Abstract
Purpose
The objective was to identify trends in surgery and the outcomes of squamous cell vulvar cancer in a population-based setting.
Methods
A total of 1113 patients with squamous cell vulvar cancer diagnosed between 1998 and 2013 in the catchment area of the Munich Cancer Registry (population approximately 4.6 million) were analysed. Trends in prognostic factors and treatment were examined by comparing patients diagnosed between 1998 and 2008 with those diagnosed between 2009 and 2013. Cumulative incidence was used to calculate time to local (LR) and lymph node recurrence (LNR). Survival was analysed by the Kaplan–Meier method, calculation of relative survival (RS), and a Cox model.
Results
The high median age at diagnosis of 75 years did not change significantly over time. In addition, no changes in the subsite of tumour or grading were noted. A decrease in patients undergoing complete vulvectomy from 27.7 to 17.8 % (p < 0.001) as well as an increase in the use of sentinel lymph node biopsy from 11.4 to 39.1 % (p < 0.001) was observed. However, time to LR (from 19 to 19 %) and time to LNR (from 9 to 9 %) as well as 5-year overall survival (from 55 to 55 %) and RS (from 66 to 63 %) were not significantly altered. After adjustment for prognostic factors, less radical locoregional surgery had no influence on survival.
Conclusion
Less radical locoregional surgery in vulvar cancer is increasingly implemented. Locoregional recurrence and survival have not been affected by these changes and are likely accompanied by an improvement in quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vulvar carcinoma is a rare gynaecological neoplasia with an incidence rate of 4.6/100,000 (European standard) in Germany (RKI 2014). Over the past 20 years, the incidence rate has increased in parts of Europe and North America (Akhtar-Danesh et al. 2014; Buttmann-Schweiger et al. 2015; Lai et al. 2014; National Cancer Institute 2015). This increase could be attributed to changes in documentation practices or an increase in the prevalence of risk factors [e.g. human papilloma virus (HPV)] (Baandrup et al. 2011; Buttmann-Schweiger et al. 2015). Mortality rates have been constant (National Cancer Institute 2015) or have slightly increased over time (RKI 2014). Despite the reported increase in younger age groups (Baandrup et al. 2011), the median age at diagnosis has remained approximately constant at 72 years in Germany (RKI 2014).
In terms of therapy, surgery is the treatment of choice. Local wide excision and sentinel lymph node biopsy offer benefits in morbidity compared with total radical vulvectomy and bilateral inguinal lymph node dissection (Wills and Obermair 2013). Furthermore, in small tumours without suspicion of lymph node involvement, evidence suggests that sentinel lymph node biopsy instead of inguinal lymph node dissection is less costly (McCann et al. 2015; Robison et al. 2014) and does not affect survival or recurrence rates (Slomovitz et al. 2015), but the technique improves quality of life (Gunther et al. 2014) by reducing morbidity, e.g. long-term lymphedema (Covens et al. 2015; van der Zee et al. 2008). In cases of positive sentinel lymph nodes, Oonk et al. (2010) suggested inguinofemoral lymphadenectomy regardless of the size of the sentinel lymph node. Mahner et al. (2015) demonstrated that patients with two or more positive lymph nodes benefited from adjuvant radiotherapy. In addition, Ignatov et al. (2015) reported on the benefits of adjuvant radiotherapy in patients with close or positive surgical margins. The evidence available has been summarised in guidelines (Alberta Health Services 2013; British Gynaecological Cancer Society 2014; DKG and DGGG 2008) that involve recommendations for the field of surgical management and adjuvant treatment to determine standard procedures that would allow for a high standard of care. Studies of guideline implementation and the subsequent effects on outcome parameters are rare. Therefore, the objective of this study was to identify population-based, long-term trends in the treatment and outcomes of patients with squamous cell carcinoma of the vulva over a 16-year period from 1998 to 2013.
