Abstract
Purpose
We aimed to examine outcomes of high-dose radiotherapy with helical tomotherapy (HT) and long-term androgen deprivation therapy (ADT) for T1–4N0M0 prostate cancer.
Methods
A total of 391 patients treated with HT between June 2006 and December 2013 were included in this retrospective study. All patients received neoadjuvant ADT for a median duration of 10 months followed by HT at a median dose of 78 Gy [interquartile range (IQR) 78–78]. The times of median adjuvant and total ADT were 19 and 27 months (IQR 20–31), respectively. The risk stratification followed the 2015 National Comprehensive Cancer Network criteria. Biochemical disease-free survival (bDFS) followed the Phoenix definition. Toxicity was scored according to the Radiation Therapy Oncology Group morbidity grading scale.
Results
Median follow-up from HT start was 60 months (IQR 42–81). Five-year bDFS rates for low-, intermediate-, high-, and very-high-risk groups were 100, 98.2, 97.7, and 87.9 %, respectively. We observed clinical relapse in nine very-high-risk patients and one high-risk patient, resulting in a 5-year clinical relapse-free survival of 100, 100, 99.4, and 91.7 %, respectively, for each risk group. Three patients died of prostate cancer, resulting in a 5-year prostate cancer-specific survival of 99.6 %. The late grade 2 or higher gastrointestinal and genitourinary toxicities were 9.7 and 10.7 %. No cardiovascular fatal events were observed.
Conclusions
This report confirmed the excellent outcomes with acceptable late toxicities with the combination of HT and long-term ADT. Longer follow-up is crucial to further determine the treatment effect and toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinical outcomes have improved substantially with high-dose external beam radiation therapy (EBRT) in patients with localized prostate cancer (Viani et al. 2009). Intensity-modulated radiation therapy (IMRT) is a technology designed to permit the safe delivery of increased doses to the target volume with concurrent dose reductions to the organs at risk (OAR). High-dose EBRT with IMRT has shown excellent long-term tumor-control outcomes in patients with localized prostate cancer (Alicikus et al. 2011), and IMRT is widely used in Japanese clinics (Tomita et al. 2014). Helical tomotherapy (HT) is a novel IMRT modality, in which treatment beams are spatially and temporally modulated to maximize the dose delivered to the target volume while minimizing the dose delivered to OAR. In addition, detectors within the tomotherapy system provide megavoltage computed tomography (MVCT), which can be obtained immediately before treatment for setup, registration, and repositioning (Kapatoes et al. 2001). Thus, image-guided IMRT (IG-IMRT) with HT provides excellent target coverage with dose uniformity while sparing OAR. We introduced HT in 2006, and HT has been used to prescribe the higher dose of 78 Gy to the prostate compared with the dose of 74 Gy in three-dimensional conformal radiotherapy (3DCRT) at our institution. We previously reported the preliminary results of HT for prostate cancer, and HT was associated with low rates of acute and late toxicities and excellent short-term biochemical control (Tomita et al. 2012). Recently, Zapatero et al. (2015) published the first report on their randomized trial, in which long-term androgen deprivation therapy (ADT) plus high-dose radiotherapy was superior to short-term ADT plus high-dose radiotherapy in terms of biochemical disease-free survival and overall survival, particularly in patients with high-risk prostate cancer. We have been combining EBRT with long-term ADT because approximately 70 % of patients were in the high-risk group at our institution (Tomita et al. 2009). Thus, promising results may be shown with combination therapy of high-dose IMRT with HT and long-term ADT. Prostate cancer shows an exponential increase against the background of the aging society and the PSA screening test in Japan. The incidence of prostatic cancer is expected to be the leading cancer in men by 2020 (Tabata et al. 2008). In this report, we examine 5-year outcomes of HT with long-term ADT.
Patients and methods
Patients
Between June 2006 and December 2013, 422 patients with clinically localized or locally advanced prostate cancer were treated with HT at our single institution. Of these, routine follow-up data of 31 patients were missing because of observation at their local hospital immediately after radiotherapy. The remaining 391 patients, who were followed regularly at our institution, were included in this retrospective study. Patients with a previous history of cancer that had been controlled at the start date of HT were also included in this study. Pretreatment diagnostic evaluations were performed using serum prostate-specific antigen (PSA), digital rectal examination (DRE), magnetic resonance imaging (MRI) of the pelvis, computed tomography (CT) of the chest to the pelvis, and bone scintigraphy. The tumor stage was decided comprehensively by both DRE and MRI. Patients had histologically confirmed prostatic adenocarcinoma, classified according to the Gleason grading system. Typically, 12 cores were collected by needle biopsy, but data of positive cores were missing for 12 patients. Patients had clinical stage T1–T4N0M0 prostate adenocarcinoma according to the American Joint Committee on Cancer clinical staging. A risk stratification followed the National Comprehensive Cancer Network (NCCN) guidelines version 1.2015 (http://www.nccn.org) in this study. Patients were classified into four prognostic risk groups as follows: low risk, pretreatment PSA < 10 ng/ml, T1-T2a, and Gleason score ≤6; intermediate risk, T2b-T2c or Gleason score 7 or PSA 10–20 ng/ml; high risk, T3a or Gleason score 8–10 or PSA > 20 ng/ml; and very-high risk, T3b-T4 or primary Gleason pattern 5 or cores >4 with Gleason score 8–10. Table 1 describes the patient characteristics. Sixty-three men (16.1 %) had a history of cancer treatment at 71 other sites before the start of HT. The most common site was the colon in 16 patients (4.1 %), and the second common site was the colon in 15 patients (3.8 %). Other sites included the rectum, bladder, and ureter in seven (1.8 %), four (1.0 %), and two patients (0.5 %), respectively.
