Abstract
Purpose
Immune checkpoint molecules are key targets for the treatment of various malignancies. Due to the heterogeneity of advanced gastric cancer (GC), the role of programmed cell death ligand 1 (PD-L1) expression as a tumor biomarker remains controversial. In this study, the prognostic value of soluble PD-L1 (sPD-L1) levels in serum samples was assessed in patients with metastatic GC.
Methods
All patients received first-line treatment with fluoropyrimidine and platinum chemotherapy, and trastuzumab was added for HER2-positive patients. Serum levels of sPD-L1 were measured by enzyme-linked immunosorbent assay.
Results
Among 75 metastatic GC patients, the median serum sPD-L1 level was 0.704 ng/ml (range <0.156–3.214). Serum sPD-L1 was significantly higher in patients with a high versus a low white blood cell count at baseline. When the cutoff value was set as the median, multivariate analyses showed that high sPD-L1 levels were associated with worse overall survival compared with low sPD-L1 levels (HR 2.218, 95 % CI 1.139–4.320, P = 0.019). Regardless of HER2 status, overall survival tended to be shorter in patients with high sPD-L1 compared with low sPD-L1. There was no significant association between sPD-L1 level and progression-free survival on the first-line treatment of metastatic GC.
Conclusions
High serum levels of sPD-L1 correlated with worse overall survival on the first-line chemotherapy in metastatic GC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is prevalent in males and females, being the third and fifth leading cause of cancer-related mortality in the two respective genders worldwide (Torre et al. 2012). The incidence of GC is highest in Eastern Asia, Central and Eastern Europe, and South America and lowest in North America and most African countries. Although endoscopic or curative resection may prolong survival in patients with early GC, the prognosis for metastatic or recurrent GC remains poor, despite the development of novel molecularly targeted drugs (Bang et al. 2010; Fuchs et al. 2014; Wilke et al. 2014).
The tumor microenvironment consists of stem, stromal, and endothelial cells, and a variety of immune cells (Hanahan and Coussens 2012). Programmed cell death 1 (PD-1) and its ligand (PD-L1) are members of the B7 family, which negatively regulates the immune system and plays important roles in maintaining self-tolerance, preventing autoimmunity, and protecting against immune collateral damage in normal tissue (Murdoch et al. 2008; Yang et al. 2008; Joyce and Pollard 2009). PD-L1 is a transmembrane protein that is often overexpressed in malignant tumors, and inhibits T cell-mediated immune attack through binding to PD-1 on tumor-specific T cells. PD-L1 expression is regulated by several intracellular mechanisms, including activation of the STAT3 pathway by inflammatory cytokines such as interferon gamma (IFN-γ), as well as the mitogen-activated protein kinase (MAPK)/nuclear factor-kappa B (NF-κB) pathway and the phosphatidylinositide 3-kinase(PI3K)/AKT pathway (Qian et al. 2008; Crane et al. 2009). PD-L1- and PD-1-targeted therapies have been developed for several cancer types. The efficacy and safety profile of an anti-PD-1 antibody has been confirmed in randomized phase 3 trials in malignant melanoma, non-small cell lung cancer, and renal cell carcinoma (Borghaei et al. 2015; Motzer et al. 2015; Robert et al. 2015).
Previous studies indicate that PD-L1 expression is a poor prognostic factor in several histological types of malignant tumor (Thompson et al. 2006; Nomi et al. 2007; Kim et al. 2013; Muenst et al. 2014; Huang et al. 2015; Paydas et al. 2015; Wang et al. 2015a, b; Wu et al. 2015; Cierna et al. 2016). Although overexpression of PD-L1 in tumor tissue, assessed by immunohistochemistry (IHC), has been linked to responses to anti-PD-L1 antibody treatment, the value of PD-L1 as a prognostic or predictive biomarker remains controversial. This may be explained by the heterogeneity of PD-L1 expression in tumor tissue, and inconsistencies in the definition of PD-L1 positivity and diagnostic antibodies (Callea et al. 2015; Madore et al. 2015; McLaughlin et al. 2016). In addition, PD-L1 expression in tumor tissue is affected by the timing of biopsy during treatment, the composition of tumor tissue, and differences between biopsies from the primary lesion and metastatic sites (Kerr et al. 2015).
PD-L1 expression can be detected on the surface of tumor and immune cells by IHC, and in blood samples by enzyme-linked immunosorbent assay (ELISA). PD-L1 is a component of normal human serum, and soluble PD-L1 (sPD-L1) is released from PD-L1-positive cell lines (Chen et al. 2011). Serum sPD-L1 levels are higher in patients with malignant cancer than in healthy individuals, and high sPD-L1 was a poor prognostic factor during standard treatment of hematopoietic malignancies in recent studies (Rossille et al. 2014; Wang et al. 2015a, b). To date, few investigations have evaluated the relationship between serum sPD-L1 levels and the prognosis of metastatic GC patients treated with systemic chemotherapy, and the present study was conducted to address this issue.
