note review showed that with appropriate supportive measures such as prophylactic GCSF and antibiotics plus close patient monitoring in a specialist centre, TPF ICT is well tolerated and associated with good outcomes. Careful planning is essential to prevent unnecessary gaps between completing ICT and starting CRT. TPF ICT should continue to be considered for very fit patients (WHO PS = 0), who are most at risk of distant metastases i.e., stage IVa/b node-positive disease. Well-designed randomised control trials comparing ICT to CRT alone in selected patient populations are warranted to validate these findings. Further larger studies need to verify our result suggesting patients with poorly differentiated tumours benefit most from ICT.
References
Adelstein DJ, Li Y, Adams GL, Wagner H Jr, Kish JA, Ensley JF, Schuller DE, Forastiere AA (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer N. Engl J Med 363:24–35. https://doi.org/10.1056/NEJMoa0912217
Ang KK, Zhang Q, Rosenthal DI, Nguyen-Tan PF, Sherman EJ, Weber RS, Galvin JM, Bonner JA, Harris J, El-Naggar AK, Gillison ML, Jordan RC, Konski AA, Thorstad WL, Trotti A, Beitler JJ, Garden AS, Spanos WJ, Yom SS, Axelrod RS (2014) Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol 32:2940–2950. https://doi.org/10.1200/jco.2013.53.5633
Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head neck cancer Lancet 371:1695–1709. https://doi.org/10.1016/s0140-6736(08)60728-x
Begg AC, Haustermans K, Hart AA, Dische S, Saunders M, Zackrisson B, Gustaffson H, Coucke P, Paschoud N, Hoyer M, Overgaard J, Antognoni P, Richetti A, Bourhis J, Bartelink H, Horiot JC, Corvo R, Giaretti W, Awwad H, Shouman T, Jouffroy T, Maciorowski Z, Dobrowsky W, Struikmans H, Wilson GD et al (1999) The value of pretreatment cell kinetic parameters as predictors for radiotherapy outcome in head and neck cancer: a multicenter analysis. Radiother Oncol 50:13–23
Bentzen SM (2003) Repopulation in radiation oncology: perspectives of clinical research. Int J Radiat Biol 79:581–585
Bentzen SM, Atasoy BM, Daley FM, Dische S, Richman PI, Saunders MI, Trott KR, Wilson GD (2005) Epidermal growth factor receptor expression in pretreatment biopsies from head and neck squamous cell carcinoma as a predictive factor for a benefit from accelerated radiation therapy in a randomized controlled trial. J Clin Oncol 23:5560–5567. https://doi.org/10.1200/jco.2005.06.411
Bernstein JM, Kershaw LE, Withey SB, Lowe NM, Homer JJ, Slevin NJ, Bonington SC, Carrington BM, West CM (2015) Tumor plasma flow determined by dynamic contrast-enhanced MRI predicts response to induction chemotherapy in head and neck cancer. Oral Oncol 51:508–513. https://doi.org/10.1016/j.oraloncology.2015.01.013
Blanchard P, Hill C, Guihenneuc-Jouyaux C, Baey C, Bourhis J, Pignon JP (2011) Mixed treatment comparison meta-analysis of altered fractionated radiotherapy and chemotherapy in head and neck cancer. J Clin Epidemiol 64:985–992. https://doi.org/10.1016/j.jclinepi.2010.10.016
Bourhis J, Sire C, Graff P, Gregoire V, Maingon P, Calais G, Gery B, Martin L, Alfonsi M, Desprez P, Pignon T, Bardet E, Rives M, Geoffrois L, Daly-Schveitzer N, Sen S, Tuchais C, Dupuis O, Guerif S, Lapeyre M, Favrel V, Hamoir M, Lusinchi A, Temam S, Pinna A, Tao YG, Blanchard P, Auperin A (2012) Concomitant chemoradiotherapy versus acceleration of radiotherapy with or without concomitant chemotherapy in locally advanced head and neck carcinoma (GORTEC 99–02): an open-label phase 3 randomised trial. Lancet Oncol 13:145–153. https://doi.org/10.1016/s1470-2045(11)70346-1
Brockstein B, Haraf DJ, Rademaker AW, Kies MS, Stenson KM, Rosen F, Mittal BB, Pelzer H, Fung BB, Witt M-E, Wenig B, Portugal L, Weichselbaum RW, Vokes EE (2004) Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 15:1179–1186. https://doi.org/10.1093/annonc/mdh308
Budach W, Bolke E, Kammers K, Gerber PA, Orth K, Gripp S, Matuschek C (2016) Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): a meta-analysis of randomized trials radiotherapy and oncology. J Eur Soc Ther Radiol Oncol 118:238–243. https://doi.org/10.1016/j.radonc.2015.10.014