All patients received written informed consent before treatment. This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or similar ethical standards. This study was approved by our institutional review board. The median follow-up time from the start date of HT was 60 months [interquartile range (IQR) 42–81, range 15–115]. The median follow-up time from the start date of ADT was 73 months (IQR 54–93, range 22–139).
Helical tomotherapy
Radiotherapy was administered with HT for all patients. All patients were immobilized in a supine position with an Esform vacuum-type immobilization system and simulated by pelvic CT with a 2.0- or 2.5-mm slice thickness. On the day of CT simulation and during IMRT, all patients defecated where possible every morning and discharged urine about 1 h before CT simulation and IMRT to minimize daily variations in the shape and anatomical location of the prostate. Outlines of the target were delineated on a three-dimensional radiation treatment planning system (Pinnacle3 workstation, Hitachi Medical Corporation, Tokyo, Japan) using the abdominal CT window setting. Clinical target volume (CTV) was defined as the entire prostate and proximal seminal vesicle. In the case of seminal vesicle invasion, CTV included the entire seminal vesicle. Planning target volume 1 (PTV1) included CTV with a 6–8-mm margin except at the prostatorectal interface, where a 4–6-mm margin was used. PTV2 was defined as the seminal vesicle with a similar margin as PTV1 outside of PTV1. Elective pelvic radiotherapy was not used in any patients. Normal structures including the rectum, bladder, femoral head, penile bulb, pubic bone, bowel, and sigmoid colon adjacent to PTV were considered to be OAR. Normal structures were constrained on an individual basis using maximum and dose-volume histogram dose constraints without compromising PTV1 coverage. The prescribed doses were as follows: (1) PTV1 D95 (i.e., dose delivered to 95 % of PTV1): 74 Gy in the low-risk group, 78 Gy in intermediate-, high-, and very-high-risk groups; (2) PTV2 D95: 64 Gy. Dose constraints for normal structures have been described previously (Tomita et al. 2012). Treatment was provided in daily 2 Gy fractions. All patients began treatment with daily MVCT acquisitions for setup, registration, and repositioning on the basis of the location of the prostate.
The prescribed dose was reduced slightly to 70–76 Gy for 21 patients (21 of 358, 5.9 %) in the intermediate- to very-high-risk groups because of their anti-thrombogenic medications, failure in OAR dose constraints, and physicians’ suggestion for their acute rectal symptoms. Nine patients in the low-risk group (9 of 33, 27.3 %) received 78 Gy at the discretion of the radiation oncologist in consideration of risk factors such as high positive cores. The median HT period was 57 days (range 48–95).
Hormonal therapy
Maximum androgen blockade consisted of a luteinizing hormone-releasing hormone (LHRH) analog (i.e., leuprorelin at 11.25 mg or goserelin at 10.8 mg subcutaneously every 12 weeks), and anti-androgen therapy (i.e., bicalutamide 80 mg per day) was performed as neoadjuvant ADT (N-ADT) for all patients. N-ADT duration depended on the HT reservation in principle, and the median time of N-ADT was 10 months (IQR 7.5–11; range 1–68). Adjuvant ADT (A-ADT) consisted of only the LHRH-analog. Patients were given A-ADT for 1–2 years at the discretion of the urologist. Seventeen patients (4.3 %) did not receive A-ADT because they experienced adverse effects associated with N-ADT, such as liver dysfunction. Seven patients continued to receive A-ADT at the time of this analysis. The median time of A-ADT was 19 months (IQR 9–21; range 0–100). The median total ADT time was 27 months (IQR 20–31; range 4–123). The median total ADT times were 20 (IQR 16–23; range 6–48), 19 (IQR 17–22; range 5–80), 29 (IQR 24–32; range 12–91), and 30 (IQR 27–32; range 4–123) months for the low-, intermediate-, high-, and very-high-risk groups.