Materials and methods
Patients and sample collection
Patients with histologically diagnosed gastric adenocarcinoma and radiologic confirmation of metastatic or recurrent lesions were enrolled. Other inclusion criteria were as follows: age ≥20 years, Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–2, adequate hematological and organ function, at least one measurable target lesion, no prior chemotherapy or radiotherapy, first-line cancer treatment, no brain or central nervous system metastases, no coexisting cancers except for gastroesophageal junction adenocarcinoma, no uncontrolled complications, sufficient serum samples at baseline, and the patient’s written informed consent for use of clinical data and materials.
Patients’ sera were obtained from residual blood samples, which were collected for other laboratory tests, between September 2011 and July 2015, within 2 weeks of initiating first-line treatment. Separated serum samples were immediately stored at –20 °C in the Biobank of the National Cancer Center Hospital, Tokyo, Japan, until analysis. In advanced GC, human endothelial growth factor receptor 2 (HER2) positivity tends to be less frequent than HER2 negativity. To enable the prognostic value of sPD-L1 levels to be evaluated in both HER2-positive and HER2-negative patients, more HER2-positive patients were enrolled in this study, regardless of the outcome of chemotherapy.
HER2 expression was determined by IHC (HercepTest, Dako, Denmark) of formalin-fixed paraffin-embedded tumor specimens from all patients. HER2 staining was graded on a scale of 0–3 as follows: 0 = no reactivity or membranous reactivity in ≥10 % of cells; 1+ = faint/barely perceptible membranous reactivity in ≥10 % of cells or reactivity in a portion of the cell membrane; 2+ = weak-to-moderate complete or basolateral membranous reactivity in ≥10 % of cells; and 3+ = strong complete or basolateral membranous reactivity in ≥10 % of cells. In tumor specimens with IHC scores of 2+, dual in situ hybridization (DISH; Ventana INFORM HER2 Dual Color ISH, Roche) was also performed. HER2 IHC3+ and IHC2+/DISH-positive (HER2/CEP17 ratio ≥2) specimens were defined as HER2 positive, and other specimens were defined as HER2 negative.
Informed consent of Biobank was obtained from all individual participants included in the study. The study protocol was approved by the local institutional review board.
Treatment
All patients received first-line chemotherapy comprising a fluoropyrimidine (S-1, capecitabine or 5-fluorouracil) and platinum (cisplatin or oxaliplatin). HER2-negative patients received either dual therapy consisting of S-1 plus cisplatin (CS), S-1 plus oxaliplatin (SOX), or capecitabine plus oxaliplatin (XELOX), or triple therapy comprising docetaxel plus CS (DCS). HER2-positive patients received triple therapy comprising trastuzumab with either 5-fluorouracil plus cisplatin (FP) or capecitabine plus cisplatin (XP). The chemotherapeutic regimens, which are summarized in Supplementary material 1A, were based on previous clinical trials (Bang et al. 2010; Koizumi et al. 2008, 2012; Kim et al. 2012; Yamada et al. 2015). Patients continued to receive treatment until disease progression or intolerable toxicity from chemotherapy. In the case of severe adverse events, dose modifications were made according to the physician’s judgment. Responses to treatment were evaluated every 2–3 months by contrast-enhanced computed tomography (CT).
ELISA
Serum PD-L1 levels were measured using a commercially available ELISA kit (USCN, Wuhan, China). ELISA was conducted as follows: (1) all reagents, standard dilutions, and samples were prepared according to the manufacturer’s instructions; (2) 100 µL of the standard and sample were added to each well; (3) plates were covered with a plate sealer and incubated at 37 °C for 2 h; (4) after aspiration, 100 μL of detection reagent A (1:100) was added to each well, and the plates were re-sealed and incubated at 37 °C for 2 h; (5) each well was aspirated and washed four times in wash buffer; (6) 100 µL of detection reagent B (1:100) was added to each well, and the plates were re-sealed and incubated for 0.5 h at 37 °C; (7) each well was aspirated and washed five times, 90 μL of substrate solution was added, and the plates were newly sealed and incubated in a dark room for 20 min at 37 °C; (8) 50 µL of stop solution was added to each well, and absorbance at 450 nm was immediately measured with a plate reader.