Material and methods
Data collection
The Munich Cancer Registry (MCR) is the population-based clinical cancer registry of Upper Bavaria and part of Lower Bavaria (in southern Germany). The registry’s catchment area has increased from 2.3 million inhabitants to 3.8 million in 2002 and to 4.6 million in 2007 (Munich Cancer Registry 2015). Pathologic reports of solid tumours from all pathology laboratories in this catchment area are available. From these reports, the total number of vulvar cancer patients in the region is systematically recorded, and the main prognostic factors are ascertained. Clinicians complete standardised forms concerning patients’ ages; primary disease characteristics, such as stage, histology, and grade; and therapies. Additionally, the life status of patients with cancer diagnoses is maintained systematically through death certificates. All data and outcome measurements (e.g. death, local recurrence, and lymph node recurrence) are documented according to the rules of the International Agency for Research in Cancer (IARC).
Depending on the year of diagnosis, tumours are classified according to the staging criteria of the Féderation Internationale de Gynécologie et d’Obstétrique (FIGO) of the 6th (1998–2008) or 7th (2009–2013) edition of the TNM Classification of Malignant Tumours (Sobin and Wittekind 2002; Sobin et al. 2009). The three main changes in 2009 included the following (British Gynaecological Cancer Society 2014):
-
Combination of FIGO stages II and IB.
-
Involvement of the vagina and/or urethra changed from FIGO stage III to FIGO stage II.
-
Number and morphology of positive nodes considered for FIGO stage III.
To have the possibility to compare cohorts based on FIGO stage, we additionally classified all patients according to the new classification criteria as of 2009.
Patients
A total of 1753 patients residing in the catchment area were diagnosed with malignant vulvar tumours over the study period from 1998 to 2013 (Fig. 1).
Patients with non-invasive carcinoma, sarcoma or lymphoma, malignant melanoma or basal cell carcinoma as well as cases of non-squamous cell cancer and cases registered by death certificates only (DCO, 3.9 %) were excluded. Thus, the analyses of the epidemiological cohort of 1113 patients provided a population-based survey of invasive vulvar squamous cell carcinomas. Patients with evidence of other previous or synchronous malignant tumours were excluded from the survival analyses to eliminate overlapping tumour effects. Thus, the cohort for the survival analyses consisted of 923 patients.
Statistical analysis
The MCR organises data in an Oracle database (Oracle, Belmont, CA). All analyses were conducted using SAS software, version 9.2 (SAS Institute, Cary, NC). To assess medical progress, the cohort was divided into two periods of time (1998–2008 and 2009–2013). The cut-off point was chosen a priori and based on the changes in tumour staging classification. Student’s t test and the Chi-square test (two-sided p values) were used to examine continuous variables and frequency data, respectively. Missing values were not considered in the percentage calculations of frequency distributions.
Overall survival (OS) was estimated by the Kaplan–Meier method and was tested with the log-rank test. Relative survival (RS) was computed by the ratio of the observed survival rate to the expected survival rate. The expected survival time of age-matched individuals was calculated using life tables for the general German population using the Ederer II method (Ederer and Heise 1959). RS can be interpreted as survival from cancer after correction for other causes of death; therefore, RS was used to estimate cancer-specific survival. The 95 % confidence intervals (95 % CIs) were used to assess significance. To account for competing risks (Kalbfleisch and Prentice 1980), cumulative incidence (CI) analysis was used to calculate time to local recurrence (LR) and time to lymph node recurrence (LNR). Differences among subgroups were assessed by Gray’s Test for Equality of Cumulative Incidence Functions. To determine the influence on overall survival, the independent factors of age, FIGO stage (FIGO stage IA vs. IB, II, III, IV), grading (grade 1 vs. grade 2, 3/4), subsite (labia vs. clitoris, overlapping lesion), type of surgery (local wide excision vs. partial vulvectomy, complete vulvectomy), and lymph node surgery [sentinel lymph node biopsy (SLNB) only vs. lymph node dissection (LND), no LND] were analysed using a Cox proportional hazards model. The significance level in all analyses was set at 5 %.
Results
The crude incidence rate of invasive vulvar cancer in the area of Munich Cancer Registry has slightly increased since 1998 and was 4.3 per 100,000 on average for 1998–2013 (European age-standardised incidence rate, ASR [E] 2.6; World age-standardised incidence rate, ASR [W] 1.7).