Follow-up
Follow-up was performed at intervals of 3 months. When PSA values were kept at a low level after 3–5 years, follow-up intervals were extended to every 4–6 months. Serum PSA was measured at each follow-up. The follow-up length was calculated from the start date of HT. Biochemical disease-free survival (bDFS) followed the Phoenix definition (i.e., a post-treatment nadir plus 2.0 ng/ml, Roach et al. 2006). A clinical relapse comprised local disease, and lymph node, bone, or parenchymal metastases, detected by CT and/or bone scintigraphy. Patients began salvage ADT after documentation of biochemical relapse. The imaging examinations were performed at the time of biochemical relapse and in cases in which clinical progression was suspected during salvage ADT. Distributions of bDFS, clinical relapse-free survival (cRFS), prostate cancer-specific survival, and overall survival (OS) were calculated according to the Kaplan–Meier method. A logrank test was done to assess the relationship between potential prognostic factors and bDFS and cRFS. Variables included in the analysis were age (<71 vs ≥71), PSA (≤20 vs >20 ng/ml), Gleason score (≤7 vs 8–10), T-stage (T1–2 vs T3–4), NCCN risk (low and intermediate vs high and very high), rate of positive biopsies (≤50 vs >50 %), N-ADT time (<10 vs ≥10 months), A-ADT time (<19 vs ≥19 months), total ADT time (<28 vs ≥28 months), and radiation dose (<78 Gy vs 78 Gy). Cox proportional hazards regression models were used to determine the effect of each factor in the multivariate analysis. All statistical analyses were performed with EZR (Kanda. 2013), which is a graphical user interface for R (The R Foundation for Statistical Computing). A p value of <0.05 was considered significant. Toxicity was scored according to the Radiation Therapy Oncology Group morbidity grading scale (Cox et al. 1995). In brief, grade 1 toxicity represents minimal side-effects not requiring medication for symptom control, grade 2 toxicity indicates symptoms requiring medication, grade 3 indicates complications requiring minor surgical intervention (i.e., transurethral resection or laser coagulation), and grade 4 requires hospitalization and major intervention.
Results
Biochemical control and clinical relapse
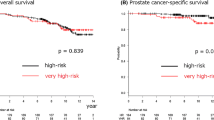
The 5-year bDFS rate was 95.0 % [95 % confidence interval (CI), 91.8–97.0 %] in all groups. The 5-year bDFS rates for low-, intermediate-, high-, and very-high-risk group patients were 100, 98.2 % (95 % CI 88.0–99.7 %), 97.7 % (95 % CI 93.0–99.3 %), and 87.9 % (95 % CI 79.0–93.2 %), respectively (p < 0.01). Figure 1 shows the bDFS for each risk group. A pretreatment PSA level (p < 0.01), Gleason score (p < 0.01), risk group (p = 0.049), and positive core (p < 0.01) were significant factors for biochemical relapse on the logrank test, as shown in Table 2. Gleason score [hazard ratio (HR), 14.5; 95 % CI 1.92–109, p < 0.01] and positive core (HR 2.86; 95 % CI 1.03–7.93, p = 0.043) were identified as significant predictors of biochemical relapse in the multivariate analysis.
We observed clinical relapse in nine very-high-risk patients and one high-risk patient, resulting in 5-year cRFSs of 100, 100, 99.4 % (95 % CI 95.6–99.9 %), and 91.7 % (95 % CI 83.2–96.0 %) for the low-, intermediate-, high-, and very-high-risk group, respectively (p < 0.01). Seven patients developed bone metastasis at a median of 53 months (range 4–75) after the start date of HT. Two patients developed lung metastasis after 42 and 51 months, and one patient developed pelvic node metastases after 27 months. Pretreatment PSA level (p < 0.01), Gleason score (p < 0.01), tumor stage (p = 0.029), risk group (p = 0.042), and positive core (p < 0.01) were significant factors for clinical relapse in the logrank test, as shown in Table 2. Factors for clinical relapse were not obtained in the multivariate analysis because of a lack of events among patients with PSA ≤ 20 and Gleason score ≤7.
An additional analysis of only high- and very-high-risk groups (N = 270) showed that pretreatment PSA level (p = 0.078), Gleason score (p < 0.01), risk group (p < 0.01), positive core (p < 0.01), and HT dose (p < 0.01) were considered to be related to biochemical relapse in the logrank test. Gleason score (HR 23; 95 % CI 2.6–199, p < 0.01), positive core (HR 5.8; 95 % CI 1.7–19.7, p < 0.01), and HT dose (HR 0.12; 95 % CI 0.04–0.37, p < 0.01) were identified as significant predictors of biochemical relapse on multivariate analysis. Gleason score (p = 0.030), risk group (p < 0.01), positive core (p = 0.030), and HT dose (p = 0.013) were considered to be related to clinical relapse in the logrank test for only the high- and very-high-risk groups. Factors for clinical relapse were not obtained in the multivariate analysis because of a lack of events among patients with Gleason score ≤7.
Survival and second malignancy
Four patients died by the date of analysis, resulting in a 5-year overall survival rate of 99.4 % (95 % CI 97.4–99.8 %). Of these, three very-high-risk patients died of prostate cancer. 5-year prostate cancer-specific survival rate was 99.6 % (95 % CI 97.4–99.9 %).
At the time of analysis, 28 men (7.2 %) had been diagnosed with new other cancers at 29 other sites. The most common site was the bladder in seven patients (1.8 %). The second most common sites were the colon in six patients (1.5 %) and the stomach in six patients (1.5 %), and other sites were the lungs in two patients (0.5 %), head and neck, esophagus, pancreas, bile duct, adrenal lymphoma, rectum, ureter, and urethra in each one patient (0.3 %). The latency period between radiation exposure and radiation-induced secondary cancers is considered to be from 5 to 15 years (Jao et al. 1987; Thompson et al. 1994). Among potentially irradiated pelvic sites such as the colon, ureter, bladder, and urethra, one patient (0.3 %) diagnosed with urethra cancer after 105 months, one patient (0.3 %) diagnosed with colon cancer after 63 months, and three patients diagnosed with bladder cancer (0.8 %) after 87, 94, and 97 months were potentially radiation-induced secondary cancers. Two patients with bladder cancer, one patient each with gastric cancer and head and neck cancer, and one patient with ureter cancer treated before IMRT had developed a terminal condition at the time of this analysis.