Byrd JK, Wilhoit CS, Fordham MT, Reeves TD, McRackan TR, Nguyen SA, Sutkowski N, Gillespie MB (2012) Predicting HPV status in head and neck cancer: the predictive value of sociodemographic and disease characteristics. Arch Otolaryngol Head Neck Surg 138:1155–1159. https://doi.org/10.1001/jamaoto.2013.850
Chang PH, Yeh KY, Huang JS, Lai CH, Wu TH, Lan YJ, Tsai JC, Chen EY, Yang SW, Wang CH (2013) Pretreatment performance status and nutrition are associated with early mortality of locally advanced head and neck cancer patients undergoing concurrent chemoradiation. Eur Arch Otorhinolaryngol 270:1909–1915. https://doi.org/10.1007/s00405-012-2290-2
Cohen EE, Karrison T, Kocherginsky M, al e (2012) Docetaxel based chemoradiotherapy plus or minus induction chemotherapy to decrease events in head and neck cancer (DeCIDE). J Clin Oncol 30:suppl 5500
Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F, Samant S, Raez LE, Mehra R, Kumar P, Ondrey F, Marchand P, Braegas B, Seiwert TY, Villaflor VM, Haraf DJ, Vokes EE (2014) Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 32:2735–2743. https://doi.org/10.1200/jco.2013.54.6309
Dahlstrom KR, Adler-Storthz K, Etzel CJ, Liu Z, Dillon L, El-Naggar AK, Spitz MR, Schiller JT, Wei Q, Sturgis EM (2003) Human papillomavirus type 16 infection and squamous cell carcinoma of the head and neck in never-smokers: a matched pair analysis. Clin Cancer Res 9:2620–2626
Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS (2010) Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2:15. https://doi.org/10.1186/1758-3284-2-15
Dische S, Saunders M, Barrett A, Harvey A, Gibson D, Parmar M (1997) A randomised multicentre trial of CHART versus conventional radiotherapy in head and neck cancer. Radiother Oncol 44:123–136
Edge SE, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2009) AJCC Cancer Staging Manual, 7th edn. Spinger, New York
Eriksen JG, Steiniche T, Overgaard J (2005) The influence of epidermal growth factor receptor and tumor differentiation on the response to accelerated radiotherapy of squamous cell carcinomas of the head and neck in the randomized DAHANCA 6 and 7study. Radiother Oncol 74:93–100
Feyer PC, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K (2011) Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer 19 Suppl 1:S5–S14. https://doi.org/10.1007/s00520-010-0950-6
Fortin A, Couture C, Doucet R, Albert M, Allard J, Tetu B (2001) Does histologic grade have a role in the management of head and neck cancers? J Clin Oncol 19:4107–4116. https://doi.org/10.1200/jco.2001.19.21.4107
Garavello W, Ciardo A, Spreafico R, Gaini RM (2006) Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 132:762–766. https://doi.org/10.1001/archotol.132.7.762
Ghi MG, Paccagnella A, D Ferrari, P Foa, MC Rocca, E Verri, F Morelli, G Azzarello, C D’Ambrosio, G Cruciani, M Guaraldi, E Massa, C Rossetto, A Bonetti, S Siena, V Minotti, H Koussis, G Pieri, V Baggio, I Floriani, Group ftGIS (2014) Concomitant chemoradiation (CRT) or cetuximab/RT (CET/RT) versus induction Docetaxel/ Cisplatin/5-Fluorouracil (TPF) followed by CRT or CET/RT in patients with Locally Advanced Squamous Cell Carcinoma of Head and Neck (LASCCHN). A randomized phase III factorial study (NCT01086826). J Clin Oncol 32:6004
Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codeca C, Nole F, Verri E, Orecchia R, Morelli F, Parisi S, Mastromauro C, Mione CA, Rossetto C, Polsinelli M, Koussis H, Loreggian L, Bonetti A, Campostrini F, Azzarello G, D’Ambrosio C, Bertoni F, Casanova C, Emiliani E, Guaraldi M, Bunkheila F, Bidoli P, Niespolo RM, Gava A, Massa E, Frattegiani A, Valduga F, Pieri G, Cipani T, Da Corte D, Chiappa F, Rulli E (2017) Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncology 28:2206–2212. https://doi.org/10.1093/annonc/mdx299
Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92:709–720
Guo T, Qualliotine JR, Ha PK, Califano JA, Kim Y, Saunders JR, Blanco RG, D’Souza G, Zhang Z, Chung CH, Kiess A, Gourin CG, Koch W, Richmon JD, Agrawal N, Eisele DW, Fakhry C (2015) Surgical salvage improves overall survival for patients with HPV-positive and HPV-negative recurrent locoregional and distant metastatic oropharyngeal cancer. Cancer 121:1977–1984. https://doi.org/10.1002/cncr.29323