Prognostic factors
Table 1 presents the patients and tumour characteristics for the two time periods and the total cohort. Only 20.1 % of the patients were younger than 60 years old. The high median age of 75.0 years did not change significantly. No important changes were noted in subsites of tumours or grading for patients diagnosed from 1998 to 2008 compared with patients diagnosed in 2009–2013. To have the possibility to compare cohorts based on FIGO stage, we classified all patients according to the new classification criteria as of 2009. The significant changes in FIGO stage (p = 0.012) result from an increase in FIGO I, as well as in FIGO III. Thus, overall a worse or better stage distribution cannot be stated. In the 2009–2013 cohort, 63.7 % of the patients were diagnosed with FIGO stage IA or IB with a tumour confined to the vulva without lymph node involvement.
Treatment
Table 2 presents the changes in vulvar cancer treatment. Of the patients, 94.8 % underwent surgery. The percentage of patients with adjuvant radio(chemo)therapy or primary radio(chemo)therapy did not change significantly over time (p = 0.164).
In surgery, a trend towards less radical locoregional procedures was noted. First, compared with patients diagnosed from 1998 to 2008 who underwent surgery, the use of local wide excision increased from 30.6 to 42.2 % in patients diagnosed from 2009 to 2013. At the same time, the percentage of patients who underwent complete vulvectomy decreased significantly from 27.7 to 17.8 % (p < 0.001). Despite less radical surgery, the proportion of patients with complete removal of the tumour (R0) increased from 83.2 to 88.8 % (p = 0.018).
Second, an increase in the use of sentinel lymph node biopsy from 11.4 to 39.1 % (p < 0.001) could be observed in the patients who underwent surgery. The increase in lymph node surgery in total was mostly attributable to the notable increase in sentinel lymph node biopsy alone (from 5.8 to 25.1 %). With the simultaneous increase in sentinel lymph node biopsy, the percentage of inguinal lymph node dissections decreased. However, in cases of lymph node dissection, the mean and categorised number of dissected lymph nodes as well as the mean and categorised number of sentinel lymph nodes did not change over time.
Table 3 presents the associations of FIGO stage and therapy with type of surgery and lymph node dissection or rather sentinel lymph node biopsy for patients diagnosed from 2009 to 2013. Approximately all patients in FIGO stages IA and IB underwent surgery alone, whereas the percentage of patients in FIGO stage III receiving adjuvant radio(chemo)therapy was 48.8 %. The percentage of patients undergoing complete vulvectomy increased from 4.2 % in FIGO stage IA to 37.2 % in FIGO stage III. In addition, 96.2 % of the FIGO stage IA patients diagnosed from 2009 to 2013 did not undergo lymph node dissection or had only the sentinel lymph node biopsied. Regarding FIGO stage IB, the percentage of patients who underwent neither lymph node dissection nor sentinel lymph node biopsy was considerably reduced (31.6 %), and 40.0 % of the patients exclusively underwent sentinel lymph node biopsy. In FIGO stage III, for which lymph node therapy is mandatory, 91.3 % underwent lymph node surgery. Of the remaining patients not undergoing lymph node surgery or undergoing sentinel lymph node biopsy only, five of seven received adjuvant radio(chemo)therapy.
Time to local recurrence and lymph node recurrence
The five-year cumulative incidence (Fig. 2a) of local recurrence was 19 % (95 % CI 16; 22). No significant difference (p = 0.833) was noted between patients diagnosed from 1998 to 2008 [19 % (95 % CI 15; 22)] and patients diagnosed from 2009 to 2013 [19 % (95 % CI 14; 23)]. The total five-year cumulative incidence of lymph node recurrence (Fig. 2b) was 9 % (95 % CI 8; 12). Similar to the time to local recurrence, no difference (p = 0.850) was noted between patients diagnosed from 1998 to 2008 [9 % (95 % CI 7; 12)] and patients diagnosed from 2009 to 2013 [9 % (95 % CI 6; 13)].
Survival
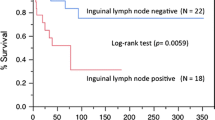
The five-year overall survival (Fig. 3a) was 55 % (95 % CI 52; 59) for the entire cohort. No significant difference was noted between the cohorts (p = 0.662) with an overall survival of 55 % (95 % CI 50; 60) for patients diagnosed from 1998 to 2008 and an overall survival of 55 % (95 % CI 48; 61) for patients diagnosed from 2009 to 2013.
The five-year relative survival (Fig. 3b) was 66 % (95 % CI 61; 70) for the entire cohort and 66 % (95 % CI 60; 71) and 63 % (95 % CI 54; 71), respectively, for the two time periods. Regarding RS, no significant difference was noted between the two cohorts.