Late toxicity
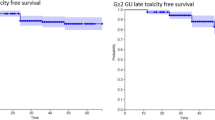
The rate of late grade 2 or higher gastrointestinal (GI) toxicities was 9.7 %. Of 29 patients (7.4 %) who developed late grade 2 GI toxicity, 24 patients (6.1 %) developed grade 2 rectal bleeding at a median of 21 months (range 9–64) after the start date of IMRT. Other symptoms were pain on defecation in three patients (0.8 %), high stool frequency in one patient (0.3 %), and subtle fecal incontinence in one patient (0.3 %). Nine patients (2.3 %) developed grade 3 rectal bleeding requiring argon plasma coagulation at a median of 19 months (range 11–51). No grade 4 late rectal toxicity was observed. The 5-year cumulative incidence of late grade 2 or higher GI toxicities was 10.4 % (grade 2, 7.8 %, grade 3, 2.6 %).
The rate of late grade 2 or higher genitourinary (GU) toxicities was 10.7 %. Of the 36 patients (9.2 %) who developed late grade 2 urinary toxicity, 29 patients (7.4 %) experienced dysuria requiring medication or addition of medication at a median of 19 months (range 7–47 months). Other symptoms were gross hematuria in four patients (1.0 %) and cystitis in three patients (0.8 %). Four patients (1.0 %) experienced grade 3 urinary retention requiring self-catheterization or dilation at 14, 17, 28, and 81 months after IMRT. Two patients developed bladder ulcer (grade 3) requiring laser coagulation after 14 and 47 months. No patients experienced late grade 4 urinary symptoms. Table 3 summarizes late GI and GU toxicities. The 5-year cumulative incidence of late grade 2 or higher GU toxicities was 11.6 % (grade 2, 10.5 %, grade 3, 1.1 %).
Cardiovascular and cerebrovascular events were occurred in 10 (2.6 %) and two patients (0.5 %), respectively. No fatal events occurred. One patient (0.3 %) developed grade 3 interstitial pneumonia due to ADT, and three patients (0.8 %) developed lumbar compression fracture after treatment.
Discussion
Our results showed that high-dose radiotherapy with HT combined with long-term ADT provided excellent biochemical control, clinical relapse-free survival, and prostate cancer-specific survival for all risk groups with prostate cancer. Our findings of excellent outcomes with HT and other clinical outcomes of radiotherapy are summarized in Table 4. Two main factors responsible for our favorable outcomes are as follows: firstly, the combination with long-term ADT. The optimum duration of ADT has been studied in men with high-risk and locally advanced prostate cancer. Findings with RTOG 92-02 showed that 4 months of ADT was inferior to 28 months of treatment in this population (Horwitz et al. 2008). Other randomized trials also have shown that ADT combined with conventional-dose radiotherapy improved overall survival, mainly in patients with intermediate- and high-risk prostate cancer (Bria et al. 2009; Schmidt-Hansen et al. 2014). Similarly, clinical outcomes have also improved substantially with high-dose radiotherapy (Viani et al. 2009). Zapatero et al. (2015) reported that long-term ADT was superior to short-term ADT in patients given high-dose radiotherapy in terms of biochemical control and overall survival, particularly in men with high-risk prostate cancer. They concluded that as the optimum ADT duration in high-dose radiotherapy in intermediate-risk disease remains to be defined, and further follow-up is needed to determine the effects of long-term ADT in this subgroup. Level I evidence supports the use of short-term ADT with conventional doses (i.e., 66–70 Gy) of EBRT for patients with intermediate-risk prostate cancer (Pollack et al. 2016; D’Amico et al. 2004; Jones et al. 2011; Denham et al. 2011). To the question of whether ADT adds benefit to dose-escalated RT for this population, Radiation Therapy Oncology Group (RTOG) 0815 (http://www.rtog.org) addresses this question. According to the review by Zumsteg and Zelefsky (2012), the randomized data are from a preliminary analysis of the French trial GETUG 14 (Dubray et al. 2011) including 366 men with intermediate-risk prostate cancer treated with high-dose radiotherapy of 80 Gy, either alone or with 4 months of ADT. ADT significantly increased 3-year biochemical progression-free survival (97 vs 91 %; p = 0.04), but the primary endpoint of the trial (combined biochemical and local tumor control) did not differ between groups (92 vs 86 %, p = 0.09). Recently, Bolla et al. (2016) have shown the results of European Organization for Research and Treatment of Cancer (EORTC) trial 22991. 