Disease assessment and statistical analyses
Therapeutic responses were categorized as complete response (CR), partial response (PR), stable disease (SD), disease progression (PD), and non-evaluable (NE), according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1. The overall response rate (ORR) was defined as the proportion of the study population with a best response of CR or PR. The disease control rate (DCR) was defined as the proportion of patients with a best response of CR, PR, or SD. Progression-free survival (PFS) was defined as the time from initiation of first-line treatment to disease progression or death without evidence of progression. Overall survival (OS) was defined as the time from initiation of first-line treatment to death or last follow-up. Survival curves for PFS and OS were estimated by the Kaplan–Meier method, and differences were evaluated by the log-rank test. Hazard ratios (HRs) and 95 % confidence intervals (CIs) were estimated with a Cox proportional hazards model. HRs for univariate analyses were calculated using the covariates of age (younger vs. older, cutoff; median), PS (0–1 vs. 2), gender (male vs. female), histological type (intestinal vs. distal and mixed), stage (stage IV vs. recurrence), HER2 status (positive vs. negative), prior gastrectomy (yes vs. no), number of metastatic sites (1 vs. ≥2), peritoneal dissemination (yes vs. no), liver metastasis (yes vs. no), chemotherapeutic regimen (dual vs. triple), and serum sPD-L1 levels (high vs. low, cutoff level: median). HRs in multivariate analyses were adjusted by first-line treatment, serum levels of PD-L1, and patient characteristics as covariates, all of which had a P-value of <0.2 in univariate analysis.
Differences in the distribution of two variables were evaluated using Fisher’s exact test or χ2 test, as appropriate. Differences in the distribution of more than two variables were evaluated by the Kruskal–Wallis test. Receiver operating characteristic (ROC) curves were generated to determine the optimal cutoff value for sPD-L1 levels as a continuous variable. All tests were two-sided and P < 0.05 was considered to denote statistical significance. All statistical analyses were performed using SPSS software, version 19 (IBM, Tokyo, Japan).
Results
The baseline characteristics of the study population, which comprised 75 patients, are summarized in Table 1. The median age was 67.0 years (range: 39–79 years), and 96.0 % were ECOG PS 0–1. Histologically, tumors were diffuse (54.7 %), intestinal (38.7 %), and mixed type (6.6 %). Analysis of HER2 status revealed that 45 (60.0 %) and 30 (40.0 %) patients were HER2 negative and HER2 positive, respectively. Among all 75 patients, 67 patients (89.3 %) received at least one subsequent treatment after failure of first-line treatment. As shown in Supplementary material 1B, subsequent treatments were a taxane (89.6 %), irinotecan (49.3 %), ramucirumab (6.0 %), and other drugs (20.9 %). The frequency of ramucirumab administration was low because of its recent approval in Japan in March 2015.
Serum sPD-L1 levels
As shown in Fig. 1, the median serum sPD-L1 value of the study population was 0.704 ng/ml (range: <0.156–3.214 ng/ml). In 29 patients, serum sPD-L1 levels were lower than the limit of detection. The distribution of serum sPD-L1 levels by response to first-line treatment was evaluated. In patients with a PR, SD, and PD, median serum sPD-L1 levels were 0.707, 0.622, and 0.751 ng/ml, respectively. There was no significant difference in sPD-L1 levels according to the response to first-line treatment.
The association between sPD-L1 levels and inflammatory markers, including white cell count (WBC), C-reactive protein (CRP), albumin, and hemoglobin, was explored. As shown in Fig. 2, patients with a high WBC (cutoff; 8000/µL) had higher serum sPD-L1 levels than those with a low WBC (P = 0.043). There were no correlations between serum sPD-L1 levels and other inflammatory markers.
Prognostic analyses of PFS and OS on the first-line treatment
The cutoff value for serum sPD-L1 was initially set as the median. Univariate and multivariate analyses of PFS are shown in Supplementary material 2. Univariate analysis showed a trend toward high sPD-L1 levels being associated with low PFS on the first-line treatment (HR 1.580, 95 % CI 0.958–2.605, P = 0.073). However, multivariate analysis demonstrated that serum sPD-L1 was not independently related to PFS (HR 1.261, 95 % CI 0.711–2.236, P = 0.427). Univariate and multivariate analyses of OS are given in Table 2. Univariate analysis identified stage IV (HR 2.237, 95 % CI 1.003–4.990, P = 0.049), HER2 positive (HR 0.537, 95 % CI 0.293–0.983, P = 0.044), and high sPD-L1 (HR 2.647, 95 % CI 1.476–4.745, P = 0.001). Multivariate analysis indicated that high serum sPD-L1 was independently associated with low OS (HR 2.218, 95 % CI 1.139–4.320, P = 0.019).
The baseline characteristics of the high and low sPD-L1 subgroups are shown in Supplementary material 3. No significant differences were found in the baseline characteristics of patients with high as opposed to low serum sPD-L1 levels, but stage IV was more frequently observed in patients with high levels of sPD-L1 compared with those with low levels of sPD-L1. The frequency of subsequent treatment after failure of first-line treatment was not different between the high and low sPD-L1 groups (Supplementary material 1B). The frequency of administration of each type of drug such as taxanes and irinotecan was similar between groups.
