Abstract
Purpose
Naringin is a natural dietary flavonoid compound. We aimed to evaluate the effects of naringin on intestinal tumorigenesis in the adenomatous polyposis coli multiple intestinal neoplasia (Apc Min/+) mouse model.
Methods
Apc Min/+ mice were given either naringin (150 mg/kg) or vehicle by p.o. gavage daily for 12 consecutive weeks. Mice were killed with ether, and blood samples were collected to assess the concentrations of IL-6 and PGE2. Total intestines were removed, and the number of polyps was examined. Tissue samples of intestinal polyps were subjected to the assays of histopathology, immunohistochemical analysis and Western blotting analysis.
Results
Apc Min/+ mice fed with naringin developed less and smaller polyps in total intestines. Naringin prevented intestinal tumorigenesis without adverse effects. Histopathologic analysis revealed the reduction of dysplastic cells and dysplasia in the adenomatous polyps. The treatments’ effects might arise from its anti-proliferation, induction of apoptosis and modulation of GSK-3β and APC/β-catenin signaling pathways. Naringin also exerted its effects on tumorigenesis through anti-chronic inflammation.
Conclusion
Naringin prevented intestinal tumorigenesis likely through a collection of activities including anti-proliferation, induction of apoptosis, modulation of GSK-3β and APC/β-catenin pathways and anti-inflammation. Naringin is a potential chemopreventive agent for reducing the risk of colonic cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a major cause of cancer-related deaths in many countries. Adenomatous polyps, or adenomas, have been considered as the major precursors for CRC, through a gradual series of histological changes, called ‘adenoma–carcinoma’ sequence. Mutation of a tumor suppressor gene, adenomatous polyposis coli (APC), is likely to be critical for initiating the ‘adenoma–carcinoma’ sequence (Bosman and Yan 2014; Liu et al. 2013). Clinical studies showed that half of population will develop at least one adenomatous polyp in their life, with 2–3 % of them going to develop CRC (Leoz et al. 2015). Fortunately, the progression of ‘adenoma–carcinoma’ sequence might last for more than 10 years (Arvelo et al. 2015). It has therefore provided an opportunity for chemoprevention of CRC through blocking the sequence of ‘adenoma–carcinoma.’
Epidemiologic studies showed that the incidence of CRC is inversely correlated with the consumption of fruits and vegetables for containing considerable amounts of biologically active compounds (Annema et al. 2011). Dietary chemoprevention has thus received attention for reducing the risk of CRC. Flavonoids include a large class of natural polyphenolic compounds and are commonly found in fresh vegetables and fruits. Among naturally occurring flavonoids, naringin (4′, 5, 7-trihydroxy flavanone-7-rhamnoglucoside), a glycone form of naringenin, can be found in most citrus fruits (Cordenonsi et al. 2015). Naringin has been reported as a multifunctional agent. It mitigates cardiac hypertrophy by reducing oxidative stress and inactivating c-Jun nuclear kinase (JNK-1) protein in type I diabetes (Adeniyi et al. 2015). Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats by modulating the activities of KIM-1, caspase-3, TGF-β and TNF-α (Adil et al. 2015). Naringin ameliorates cognitive deficits via oxidative stress, proinflammatory factors and the PPARγ signaling pathway in a type 2 diabetic rat model (Qi et al. 2015). Naringin protects neural cells on 3-nitropropionic acid-induced mitochondrial dysfunction through modulation of Nrf2 signaling pathway (Kulasekaran and Ganapasam 2015). Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells (Raha et al. 2015). Naringin prevents the cisplatin-induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing the ROS-mediated NF-κB and P53 signaling pathways (Chtourou et al. 2015). Recently, naringin has been reported to have a function of anti-tumorigenesis. Naringin inhibited the growth of hepatocellular carcinoma HepG2 cells through the induction of apoptosis (Banjerdpongchai et al. 2015). Naringin induces apoptosis in human DU145 prostate cancer cell line (Lewinska et al. 2015). Naringin inhibits potential growth of human triple-negative breast cancer cells by targeting β-catenin signaling pathway (Li et al. 2013). These biological activities were all evaluated by in vitro assays. Herein, we aimed to evaluate the effects of naringin on tumorigenesis in the Apc Min/+ mouse model, which represent the phenotypes of familial adenomatous polyposis (FAP) in humans (Merritt et al. 1997). This model is advantageous for evaluating the chemopreventive agents against the ‘adenoma–carcinoma’ sequence in intestines. Our results showed that Apc Min/+ mice fed with naringin developed less and smaller intestinal polyps without adverse effects. Naringin prevented intestinal tumorigenesis through a collection of biological activities including anti-proliferation, induction of apoptosis and anti-inflammation. These biological activities of naringin characterized the basic requirements as a chemopreventive agent to reduce the risk of cancers.