74.8 % of the 819 men were at intermediate-risk and 24.8 % were at high-risk by D’Amico risk group. At 7.2 years median follow-up, RT plus androgen suppression significantly improved bDFS (HR 0.52; 95 % CI 0.41–0.66; p = 001), as well as clinical progression-free survival (HR 0.63; 95 % CI 0.48–0.84; p = 001). Six months of concomitant and adjuvant androgen suppression improves biochemical and clinical DFS of intermediate- and high-risk cT1b-c to cT2a prostate cancer, treated by EBRT. On the other hand, in the recent study, the National Cancer Data Base was used to evaluate whether the addition of ADT to high-dose EBRT improves OS for patients with intermediate-risk prostate cancer (Amini et al. 2016). Their results show no improvement in OS when ADT is combined with high-dose EBRT. The results of their study suggest that the addition of ADT to dose-escalated RT for patients with intermediate-risk prostate cancer is only beneficial in those patients who exhibit all three intermediate-risk factors. The 5-year bDFS, cRFS, and prostate cancer-specific survival of the intermediate-risk group were favorable in our study. At least, as for our favorable biochemical control in patients with intermediate-risk disease, long-term ADT was considered to be effective. Longer follow-up and more events will enable us to provide more information about the effect of long-term ADT in patients with intermediate-risk prostate cancer. As there are no reported trials that have defined the role of ADT for patients with low-risk prostate cancer in EBRT, the NCCN guidelines recommend that patients with low-risk cancer should not receive ADT with EBRT. We may require a rethinking of ADT for patients with low-risk cancer in our institution. Secondly, differences in the evaluation method of the prescribed dose. The median dose of PTV1 prescribed 78 Gy in D95 was approximately 80 Gy in our HT plan, whereas the isocenter dose to the prostate is generally used in 3DCRT according to the guidelines of the International Commission on Radiation Units. Viani et al. (2009) reported that their dose–response model can hypothetically predict that radiation doses of approximately 86.5, 90.4, and 95.5 Gy would need to be delivered to low-, intermediate-, and high-risk patients with localized prostate cancer, respectively, to achieve a 100 % biochemical control rate. Therefore, we think that the difference between 78 and 80 Gy can impact the outcome. In addition, we considered that excellent target coverage with HT and accurate setup and repositioning with MVCT could contribute to this favorable outcome.
This study failed to demonstrate an improvement in outcome as a result of longer ADT (<28 vs ≥28 months). An additional univariate analysis of only the high- and very-high-risk groups also did not show any difference in outcomes with the ADT duration. Perhaps, this is believed to be due primarily to the use of long-term ADT in most patients. On the other hand, HT dose was considered to be related to biochemical control and clinical relapse in a multivariate analysis of only the high- and very-high-risk groups. Therefore, this indicated that the significance of dose escalation also remained in the combination of long-term ADT. The results of our study showed that the very-high-risk group (i.e., T3b–T4 or primary Gleason pattern 5 or cores >4 with Gleason score 8–10) had significantly poorer prognosis of bDFS, cRFS, and prostate cancer-specific survival compared with the other groups, as shown in Figs. 1 and 2. Narang et al. (2016) showed in a study of men consecutively treated with definitive radiation by a single provider from 1993 to 2006 and who fulfilled criteria for NCCN high-risk disease that NCCN high-risk prostate cancer patients who meet the very-high-risk criteria (multiple NCCN high-risk factors; primary Gleason pattern 5 disease; and/or ≥5 biopsy cores with Gleason sums of 8–10) experience distinctly worse outcomes following definitive radiation and long-term ADT. They concluded that the very-high-risk definition may, therefore, serve as a useful tool for identifying patients in whom to reconsider the optimal application of current therapies and to explore novel agents. The results of our study were consistent with their study.
The rates of late grade 2 or higher GI (9.7 %) and GU (10.7 %) toxicity in our study were satisfactorily low. Data indicate that late rectal toxicity profiles were excellent compared with the incidence of late grade 2 or higher GI and GU toxicity that reportedly ranged from 3 to 15 % and from 17 to 32 %, respectively, in recent studies with the use of high-dose IMRT (Wong et al. 2009; Sharma et al. 2011; Alicikus et al. 2011). We considered that our favorable toxicity rates were partly as a result of MVCT with HT. However, careful attention is required to compare data on late toxicity because of the retrospective nature of the study and different follow-up times.