In the Cox regression model (Table 4), age (p < 0.001), and FIGO stage (p < 0.001) exhibited significant effects on overall survival. Subsite (p = 0.663), grading (p = 0.588), and type of surgery (p = 0.391) did not significantly influence overall survival. The analysis of type of lymph node dissection showed similar hazard ratios of sentinel lymph node biopsy (reference category) and complete lymph node dissection (1.365 [0.822; 2.269]).
Discussion
From 1998 to 2013, the incidence of vulvar cancer slightly increased in the area of the Munich Cancer Registry, consistent with the trend that was observed in Germany (RKI 2014) and other countries (Akhtar-Danesh et al. 2014; Baandrup et al. 2011; Lai et al. 2014). However, Schuurman et al. (2013) and Hampl et al. (2008) reported an increase in the proportion of younger patients, which could not be confirmed in this study.
Although the patient characteristics and prognostic factors did not change over time, a change in the extent of surgery towards less radical procedures was noted with a decrease in the percentage of patients who underwent radical vulvectomy. Similar results were observed in lymph node surgery: the proportion of patients undergoing sentinel lymph node biopsy increased, and at the same time, inguinal and pelvic lymph node dissection decreased. In total, the proportion of patients with clarification of lymph node status increased. In the cohort from 2009 to 2013, 40.0 % of the patients in FIGO stage IB underwent sentinel lymph node biopsy only with no further lymph node dissection. However, among patients with FIGO stage IA, in which lymph node dissection or sentinel lymph node biopsy only lead to more morbidity and therefore should be omitted according to the guidelines, 4.8 % received lymph node dissection.
Despite these irregularities, therapy for vulvar cancer in the area of the Munich Cancer Registry is less radical compared to the older cohort and seems to increasingly meet latest guideline recommendations. Individual variations were mostly attributed to the high median age at diagnosis and were likely associated with comorbidities. The number of patients in FIGO IB with neither sentinel lymph node biopsy nor lymph node dissection can probably be explained by the poor health status of these patients. Regarding FIGO stage III, in which 8.8 % of the patients were not treated regularly with lymph node dissection, 65 % were 70 years old or older.
Despite the trend towards less radical procedures in locoregional surgery, time to locoregional recurrence and survival did not change significantly, indicating that these changes did not affect prognosis. These results were confirmed by the Cox regression analysis, which showed no significant effect on the type of surgery. Nevertheless, type of lymph node dissection reached statistical significance (p < 0.001) probably due to a worse health status in the group of patients without lymph node dissection. This trend towards more limited surgery likely led to an improvement in quality of life for a large proportion of patients (Gunther et al. 2014). The results supported the findings from the Netherlands, where no changes in survival rates were reported despite the introduction of less radical surgery, i.e. local excision and sentinel lymph node dissection, in the treatment for vulvar cancer (Schuurman et al. 2013).
Nevertheless, prognosis has remained moderate, with relative five-year survival rates of 66 %, which could be attributed to the high percentage (28.3 % in the cohort from 2009 to 2013) of patients diagnosed with lymph node recurrence or distant metastases in stage III or IV. Because positive lymph nodes or distant metastases are the most important prognostic factors (Mahner et al. 2015), efforts are necessary to diagnose vulvar cancer in earlier stages, which is a problem that likely originates in the high median age of the patients who do not believe that they need to see a gynaecologist. In a systematic review, Reade et al. (2014) concluded that the use of radiotherapy simultaneously combined with chemotherapy, i.e. radiochemotherapy, appeared to improve response compared with radiotherapy alone. In the presented cohort diagnosed between 2009 and 2013, approximately 20 % of FIGO III patients who received primary or adjuvant radiotherapy were treated with radiochemotherapy.
Notably, with a five-year relative survival rate of 66 % (1998–2013), the survival rate in the Munich area was reduced compared with the reported survival rates of 71.0 % in Germany (RKI 2014), 70.0 % in the USA (2005–2011) (National Cancer Institute 2015), and 69.9 % in England (2003–2005) (Lai et al. 2014) because malignant melanoma and basal cell carcinoma were excluded from the analysis to compare guideline-driven changes. Furthermore, there has been underreporting of basal cell carcinoma cases in the area of the Munich Cancer Registry. The Belgian Cancer Registry (Belgian Cancer Registry 2015) analysed squamous cell carcinoma cases in the Flemish region (2004–2007) and reported a five-year relative survival rate of 66.3 %, which was comparable to our results.