Hansen O, Overgaard J, Hansen HS, Overgaard M, Hoyer M, Jorgensen KE, Bastholt L, Berthelsen A (1997) Importance of overall treatment time for the outcome of radiotherapy of advanced head and neck carcinoma: dependency on tumor differentiation. Radiother Oncol 43:47–51
Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, Sastre J, Martinez-Trufero J, Brandariz Castelo JA, Verger E, Cruz-Hernandez JJ (2014) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25:216–225. https://doi.org/10.1093/annonc/mdt461
Huang SH, Perez-Ordonez B, Liu FF, Waldron J, Ringash J, Irish J, Cummings B, Siu LL, Kim J, Weinreb I, Hope A, Gullane P, Brown D, Shi W, O’Sullivan B (2012) A typical clinical behavior of p16-confirmed HPV-related oropharyngeal squamous cell carcinoma treated with radical radiotherapy. Int J Radiat Oncol Biol Phys 82:276–283. https://doi.org/10.1016/j.ijrobp.2010.08.031
Kies MS, Holsinger FC, Lee JJ, William WN Jr, Glisson BS, Lin HY, Lewin JS, Ginsberg LE, Gillaspy KA, Massarelli E, Byers L, Lippman SM, Hong WK, El-Naggar AK, Garden AS, Papadimitrakopoulou V (2010) Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 28:8–14. https://doi.org/10.1200/jco.2009.23.0425
Lorch JH, Goloubeva O, Haddad RI, Cullen K, Sarlis N, Tishler R, Tan M, Fasciano J, Sammartino DE, Posner MR (2011) Induction chemotherapy with cisplatin and fluorouracil alone or in combination with docetaxel in locally advanced squamous-cell cancer of the head and neck: long-term results of the TAX 324 randomised phase 3 trial. Lancet Oncol 12:153–159. https://doi.org/10.1016/s1470-2045(10)70279-5
Ma J, Liu Y, Huang XL, Zhang ZY, Myers JN, Neskey DM, Zhong LP (2012) Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis. Oral Oncol 48:1076–1084. https://doi.org/10.1016/j.oraloncology.2012.06.014
Miah AB, Schick U, Bhide SA, Guerrero-Urbano MT, Clark CH, Bidmead AM, Bodla S, Del Rosario L, Thway K, Wilson P, Newbold KL, Harrington KJ, Nutting CM (2015) A phase II trial of induction chemotherapy and chemo-IMRT for head and neck squamous cell cancers at risk of bilateral nodal spread: the application of a bilateral superficial lobe parotid-sparing IMRT technique and treatment outcomes. Br J Cancer 112:32–38. https://doi.org/10.1038/bjc.2014.553
Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS, Rosenthal DI, Soulieres D, Kim H, Silverman C, Raben A, Galloway TJ, Fortin A, Gore E, Westra WH, Chung CH, Jordan RC, Gillison ML, List M, Le Q-T (2014) Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the radiation therapy oncology group 0129 trial: long-term report of efficacy and toxicity. J Clin Oncol. https://doi.org/10.1200/jco.2014.55.3925
O’Sullivan B, Huang SH, Perez-Ordonez B, Massey C, Siu LL, Weinreb I, Hope A, Kim J, Bayley AJ, Cummings B, Ringash J, Dawson LA, Cho BC, Chen E, Irish J, Gilbert RW, Hui A, Liu FF, Zhao H, Waldron JN, Xu W (2012) Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. Radiother Oncol 103:49–56. https://doi.org/10.1016/j.radonc.2012.02.009
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Petrelli F, Sarti E, Barni S (2014) Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: An updated systematic review and meta-analysis of 30 trials. Head Neck 36:750–759. https://doi.org/10.1002/hed.23351
Pignon J-P, Maître A, Maillard E, Bourhis J (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14. https://doi.org/10.1016/j.radonc.2009.04.014
Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, Faivre S, Guerrif S, Alfonsi M, Calais G (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101:498–506. https://doi.org/10.1093/jnci/djp007
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM Jr, Haddad RI (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715. https://doi.org/10.1056/NEJMoa070956
Posner MR, Lorch JH, Goloubeva O, Tan M, Schumaker LM, Sarlis NJ, Haddad RI, Cullen KJ (2011) Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III. trial Ann Oncol 22:1071–1077. https://doi.org/10.1093/annonc/mdr006