Materials and methods
Ethics statement
The research protocol was approved by the Animal Care and Use Committee at Capital Medical University. The permit number is AEEI-2014-101. We declared that the following experiment complied with the principles of laboratory animal care issued by NIH.
Naringin
Naringin (purity ≥98 %) was purchased from Sigma Chemical Company and was solved in 5 % sodium carboxymethyl cellulose (CMC-Na) (Sigma Chemical Co).
Mice model and drug administration
Apc min/+ male mice (5 weeks old) were purchased from Model Animal Research Center of Nanjing University. Mice were crossed with wild-type C57BL/6 female mice to generate Apc Min/+ mice (Liu et al. 2013). A total of 18 mice (both male and female, 4 weeks old) were randomly divided into two groups. They were fed with standard mouse pellet diet, which contains 52 % carbohydrate, 12 % fat, 23 % protein, 4 % fiber, 6 % ash and 3 % moisture (Meidenbauer et al. 2014). After 1 week of acclimation, Apc Min/+ mice (6 weeks old) were administrated either vehicle (CMC-Na 5 %, v/v) or naringin (150 mg/kg) by p.o. gavage daily (0.2 ml/10 g body weight). Administrations were performed for 12 consecutive weeks. Mice were weighed weekly and checked daily for any health problem. At age of 18 weeks, mice were anesthetized with ether and blood samples were collected by exsanguination from inferior vein.
Intestinal polyps score
Total intestines were removed from each mouse and were sliced longitudinally and rinsed with saline. Total number and size of intestinal polyps were examined under a dissecting microscope. Intestinal polyps were categorized by size into 1–2 mm, 2–3 mm and >3 mm. After number counting, parts of small and colonic intestines were frozen in liquid nitrogen for Western blotting assay. Other parts of intestines were fixed in 10 % phosphate-buffered formalin for histopathology/immunohistochemical (IHC) analysis.
Histopathology and immunohistochemical analysis (IHC)
The paraffin-embedded intestinal polyps were cut into sections with into 5-μm-thick sections. Sections were stained with hematoxylin and eosin (H&E) for histopathology. IHC analysis was performed as previously described (Huang et al. 2015). Sections were deparaffinizated, and endogenous peroxidase was blocked by exposure to 3 % hydrogen peroxide in methanol. Primary antibodies included cyclin D1 (2922), β-catenin (9582), p-NFκB (4810, Cell Signaling Technology), Cox-2 (sc-23984, Santa Cruz), GSK3β (ab32391) and PCNA (ab29, Abcam). Second antibodies were anti-mouse IgG and anti-rabbit IgG (Santa Cruz). Positive staining with PCNA, cyclin D1, GSK3β and β-catenin was defined as brown staining in nuclei of adenomatous cells. Positive staining with COX-2 and p-NFκB was identified based on brown intensity of membrane, cytoplasm and nuclei of adenomatous cells (Liu et al. 2013; Huang et al. 2015). The stained adenomatous cells were compared with total number cells at five selected fields from each polyp.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining assay
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining assay was performed to identify apoptotic cells in the adenomatous polyps. TUNEL kit was purchased from Roche Diagnostics (Germany). Sections were prepared as described in the immunohistochemical analysis (IHC). The staining assay was performed according to the manufacturer’s instruction. TUNEL-positive cells were recognized by their dark brown nuclear staining. The proportion of apoptotic cells in six mice was scored in randomly chosen fields under a microscope.