PFS and OS curves using median sPD-L1 as the cutoff value
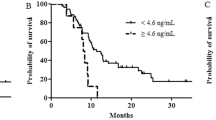
Figure 3 shows PFS and OS curves using median sPD-L1 as the cutoff value. Median PFS for high and low sPD-L1 was 5.2 months (95 % CI 3.7–7.5) and 7.4 months (95 % CI 4.6–10.3), respectively. There was no significant difference in PFS, but patients with high sPD-L1 tended to have shorter PFS than those with low sPD-L1 levels (log-rank test, P = 0.070). Median OS for high and low sPD-L1 was 13.2 months (95 % CI 11.1–16.7) and 21.6 months (95 % CI 11.1–35.3), respectively. Patients with high sPD-L1 levels had shorter OS than those with low sPD-L1 s (log-rank test, P = 0.0009).
Survival curves for serum sPD-L1 levels on the first-line treatment when the cutoff was the median (a, b) and the optimal (c, d) sPD-L1 value. Survival curves for progression-free survival (PFS) and overall survival (OS) are shown. High sPD-L1 was associated with shorter OS compared with low sPD-L1 when the cutoff value was the median (a, b). High sPD-L1 was associated with shorter PFS and OS compared with low sPD-L1 when the cutoff value was the optimum of 1.081 ng/ml (c, d)
The difference between survival curves in HER2-negative and HER2-positive patients was evaluated, as illustrated in Fig. 4. In HER2-negative patients, median PFS for high and low sPD-L1 was not different (4.9 vs. 6.5 months, P = 0.562), while median OS tended to be shorter in those with high sPD-L1 (14.0 vs 21.6 months, P = 0.076). In HER2-positive patients, median PFS was similar for high and low sPD-L1 levels (7.8 vs. 7.9 months, P = 0.379), whereas median OS was shorter for high sPD-L1 compared with low sPD-L1 (13.2 months vs. not reached, P = 0.0196).
Survival curves for serum sPD-L1 levels by HER2 status on the first-line treatment using median sPD-L1 as the cutoff value. Survival curves of progression-free survival (PFS) and overall survival (OS) in HER2-negative patients (a, c) and HER2-positive patients (b, d). OS of patients with high level of sPD-L1 tended to be associated with low level of sPD-L1 in both HER2-negative and HER2-positive patients
Survival curves using the optimal cutoff value for sPD-L1
The optimal cutoff value of sPD-L1 was estimated by ROC curve analysis to set at that for the best DCR. As a result, the cutoff value of sPD-L1 was 1.081 ng/ml. Survival curves for PFS and OS using the optimal cutoff value are shown in Fig. 3. In patients with high (n = 27) and low (n = 48) sPD-L1 levels, median PFS was 5.4 months (95 % CI 4.4–7.5) and 7.2 months (95 % CI 3.7–10.3), respectively, and high sPD-L1 correlated with shorter PFS (log-rank test, P = 0.045). Median OS in patients with high and low sPD-L1 levels was 12.7 months (95 % CI 9.8–15.8) and 20.4 months (95 % CI 15.8–22.9), respectively, and high sPD-L1 correlated with shorter OS (log-rank test, P < 0.0001). Multivariate analyses showed that there was no significant difference statistically in PFS between high and low levels of PD-L1 (HR 1.485, 95 % CI 0.6375–3.458, P = 0.360); meanwhile, high level of PD-L1 was significantly associated with worse OS compared with low level of PD-L1 (HR 3.307, 1.4470–7.559, P = 0.0046).
Discussion
Only a few reports have assessed the clinical role of serum sPD-L1 as a biomarker to predict the survival of patients with metastatic cancer (Rossille et al. 2014; Zheng et al. 2014; Cheng et al. 2015; Wang et al. 2015a, b). This study focused on evaluating the prognostic relevance of serum sPD-L1 levels in patients with metastatic GC. To the best of the authors’ knowledge, the relationship between serum sPD-L1 levels at baseline and clinical responses to first-line systemic chemotherapy in metastatic GC has not been previously investigated. As a novel finding, this study identified a negative association between OS of the first-line chemotherapy and high sPD-L1 levels at baseline. Current guidelines suggest that the eligibility criterion for antibody therapy against PD-1 or PD-L1 in metastatic GC is PD-L1 expression in tumor tissue (de Guillebon et al. 2015). Therefore, patients with high levels of sPD-L1 may obtain clinical benefit by the blockade of PD-1 or PD-L1 in metastatic GC.