Obviously, there are factors that limit the interpretation of a single institutional retrospective review. We recognize the potential underestimation of toxicity, especially from the use of long-term ADT. The rate of cardiovascular events in our study (2.6 %) was lower than those reported elsewhere. A recent randomized controlled trial reported that the rates of cardiovascular events were 20 % in the long-term ADT group and 14 % in the short-term group (Zapatero et al. 2015). The EORTC 22863 group reported a 10-year risk of death from cardiac events of 6 % in the group receiving radiotherapy and long-term ADT compared with 4.2 % in the group receiving radiotherapy alone (Bolla et al. 2010). Findings of major randomized controlled trials of ADT and radiation do not show an increase in incidence of cardiovascular mortality from ADT with 8–10 years of follow-up, nor do data from a meta-analysis (Bolla et al. 2009; Roach et al. 2008; Efstathiou et al. 2008, 2009; Nguyen et al. 2011). The incidence of ischemic cardiac disease is lower in Japan than in Europe or the USA (Ueshima 2007; Ueshima et al. 2008). In fact, the patterns of care study for prostate cancer showed that ADT was a popular initial treatment option in localized or locally advanced disease in the Japanese population compared with the USA (Onozawa et al. 2014; Cooperberg et al. 2010), because more Japanese patients had advanced disease and Japanese patients were older. Instead, we failed to evaluate non-critical events in comparison with cardiovascular events such as hot flush, gynecomastia, and erectile dysfunction, although the patient charts were scrutinized thoroughly. ADT can be associated with deleterious medical and quality-of-life sequelae, including hot flushes, decreased libido, muscle loss, anemia, osteoporosis and fracture, and erectile dysfunction. Recent two articles highlight the possible impact that ADT may have on cognition (Gonzalez et al. 2015; Nead et al. 2016). Other difficulties associated with this study were the insufficient follow-up times and the small number of events. In particular, our study included 28 lost-to-follow-up patients within 3 years, whereas the study by Zapatero et al. included only eight lost-to-follow-up throughout the whole study period. It is possible that this loss to follow-up to seem to raise bDFS and cRFS of our study. Longer follow-up is crucial to determine this treatment effect and toxicity.
Zapatero et al. (2015) reported that an unexpected finding of their randomized trial was that almost five times as many patients died of cancers other than of the prostate. The results of our study also showed that 28 patients developed 29 subsequent cancers other than of the prostate after HT. On the other hand, potentially radiation-induced related secondary cancers, which developed at least 5 years after HT, were observed in five patients (1.3 %; 3 bladder, 1 urethra, and 1 colon). It is difficult to evaluate the rate of radiation-induced related secondary cancers in our study because of the small numbers of patients, limited follow-up time, and the lack of appropriate comparison groups. Several registry-based studies have shown an increased risk of second malignancies with radiotherapy compared to surgery or no treatment. Brenner et al. (2000) reported that radiotherapy for prostate carcinoma was associated with a small, statistically significant increase in the risk of solid tumors relative to treatment with surgery. Among patients who survived for more than 5 years, the increased relative risk reached 15 %, and was 34 % for patients surviving more than 10 years. The most significant contributors to the increased risk in the irradiated group were carcinomas of the bladder, rectum, and lung, and sarcomas within the treatment field. Compared with men who received no prostate cancer-directed radiation, men who received EBRT had statistically significant increased odds of developing secondary cancers at several sites potentially related to radiation therapy, including the bladder [odds ratio (OR), 1.63; 95 % CI 1.44–1.84] and rectum (OR 1.60; 95 % CI 1.29–1.99) (Moon et al. 2006). The cumulative incidence of any second solid cancers (both spontaneous and possibly radiation-related, with adjustment for competing cases of death) was 8 % by 10 years after prostate cancer diagnosis and 15 % by 15 years after diagnosis in all types of radiotherapy (Berrington de Gonzalez et al. 2015). The cohort was comprised of 34,889 prostate carcinoma patients who had undergone RT, and 106,872 who had not. After 8 years, the risk of bladder carcinoma was elevated for the RT group [relative risk (RR) 1.5; 95 % CI 1.1–2.0], but not for the non-RT group (RR 1.0; 95 % CI 0.7–1.2) (Neugut et al. 1997). For reference, the rate of radiation-induced related secondary bladder cancer in our study was similar to previous data of 1.4 % (568 of 39805, Moon et al. 2006) and 0.8 % (455 of 51584, Brenner et al. 2000).
In conclusion, this report confirmed the excellent 5-year outcomes with acceptable late toxicities associated with the combination of HT and long-term ADT. Superior dose distributions and IGRT with HT was an efficacious option for high-dose EBRT. The combination of long-term ADT was effective mainly for high-risk disease, and longer follow-up is crucial to further determine the treatment effect and toxicity. Outcomes of the very-high-risk group were apparently inferior to other groups, so that the very-high-risk group may require the development of new combination therapies with high-dose IMRT.