Prestwich RJ, Öksüz D, Dyker K, Coyle C, Şen M (2011) Feasibility and efficacy of induction docetaxel, cisplatin, and 5-fluorouracil chemotherapy combined with cisplatin concurrent chemoradiotherapy for nonmetastatic stage iv head-and-neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys 81:e237–e243 https://doi.org/10.1016/j.ijrobp.2011.03.043
Sinha P, Thorstad WT, Nussenbaum B, Haughey BH, Adkins DR, Kallogjeri D, Lewis Jr (2014) JS Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: a critical analysis of patterns and outcomes. Oral oncology 50:45–51. https://doi.org/10.1016/j.oraloncology.2013.10.007
Slevin NJ, West CM, Wilson GD, Hendry JH (1999) The potential benefit from individualised radiotherapy scheduling for head and neck tumours on the basis of both histological grade and kinetics. Radiother Oncol 51:109–111
Stokes WA, Amini A, Jones BL, McDermott JD, Raben D, Ghosh D, Goddard JA, Bowles DW, Karam SD (2017) Survival impact of induction chemotherapy in advanced head and neck cancer: A National Cancer Database analysis. Head Neck 39:1113–1121. https://doi.org/10.1002/hed.24739
Takacsi-Nagy Z, Hitre E, Remenar E, Oberna F, Polgar C, Major T, Godeny M, Fodor J, Kasler M (2015) Docetaxel, cisplatin and 5-fluorouracil induction chemotherapy followed by chemoradiotherapy or chemoradiotherapy alone in stage III-IV unresectable head and neck cancer: Results of a randomized phase. II study Strahlenther Onkol 191:635–641. https://doi.org/10.1007/s00066-015-0829-z
Trosman SJ, Koyfman SA, Ward MC, Al-Khudari S, Nwizu T, Greskovich JF, Lamarre ED, Scharpf J, Khan MJ, Lorenz RR, Adelstein DJ, Burkey BB (2015) Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol Head Neck Surg 141:457–462. https://doi.org/10.1001/jamaoto.2015.136
Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W (2000) Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47:13–47
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Weyngaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704. https://doi.org/10.1056/NEJMoa071028
Vidal L, Ben Aharon I, Limon D, Cohen E, Popovtzer A (2017) Role of induction chemotherapy prior to chemoradiation in head and neck squamous cell cancer—systematic review and meta-analysis. Cancer J 23:79–83. https://doi.org/10.1097/ppo.0000000000000253
Wilson GD, Saunders MI, Dische S, Daley FM, Buffa FM, Richman PI, Bentzen SM (2006) Pre-treatment proliferation and the outcome of conventional and accelerated radiotherapy. Eur J Cancer 42:363–371. https://doi.org/10.1016/j.ejca.2005.10.022
Won HS, Lee YS, Jeon EK, Hong SH, Kang JH, Kim YS, Yoo le R, Sun DI, Kim MS (2014) Clinical outcome of induction chemotherapy in locally advanced head and neck squamous cell carcinoma. Anticancer Res 34:5709–5714
Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y (2015) Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta-analysis. Sci Rep 5:10798. https://doi.org/10.1038/srep10798
National Cancer Institute (NCI, NIH, DHHS. May 29, 2009) Common Terminology Criteria for Adverse Events v4.0
Acknowledgements
The authors acknowledge Jason Kennedy and Lucy E. Kershaw for help and support on the database management, Clare Hodgson for statistical review and Helen Valentine for p16 immunostaining.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by Cancer Research UK Major Centre funding, the Christie NHS Foundation Trust and NIHR Manchester Biomedical Research Centre.
Conflict of interest
All authors report no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. For this type of study, formal consent is not required. Ethics approval for the staining of tumour samples for p16 was granted by National Research Ethics Service; NRES Committee North West–Greater Manchester East, reference number: 03/TG/076.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lowe, N.M., Bernstein, J.M., Mais, K. et al. Taxane, platinum and 5-FU prior to chemoradiotherapy benefits patients with stage IV neck node-positive head and neck cancer and a good performance status. J Cancer Res Clin Oncol 144, 389–401 (2018). https://doi.org/10.1007/s00432-017-2553-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-017-2553-9