The prognostic role of measuring PD-L1 expression by IHC has been reported for several tumor types, and the majority of data suggest that PD-L1 expression is associated with shorter OS (Thompson et al. 2006; Nomi et al. 2007; Kim et al. 2013; Muenst et al. 2014; Huang et al. 2015; Paydas et al. 2015; Wang et al. 2015a, b; Wu et al. 2015; Cierna et al. 2016). In gastric cancer, PD-L1 expression by IHC has been investigated by several studies, all conducted in Asian countries (Wu et al. 2006; Geng et al. 2014; Hou et al. 2014; Jiang et al. 2014; Qing et al. 2015; Kim et al. 2016). Three studies evaluated the prognostic role of PD-L1 expression. Although interpretation of the findings is limited by differences in the definition of PD-L1 positivity and retrospective analyses, two studies indicated that high PD-L1 expression was associated with a worse prognosis in advanced GC (Wu et al. 2006; Qing et al. 2015), while the other study could not confirm the prognostic role of PD-L1 expression (Kim et al. 2016). This may be due to the heterogeneity of PD-L1 expression in GC, with separate tumor biopsies from the same patient producing conflicting evidence. Consistent with two of the above studies, the present study demonstrated that high sPD-L1 levels correlated with shorter OS compared with low sPD-L1 levels. This result suggests that serum sPD-L1 may be a valuable and less invasive biomarker than tumor PD-L1 expression for predicting OS.
B7-H immune checkpoint molecules usually exist as membrane-bound ligands on myeloid cells and activated lymphocytes. In previous reports, B7-H was detected in the sera of patients with malignancies such as renal cell carcinoma (Simon et al. 2006, 2007; Frigola et al. 2011). Preclinical data suggest that sPD-L1 is produced and released by activated mature dendritic cells and was associated with a poor prognosis in patients with renal cell carcinoma (Frigola et al. 2012). Specific binding of sPD-L1 to PD-1 has been verified by ELISA (Wang et al. 2015a, b). Taken together, these findings demonstrate that serum sPD-L1 may play an important role in the immune microenvironment of tumors. Similar to high expression of PD-L1 in tumor tissue, sPD-L1 levels detected by ELISA were significantly higher in patients with malignancies compared with healthy individuals (Rossille et al. 2014; Zheng et al. 2014; Wang et al. 2015a, b). To enable an indirect comparison of sPD-L1 levels in patients with malignancies, the present study used the same ELISA kit as that previous studies. Of note, the mean sPD-L1 level in this study (0.883 ng/ml) was very similar to that of patients with any stage of GC in another report (0.893 ng/mL) (Zheng et al. 2014). However, the predictive value of serum sPD-L1 as a prognostic marker of antibody treatment against PD-L1 or PD-1 in patients with malignancies remains to be clarified. With respect to anti-PD-1 antibody treatment, recent research demonstrated that serum sPD-L1 levels at pre-treatment were significantly higher in responders than in non-responders in melanoma (Dronca et al. 2015). This indicates that serum sPD-L1 may have to the potential to be not only a prognostic factor, but also a predictive marker of eligibility for anti-PD-1 antibody in metastatic GC patients.
Newly developed drugs that inhibit immune checkpoints have led to dramatic changes to the conventional treatment strategy for malignant tumors. PD-L1 is up-regulated in a variety of solid tumors, and it can be detected by IHC on both tumor and immune cells. In a phase Ib clinical trial of the anti-PD-1 antibody pembrolizumab for metastatic GC, approximately 50 % of patients achieved tumor shrinkage of target lesions and a long duration of response, despite the study population comprising patients who had previously failed on chemotherapy (Meng et al. 2015). About 40 % of patients were diagnosed as PD-L1 positive, defined as PD-L1 staining in the stroma and ≥1 % of tumor cells. The definition of positivity for PD-L1 expression has not been rigorously determined in previous studies of PD-1 or PD-L1 inhibitors, and a wide spectrum of PD-L1 expression levels is observed in different tumor types (Muro et al. 2014). Reasons for the lack of clarity include differences in the cutoff value of PD-L1 positivity (proportion of positive cells: 1, 5, 10 %), positive cells (tumor cells and/or immune cells), and the manufacturer of diagnostic antibodies. Therefore, unification of IHC techniques and definitions of optimal cutoff values are required for clinical use of PD-L1 staining as a predictive marker of immune checkpoint inhibitor treatment. Recent studies have assessed immune-related biomarkers obtained by liquid biopsy (Dronca et al. 2015; Lote et al. 2015). This technique is less invasive than tissue biopsy, and it may enable the evaluation of immune status before and during treatment as opposed to archival tissue samples.