References
Alicikus ZA, Yamada Y, Zhang Z, Pei X, Hunt M, Kollmeier M, Cox B, Zelefsky MJ (2011) Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer 117(7):1429–1437
Amini A, Rusthoven CG, Jones BL, Armstrong H, Raben D, Kavanagh BD (2016) Survival outcomes of radiotherapy with or without androgen-deprivation therapy for patients with intermediate-risk prostate cancer using the National Cancer Data Base. Urol Oncol 34(4):165.e1–165.e9
Berrington de Gonzalez A, Wong J, Kleinerman R, Kim C, Morton L, Bekelman JE (2015) Risk of second cancers according to radiation therapy technique and modality in prostate cancer survivors. Int J Radiat Oncol Biol Phys 91(2):295–302
Bolla M, de Reijke TM, Van Tienhoven G, Van den Bergh AC, Oddens J, Poortmans PM, Gez E, Kil P, Akdas A, Soete G, Kariakine O, van der Steen-Banasik EM, Musat E, Pierart M, Mauer ME, Collette L (2009) Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 360(24):2516–2527
Bolla M, Van Tienhoven G, Warde P, Dubois JB, Mirimanoff RO, Storme G, Bernier J, Kuten A, Sternberg C, Billiet I, Torecilla JL, Pfeffer R, Cutajar CL, Van der Kwast T, Collette L (2010) External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 11(11):1066–1073
Bolla M, Maingon P, Carrie C, Villa S, Kitsios P, Poortmans PM, Sundar S, van der Steen-Banasik EM, Armstrong J, Bosset JF, Herrera FG, Pieters B, Slot A, Bahl A, Ben-Yosef R, Boehmer D, Scrase C, Renard L, Shash E, Coens C, van den Bergh AC, Collette L (2016) Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC Trial 22991. J Clin Oncol. doi:10.1200/JCO.2015.64.8055
Brenner DJ, Curtis RE, Hall EJ, Ron E (2000) Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer 88(2):398–406
Bria E, Cuppone F, Giannarelli D, Milella M, Ruggeri EM, Sperduti I, Pinnaro P, Terzoli E, Cognetti F, Carlini P (2009) Does hormone treatment added to radiotherapy improve outcome in locally advanced prostate cancer? Meta-analysis of randomized trials. Cancer 115(15):3446–3456
Cooperberg MR, Broering JM, Carroll PR (2010) Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 28(7):1117–1123
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW (2004) 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA 292(7):821–827
Denham JW, Steigler A, Lamb DS, Joseph D, Turner S, Matthews J, Atkinson C, North J, Christie D, Spry NA, Tai KH, Wynne C, D’Este C (2011) Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol 12(5):451–459
Dubray BM, Beckendorf V, Guerif S et al (2011) Does short-term androgen depletion add to high-dose radiotherapy (80 Gy) in localized intermediate-risk prostate cancer? Intermediate analysis of GETUG 14 randomized trial (EU-20503/NCT00104741). Proc Am Soc Clin Oncol 29(suppl):4521 (abstr)
Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR (2008) Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92-02. Eur Urol 54(4):816–823
Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, Smith MR (2009) Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol 27(1):92–99
Feng FY, Blas K, Olson K, Stenmark M, Sandler H, Hamstra DA (2013) Retrospective evaluation reveals that long-term androgen deprivation therapy improves cause-specific and overall survival in the setting of dose-escalated radiation for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 86(1):64–71
Gonzalez BD, Jim HS, Booth-Jones M, Small BJ, Sutton SK, Lin HY, Park JY, Spiess PE, Fishman MN, Jacobsen PB (2015) Course and predictors of cognitive function in patients with prostate cancer receiving androgen-deprivation therapy: a controlled comparison. J Clin Oncol 33(18):2021–2027
Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, Venkatesan V, Lawton CA, Rosenthal SA, Sandler HM, Shipley WU (2008) Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol 26:2497–2504
Jao SW, Beart RW Jr, Reiman HM, Gunderson LL, Ilstrup DM (1987) Colon and anorectal cancer after pelvic irradiation. Dis Colon Rectum 30:953–958
Jones CU, Hunt D, McGowan DG, Amin MB, Chetner MP, Bruner DW, Leibenhaut MH, Husain SM, Rotman M, Souhami L, Sandler HM, Shipley WU (2011) Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med 365(2):107–118
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458
Kapatoes JM, Olivera GH, Ruchala KJ, Smilowitz JB, Reckwerdt PJ, Mackie TR (2001) A feasible method for clinical delivery verification and dose reconstruction in tomotherapy. Med Phys 28(4):528–542
Moon K, Stukenborg GJ, Keim J, Theodorescu D (2006) Cancer incidence after localized therapy for prostate cancer. Cancer 107(5):991–998
Narang AK, Gergis C, Robertson AK, He P, Ram AN, McNutt TR, Griffith E, DeWeese TA, Honig S, Singh H, Song DY, Tran PT, DeWeese TL (2016) Very high-risk localized prostate cancer: outcomes following definitive radiation. Int J Radiat Oncol Biol Phys 94(2):254–262
National Comprehensive Cancer Network (2015) NCCN clinical practice guidelines in oncology: prostate cancer V1 2015. http://www.nccn.org/. Accessed 10 April 2015
Nead KT, Gaskin G, Chester C, Swisher-McClure S, Dudley JT, Leeper NJ, Shah NH (2016) Androgen deprivation therapy and future Alzheimer’s disease risk. J Clin Oncol 34(6):566–571
Neugut AI, Ahsan H, Robinson E, Ennis RD (1997) Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer 79(8):1600–1604
Nguyen PL, Je Y, Schutz FA, Hoffman KE, Hu JC, Parekh A, Beckman JA, Choueiri TK (2011) Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA 306(21):2359–2366