Although the arguments were coherent and the study cohort appeared to be representative, some limitations of this study should be noted. One limitation of cohort studies such as this study is the absence of randomisation, especially with regard to unknown confounders, such as comorbidity or health status. To adjust for patients with comorbidities often undergoing therapeutic approaches that differ from the standard (Rauh-Hain et al. 2014), it is desirable that this prognostic factor be included in further studies. In addition, missing data were a problem, especially in the older cohort. Fortunately, missing data appeared to occur randomly and therefore should not have affected the results. Nevertheless, in some cases, it is not possible to know the exact number of missing values. The high number of patients without any treatment for the lymph nodes in stage IB could for example be attributable to missing data, caused by the fact that some of the patients had a lymph node dissection but were reported without any information about it.
Furthermore, the surgical procedures were divided into local wide excision, partial vulvectomy, and total vulvectomy. Given the lack of definitions for these procedures, it was difficult to differentiate between local wide excision and partial vulvectomy in some cases, even using the information in the medical or surgery report. However, even if local wide excision and partial vulvectomy were combined into one category, there was no change in the stated decrease in the proportion of complete vulvectomy.
Conclusion
Less radical locoregional surgery in squamous cell vulvar cancer seems to have increasingly been implemented in the area of the Munich Cancer Registry. Variations are likely due to the advanced age of the patients. Survival outcome measurements were not affected by these changes; thus, prognosis remained the same, likely accompanied by improved quality of life. Further efforts should focus on the improvement of moderate prognosis.
References
Akhtar-Danesh N, Elit L, Lytwyn A (2014) Trends in incidence and survival of women with invasive vulvar cancer in the United States and Canada: a population-based study. Gynecol Oncol 134(2):314–318
Alberta Health Services (2013) Clinical practice guideline. Squamous cell carcinoma of the vulva
Baandrup L, Varbo A, Munk C, Johansen C, Frisch M, Kjaer SK (2011) In situ and invasive squamous cell carcinoma of the vulva in Denmark 1978–2007—a nationwide population-based study. Gynecol Oncol 122(1):45–49
Belgian Cancer Registry (2015) Zeldsame tumoren. http://www.kankerregister.org/Zeldzame_tumoren. 04 Nov 2015
British Gynaecological Cancer Society (2014) Guidelines for the diagnosis and management of vulval cancer. Royal College of Obstetricians & Gynaecologists
Buttmann-Schweiger N, Klug SJ, Luyten A, Holleczek B, Heitz F, du Bois A et al (2015) Incidence patterns and temporal trends of invasive nonmelanotic vulvar tumors in Germany 1999–2011. A population-based cancer registry analysis. PLoS ONE 10(5):e0128073
Covens A, Vella ET, Kennedy EB, Reade CJ, Jimenez W, Le T (2015) Sentinel lymph node biopsy in vulvar cancer: systematic review, meta-analysis and guideline recommendations. Gynecol Oncol 137(2):351–361
Deutsche Gesellschaft für Gynäkologie und Geburtshilfe (DGGG)/Deutsche Krebsgesellschaft (DKG) (2008) Arbeitsgemeinschaft Gynäkologische Onkologie. Interdisziplinäre S2 k-Leitlinie für die Diagnostik und Therapie des Vulvakarzinoms und seiner Vorstufen. Zuckschwerdt, München
Ederer F, Heise H (1959) Instructions to IBM 650 programmers in processing survival computations. Methodological note no. 10. End results evaluation section. National Cancer Institute
Gunther V, Malchow B, Schubert M, Andresen L, Jochens A, Jonat W et al (2014) Impact of radical operative treatment on the quality of life in women with vulvar cancer—a retrospective study. Eur J Surg Oncol 40(7):875–882
Hampl M, Deckers-Figiel S, Hampl JA, Rein D, Bender HG (2008) New aspects of vulvar cancer: changes in localization and age of onset. Gynecol Oncol 109(3):340–345
Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A (2015) Adjuvant radiotherapy for vulvar cancer with close or positive surgical margins. J Cancer Res Clin Oncol 142(2):489–495
Kalbfleisch J, Prentice R (1980) The statistical analysis of failure time data. Wiley, New York
Lai J, Elleray R, Nordin A, Hirschowitz L, Rous B, Gildea C et al (2014) Vulval cancer incidence, mortality and survival in England: age-related trends. BJOG 121(6):728–738 (discussion 739)
Mahner S, Jueckstock J, Hilpert F, Neuser P, Harter P, de Gregorio N et al (2015) Adjuvant therapy in lymph node-positive vulvar cancer: the AGO-CaRE-1 study. J Natl Cancer Inst 107(3):dju426
McCann GA, Cohn DE, Jewell EL, Havrilesky LJ (2015) Lymphatic mapping and sentinel lymph node dissection compared to complete lymphadenectomy in the management of early-stage vulvar cancer: a cost-utility analysis. Gynecol Oncol 136(2):300–304
Munich Cancer Registry (2015) Catchment area of MCR. http://www.tumorregister-muenchen.de/en/area.php. 04 Nov 15
National Cancer Institute. Surveillance, epidemiology, and end results program. http://seer.cancer.gov/statfacts/html/vulva. 04 Nov 15
Oonk MH, van Hemel BM, Hollema H, de Hullu JA, Ansink AC, Vergote I et al (2010) Size of sentinel-node metastasis and chances of non-sentinel-node involvement and survival in early stage vulvar cancer: results from GROINSS-V, a multicentre observational study. Lancet Oncol 11(7):646–652
Rauh-Hain JA, Clemmer J, Clark RM, Bradford LS, Growdon WB, Goodman A et al (2014) Management and outcomes for elderly women with vulvar cancer over time. BJOG 121(6):719–727 (discussion 727)
Reade CJ, Eiriksson LR, Mackay H (2014) Systemic therapy in squamous cell carcinoma of the vulva: current status and future directions. Gynecol Oncol 132(3):780–789
Robert Koch Institute (RKI) (2014) German Centre for Cancer Registry Data (ZfKD). Cancer in Germany 2009/2010. 9th edn. http://www.krebsdaten.de/Krebs/EN. 04 Nov 15
Robison K, Roque D, McCourt C, Stuckey A, DiSilvestro PA, Sung CJ et al (2014) Long-term follow-up of vulvar cancer patients evaluated with sentinel lymph node biopsy alone. Gynecol Oncol 133(3):416–420
Schuurman MS, van den Einden LC, Massuger LF, Kiemeney LA, van der Aa MA, de Hullu JA (2013) Trends in incidence and survival of Dutch women with vulvar squamous cell carcinoma. Eur J Cancer 49(18):3872–3880
Slomovitz BM, Coleman RL, Oonk MH, van der Zee A, Levenback C (2015) Update on sentinel lymph node biopsy for early-stage vulvar cancer. Gynecol Oncol 138(2):472–477
Sobin L, Wittekind C (2002) TNM classification of malignant tumours. International Union Against Cancer (UICC), 6th edn. Wiley-Liss, New York
Sobin L, Gospodarowicz M, Wittekind C (2009) TNM classification of malignant tumours. International union against cancer (UICC), 7th edn. Wiley-Liss, New York
Van der Zee AG, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH et al (2008) Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol 26(6):884–889
Wills A, Obermair A (2013) A review of complications associated with the surgical treatment of vulvar cancer. Gynecol Oncol 131(2):467–479
Acknowledgments
We thank all the hospitals, departments, and practitioners who participated in the documentation of the data. Funding body: The Munich Cancer Registry (MCR) is part of the Munich Tumour Centre (TZM) at the Institute for Medical Information Processing, Biometry, and Epidemiology (IBE) of the Ludwig-Maximilians-Universität (LMU) and the University Hospital of Munich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Welfare of animals
This article does not contain any studies with animals performed by one of the authors.
Rights and permissions
About this article
Cite this article
Rottmann, M., Beck, T., Burges, A. et al. Trends in surgery and outcomes of squamous cell vulvar cancer patients over a 16-year period (1998–2013): a population-based analysis. J Cancer Res Clin Oncol 142, 1331–1341 (2016). https://doi.org/10.1007/s00432-016-2135-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2135-2