In our study, there was no difference in PFS between patients with high and low level of sPD-L1. On the other hand, patients with higher sPD-L1 level had shorter OS compared with lower sPD-L1. There were no reports of prognostic analyses of both PFS and OS according to the sPD-L1 level on the treatment of metastatic GC patients. This may indicate that sPD-L1 may be a prognostic factor but not a predictive factor of first-line treatment in metastatic gastric cancer. Combination of fluoropyrimidine and platinum is known to achieve high response rate (Bang et al. 2010; Koizumi et al. 2008, 2012; Kim et al. 2012; Yamada et al. 2015); thus, even patients with high level of sPD-L1 may obtain the clinical benefit of treatment. In addition, high WBC counts were observed in patients with high sPD-L1 level in our study. The other inflammatory findings were not associated with sPD-L1 level. To our knowledge, there were no previous reports that evaluated the association between sPD-L1 level and WBC counts of peripheral blood in patients with malignant tumors. We additionally checked leukocyte counts and neutrophil counts of peripheral blood, and neutrophil count was high in patients with high sPD-L1 level (data not shown). Neutrophil-to-leukocyte ratio (NLR) was well known as a prognostic factor in advanced gastric cancer according to previous reports (Grenader et al. 2016; Ock et al. 2016), and our study indicated that NLR was also a prognostic factors in OS by multivariate analyses in our study (data not shown). This indicated that both neutrophil counts (WBC counts) and sPD-L1 may be higher in patients with poor prognosis compared with those with better prognosis.
With respect to other biomarkers for predicting the response to anti-PD-1 or PD-L1 antibody therapy, a clinical trial in patients with GC indicated that microsatellite instability (MSI) status, a higher number of somatic mutations and antigen burden, and tumor-infiltrating lymphocytes (TILs) were considered as main candidates (Wang et al. 2013). MSI status is considered to be a particularly promising predictive biomarker of anti-PD-1 agents in gastrointestinal cancers. In colorectal cancer, pembrolizumab was associated with a high response rate in patients with MSI-high (MSI-H), whereas no responses were observed in patients with MSI-stable (MSS) (Le et al. 2015). In colorectal and non-colorectal cancers, MSI-H tumors display a higher number of somatic mutations and neo-antigen burden than MSS tumors, which strongly induces TILs and cytokine-rich tumor microenvironment, as well as expression of negative immune checkpoints, including PD-1, PD-L1, cytotoxic T-lymphocyte-associated protein 4, lymphocyte activation gene 3, and indoleamine 2,3-dioxygenase (Le et al. 2015; Llosa et al. 2015). In advanced GC, four molecular subtypes were categorized by comprehensive evaluation of a section of the Cancer Genome Atlas project (Cancer Genome Atlas Research Network 2014). Two of the four GC subtypes, tumors positive for Epstein–Barr virus harboring PIK3CA mutations as well as amplification of JAK2, CD274 and PDCD1LG2 (9 %) and microsatellite unstable tumors with elevated gene mutation rates (22 %), were considered to be vulnerable to blockade of PD-1 or PD-L1.
The present study has several limitations. The potential for selection bias cannot be excluded, and this was an exploratory study with a limited sample size. Patients with insufficient serum samples for ELISA were excluded, while a higher proportion of HER2-positive than HER2-negative patients were included. Of note, age and ECOG PS, which are well-known prognostic factors, were not included in multivariate analysis because these covariates had P-values ≥0.2 in univariate analysis. Furthermore, prospective data are required to validate the results of this study. Considering the heterogeneity of PD-L1 expression in GC tissue, it is necessary to determine the level of concordance between PD-L1 expression by IHC and serum sPD-L1 by ELISA.
This study revealed that the detection of high levels of serum sPD-L1 was associated with a poor survival prognosis in patients with metastatic GC. Although the potential for differences between sPD-L1 in blood and PD-L1 expression in tissue remains unclear, it is possible that serum sPD-L1 is a useful and less invasive biomarker in metastatic GC patients. Serum level of sPD-L1 in 40 % of enrolled patients was lower than measurable range in our study, and more sensitive ELISA kit may be required for strict quantitative analyses. Further investigations are warranted to confirm the relevance of sPD-L1 as a prognostic or predictive biomarker of systemic chemotherapy as well as immune checkpoint inhibitors.