Onozawa M, Hinotsu S, Tsukamoto T, Oya M, Ogawa O, Kitamura T, Suzuki K, Naito S, Namiki M, Nishimura K, Hirao Y, Akaza H (2014) Recent trends in the initial therapy for newly diagnosed prostate cancer in Japan. Jpn J Clin Oncol 44(10):969–981
Pickles T, Phillips N (2002) The risk of second malignancy in men with prostate cancer treated with or without radiation in British Columbia, 1984–2000. Radiother Oncol 65:145–151
Pollack A, Abramowitz MC (2016) Weighing the addition of androgen suppression therapy to radiotherapy dose escalation for intermediate-risk prostate cancer. J Clin Oncol. doi:10.1200/JCO.2015.66.2320
Roach M 3rd, Hanks G, Thames H Jr, Schellhammer P, Shipley WU, Sokol GH, Sandler H (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65(4):965–974
Roach M 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, Lawton C, Valicenti R, Grignon D, Pilepich MV (2008) Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol 26(4):585–591
Schmidt-Hansen M, Hoskin P, Kirkbride P, Hasler E, Bromham N (2014) Hormone and radiotherapy versus hormone or radiotherapy alone for non-metastatic prostate cancer: a systematic review with meta-analyses. Clin Oncol (R Coll Radiol) 26(10):e21–e46
Sharma NK, Li T, Chen DY, Pollack A, Horwitz EM, Buyyounouski MK (2011) Intensity-modulated radiotherapy reduces gastrointestinal toxicity in patients treated with androgen deprivation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 80(2):437–444
Tabata N, Ohno Y, Matsui R, Sugiyama H, Ito Y, Tsukuma H, Oshima A (2008) Partial cancer prevalence in Japan up to 2020: estimates based on incidence and survival data from population-based cancer registries. Jpn J Clin Oncol 38(2):146–157
Tendulkar RD, Reddy CA, Stephans KL, Ciezki JP, Klein EA, Mahadevan A, Kupelian PA (2012) Redefining high-risk prostate cancer based on distant metastases and mortality after high-dose radiotherapy with androgen deprivation therapy. Int J Radiat Oncol Biol Phys 82(4):1397–1404
Thompson DE, Mabuchi K, Ron E, Soda M, Tokunaga M, Ochikubo S, Sugimoto S, Ikeda T, Terasaki M, Izumi S et al (1994) Cancer incidence in atomic bomb survivors. Part II: solid tumors, 1958–1987. Radiat Res 137(2 Suppl):S17–S67
Tomita N, Kodaira T, Tachibana H, Nakamura T, Tomoda T, Nakahara R, Inokuchi H, Hayashi N, Fuwa N (2009) Dynamic conformal arc radiotherapy with rectum hollow-out technique for localized prostate cancer. Radiother Oncol 90(3):346–352
Tomita N, Soga N, Ogura Y, Hayashi N, Shimizu H, Kubota T, Ito J, Hirata K, Ohshima Y, Tachibana H, Kodaira T (2012) Preliminary results of intensity-modulated radiation therapy with helical tomotherapy for prostate cancer. J Cancer Res Clin Oncol 138(11):1931–1936
Tomita N, Kodaira T, Teshima T, Ogawa K, Kumazaki Y, Yamauchi C, Toita T, Uno T, Sumi M, Onishi H, Kenjo M, Nakamura K (2014) Japanese structure survey of high-precision radiotherapy in 2012 based on institutional questionnaire about the patterns of care. Jpn J Clin Oncol 44(6):579–586
Ueshima H (2007) Explanation for the Japanese paradox: prevention of increase in coronary heart disease and reduction in stroke. J Atheroscler Thromb 14:278–286
Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, Nakamura Y, Okamura T (2008) Cardiovascular disease and risk factors in Asia: a selected review. Circulation 118:2702–2709
Viani GA, Stefano EJ, Afonso SL (2009) Higher-than-conventional radiation doses in localized prostate cancer treatment: a meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 74:1405–1418
Wong WW, Vora SA, Schild SE, Ezzell GA, Andrews PE, Ferrigni RG, Swanson SK (2009) Radiation dose escalation for localized prostate cancer: intensity-modulated radiotherapy versus permanent transperineal brachytherapy. Cancer 115(23):5596–5606
Zapatero A, Guerrero A, Maldonado X, Alvarez A, Gonzalez San Segundo C, Cabeza Rodriguez MA, Macias V, Pedro Olive A, Casas F, Boladeras A, de Vidales CM, Vazquez de la Torre ML, Villa S, Perez de la Haza A, Calvo FA (2015) High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): a randomised, controlled, phase 3 trial. Lancet Oncol 16(3):320–327
Zelefsky MJ, Levin EJ, Hunt M, Yamada Y, Shippy AM, Jackson A, Amols HI (2008a) Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 70(4):1124–1129
Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z, Hunt M, Cahlon O, Park J, Shippy A (2008b) Long-term results of conformal radiotherapy for prostate cancer: impact of dose escalation on biochemical tumor control and distant metastases-free survival outcomes. Int J Radiat Oncol Biol Phys 71(4):1028–1033
Zumsteg ZS, Zelefsky MJ (2012) Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: the standard of care? Lancet Oncol 13(6):e259–e269. doi:10.1016/S1470-2045(12)70084-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or similar ethical standards.
Informed consent
Informed consent was obtained from all patients included in the study.
Rights and permissions
About this article
Cite this article
Tomita, N., Soga, N., Ogura, Y. et al. High-dose radiotherapy with helical tomotherapy and long-term androgen deprivation therapy for prostate cancer: 5-year outcomes. J Cancer Res Clin Oncol 142, 1609–1619 (2016). https://doi.org/10.1007/s00432-016-2173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2173-9