References
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR (2015) Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627–1639
Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, Hodi FS, Choueiri TK, McDermott DF, Freeman GJ, Signoretti S (2015) Differential expression of PD-L1 between primary and metastatic sites in clear-cell renal cell carcinoma. Cancer Immunol Res 3:1158–1164
Cancer Genome Atlas Research Network (2014) Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513:202–209
Chen Y, Wang Q, Shi B, Xu P, Hu Z, Bai L, Zhang X (2011) Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 56:231–238
Cheng S, Zheng J, Zhu J, Xie C, Zhang X, Han X, Song B, Ma Y, Liu J (2015) PD-L1 gene polymorphism and high level of plasma soluble PD-L1 protein may be associated with non-small cell lung cancer. Int J Biol Markers 30:e364–e368
Cierna Z, Mego M, Miskovska V, Machalekova K, Chovanec M, Svetlovska D, Hainova K, Rejlekova K, Macak D, Spanik S, Ondrus D, Kajo K, Mardiak J, Babal P (2016) Prognostic value of programmed-death-1 receptor (PD-1) and its ligand 1 (PD-L1) in testicular germ cell tumors. Ann Oncol 27:300–305
Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT (2009) PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene 28:306–312
de Guillebon E, Roussille P, Frouin E, Tougeron D (2015) Anti program death-1/anti program death-ligand 1 in digestive cancers. World J Gastrointest Oncol 7:95–101
Dronca RS, Markovic SN, Kottschade LA, Nevala W, Dong H (2015) Bim as a predictive T cell biomarker for response to anti-PD-1 therapy in metastatic melanoma (MM). CRI-CIMT-EATI-AACR; September 16–19, New York, NY, Abstract A007
Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED (2011) Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res 17:1915–1923
Frigola X, Inman BA, Krco CJ, Liu X, Harrington SM, Bulur PA, Dietz AB, Dong H, Kwon ED (2012) Soluble B7-H1: differences in production between dendritic cells and T cells. Immunol Lett 142:78–82
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry DR, Melichar B, Tehfe M, Topuzov E, Zalcberg JR, Chau I, Campbell W, Sivanandan C, Pikiel J, Koshiji M, Hsu Y, Liepa AM, Gao L, Schwartz JD, Tabernero J, Trial Investigators REGARD (2014) Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 383:31–39
Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C (2014) Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 20:273–281
Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, Bridgewater J (2016) Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol 2016(27):687–692
Hanahan D, Coussens LM (2012) Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21:309–322
Hou J, Yu Z, Xiang R, Li C, Wang L, Chen S, Li Q, Chen M, Wang L (2014) Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 96:284–291
Huang B, Chen L, Bao C, Sun C, Li J, Wang L, Zhang X (2015) The expression status and prognostic significance of programmed cell death 1 ligand 1 in gastrointestinal tract cancer: a systematic review and meta-analysis. Onco Targets Ther 8:2617–2625
Jiang D, Xu YY, Li F, Xu B, Zhang XG (2014) The role of B7-H1 in gastric carcinoma: clinical significance and related mechanism. Med Oncol 31:268
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252
Kerr KM, Tsao MS, Nicholson AG, Yatabe Y, Wistuba II, Hirsch FR, Pathology Committee IASLC (2015) Programmed death-ligand 1 immunohistochemistry in lung cancer: In what state is this art? J Thorac Oncol 10:985–989
Kim GM, Jeung HC, Rha SY, Kim HS, Jung I, Nam BH, Lee KH, Chung HC (2012) A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer 48:518–526
Kim JR, Moon YJ, Kwon KS, Bae JS, Wagle S, Kim KM, Park HS, Lee H, Moon WS, Chung MJ, Kang MJ, Jang KY (2013) Tumor infiltrating PD1-positive lymphocytes and the expression of PD-L1 predict poor prognosis of soft tissue sarcomas. PLoS One 8:e82870
Kim JW, Nam KH, Ahn SH, Park do J, Kim HH, Kim SH, Chang H, Lee JO, Kim YJ, Lee HS, Kim JH, Bang SM, Lee JS, Lee KW (2016) Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 19:42–52
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Koizumi W, Nakayama N, Tanabe S, Sasaki T, Higuchi K, Nishimura K, Takagi S, Azuma M, Ae T, Ishido K, Nakatani K, Naruke A, Katada C (2012) A multicenter phase II study of combined chemotherapy with docetaxel, cisplatin, and S-1 in patients with unresectable or recurrent gastric cancer (KDOG 0601). Cancer Chemother Pharmacol 69:407–413
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 372:2509–2520
Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, Zhang M, Papadopoulos N, Kinzler KW, Vogelstein B, Sears CL, Anders RA, Pardoll DM, Housseau F (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5:43–51
Lote H, Cafferkey C, Chau I (2015) PD-1 and PD-L1 blockade in gastrointestinal malignancies. Cancer Treat Rev 41:893–903
Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, Hersey P, Scolyer RA (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 28:245–253
McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL (2016) Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol 2:46–54
Meng X, Huang Z, Teng F, Xing L, Yu J (2015) Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 41:868–876
Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P, CheckMate 025 Investigators (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803–1813
Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, Weber WP, Soysal SD (2014) Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 146:15–24
Murdoch C, Muthana M, Coffelt SB, Lewis CE (2008) The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8:618–631
Muro K, Bang YJ, Shankaran V, Geva R, Catenacci DVT, Gupta S, Eder JP, Berger R, Gonzalez EJ, Ray A, Dolled-Filhart M, Emancipator K, Pathiraja K, Lunceford JK, Cheng JD, Koshiji M, Chung HC (2014) Relationship between PD-L1 expression and clinical outcomes in patients (Pts) with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab (Pembro; MK-3475) in KEYNOTE-012. Ann Oncol 25:1–41
Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y (2007) Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 13:2151–2157
Ock CY, Nam AR, Lee J, Bang JH, Lee KH, Han SW, Kim TY, Im SA, Kim TY, Bang YJ, Oh DY (2016) Prognostic implication of antitumor immunity measured by the neutrophil-lymphocyte ratio and serum cytokines and angiogenic factors in gastric cancer. Gastric Cancer. Epub ahead of print
Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M (2015) Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in hodgkin lymphoma. Ann Hematol 94:1545–1552
Qian Y, Deng J, Geng L, Xie H, Jiang G, Zhou L, Wang Y, Yin S, Feng X, Liu J, Ye Z, Zheng S (2008) TLR4 signaling induces B7-H1 expression through MAPK pathways in bladder cancer cells. Cancer Investig 26:816–821
Qing Y, Li Q, Ren T, Xia W, Peng Y, Liu GL, Luo H, Yang YX, Dai XY, Zhou SF, Wang D (2015) Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Dev Ther 9:901–909
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, Savage KJ, Hernberg MM, Lebbé C, Charles J, Mihalcioiu C, Chiarion-Sileni V, Mauch C, Cognetti F, Arance A, Schmidt H, Schadendorf D, Gogas H, Lundgren-Eriksson L, Horak C, Sharkey B, Waxman IM, Atkinson V, Ascierto PA (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372:320–330
Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T, Groupe Ouest-Est des Leucémies et Autres Maladies du Sang; Groupe Ouest-Est des Leucémies et Autres Maladies du Sang (2014) High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia 28:2367–2375
Simon I, Zhuo S, Corral L, Diamandis EP, Sarno MJ, Wolfert RL, Kim NW (2006) B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res 66:1570–1575
Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW, McIntosh MW (2007) Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol 106:112–118
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED (2006) Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 66:3381–3385
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2012) Global cancer statistics, 2012. CA Cancer J Clin 65:87–108
Wang L, Chang EW, Wong SC, Ong SM, Chong DQ, Ling KL (2013) Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 190:794–804
Wang A, Wang HY, Liu Y, Zhao MC, Zhang HJ, Lu ZY, Fang YC, Chen XF, Liu GT (2015a) The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol 41:450–456
Wang L, Wang H, Chen H, Wang WD, Chen XQ, Geng QR, Xia ZJ, Lu Y (2015b) Serum levels of soluble programmed death ligand 1 predict treatment response and progression free survival in multiple myeloma. Oncotarget 6:41228–41236
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A, RAINBOW Study Group (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15:1224–1235
Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N (2006) Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 108:19–24
Wu P, Wu D, Li L, Chai Y, Huang J (2015) PD-L1 and survival in solid tumors: a meta-analysis. PLoS One 10:e0131403
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, Tsuji A, Imamura H, Tsuda M, Yasui H, Fujii H, Yamaguchi K, Yasui H, Hironaka S, Shimada K, Miwa H, Hamada C, Hyodo I (2015) Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 26:141–148
Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL (2008) Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+ CD11b+ myeloid cells that promote metastasis. Cancer Cell 13:23–35
Zheng Z, Bu Z, Liu X, Zhang L, Li Z, Wu A, Wu X, Cheng X, Xing X, Du H, Wang X, Hu Y, Ji (2014) Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin J Cancer Res 26:104–111
Acknowledgments
We gratefully appreciate the participation of the patients and their families in this study, and we would like to thank all co-investigators for their contributions: Ms. Hideko Morita and Mrs. Noriko Abe (serum sample preparation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed were in accordance with the ethical standards of the national regulations and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent of Biobank of National Cancer Center and using the clinical data was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2016_2184_MOESM2_ESM.docx
Supplementary material 2. Prognostic analyses of progression-free survival by uni- and multivariate analyses (DOCX 20 kb)
432_2016_2184_MOESM3_ESM.docx
Supplementary material 3. Difference in patient characteristics by serum sPD-L1 levels using the median as the cutoff value (DOCX 22 kb)
Rights and permissions
About this article
Cite this article
Takahashi, N., Iwasa, S., Sasaki, Y. et al. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol 142, 1727–1738 (2016). https://doi.org/10.1007/s00432-016-2184-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2184-6