Western blotting assay
The intestinal polyps were excised and pooled together based on groups. Three–five polyps were incubated with 80 μl RIPA lysis buffer at 4 °C for 30 min to obtain lysates of adenomatous cells as previously described (Liu et al. 2013). The supernatants were diluted, and the concentration of protein was determined by using bicinchoninic acid kit (Pierce). Lysates (30 μg of protein per lane) were fractionated by SDS-PAGE. Protein was electro-transferred onto poly (vinylidene fluoride) (PVDF) membranes and was then determined by using the primary antibodies. The primary antibodies included β-catenin (9582), p-β-catenin Ser37 (9561), cleaved caspase-3 (Asp175) (9664), Bcl-xL (2764), cyclin D1 (2922), p-NFκB (4810, Cell Signaling Technology), p-GSK3β Ser9 (ab75814), TNF-α (ab6671), PCNA (ab29), β-actin (ab6276, Abcam), c-Myc (sc-42) and Bax (sc-493, Santa Cruz). PVDF membranes were washed in 0.05 % Tween-20/Tris-buffered saline and incubated with horseradish peroxidase-conjugated secondary antibody. The bound antibodies were visualized and quantified under FluorChem FC3 image analyzer (molecular devices). Densitometric analyses of bands were adjusted with β-actin functioning as a loading control.
ELISA
Plasma IL-6 was detected by using sandwich ELISA kits according to manufacturer’s protocol (EZMIL6, Meck Millipore). Plasma PGE2 was quantified by an enzyme immunoassay (EIA) kit (ADI-900-001, Enzo Life Science). Briefly, serum and PGE2 conjugates were added to a 96-well plate pre-coated with goat anti-mouse IgG for 2 h. Plate was washed with PBS to remove unbound antibody–enzyme reagent. The substrate solution was added, and the intensity of color developed was read at a wavelength of 405 nm.
Statistical analysis
Statistical analysis was done with SPSS/Win13.0 software (SPSS, Inc., Chicago, Illinois). Data were described as mean ± SD. Comparisons between control and treatment group were conducted by two-tailed Student’s t test. A p value less than 0.05 was considered statistically significant.
Results
Naringin prevented intestinal adenomatous polyps without causing health problems to mice
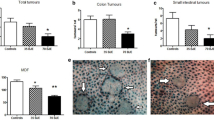
In control group, Apc Min/+ mice at age of 18 weeks developed 31.2 and 8.3 polyps, respectively, on average in small intestine and colon (Fig. 1a). Mice fed with naringin developed less and smaller intestinal polyps (Fig. 1b). Total intestinal polyps were reduced by 52.6 % (p < 0.01 vs. vehicle control) in small intestines and 62.5 % (p < 0.01 vs. vehicle control) in colons (Fig. 1c). Further analysis showed differential naringin effects depending upon polyp sizes and segments. Naringin reduced larger polyps (>2 mm) more significantly than smaller polyps. Naringin reduced the number of polyps between 1 and 2 mm by 58.8 % (p < 0.01 vs. vehicle control) and the number of polyps between 2 and 3 mm by 66.5 % (p < 0.01 vs. vehicle control), in small intestine (Fig. 1d). Naringin reduced the colonic polyps by 64.1 % (p < 0.01 vs. vehicle control) in the number of polyps between 1 and 2 mm, 72.4 % (p < 0.01 vs. vehicle control) in the number of polyps between 2 and 3 mm and 84.1 % (p < 0.01 vs. vehicle control) in the number of polyps >3 mm (Fig. 1e).
Naringin prevented the intestinal tumorigenesis in Apc Min/+ mice. At age of 18 weeks, mice were killed and intestinal polyps and size were counted. a Vehicle control intestines. b Mice fed with naringin developed less and smaller intestinal polyps. c Naringin prevented intestinal polyps. d Naringin reduced sizes in different sections of intestines. *p < 0.05; **p < 0.01 versus vehicle control. Bars represent mean ± SD of 9 mice
During the process of the experiment, mice did not show considerable change in food consumption and body weight. There was no any significant difference in liver function, blood element counts and other biological signs between naringin-fed and vehicle control mice (data not shown).
Naringin blocked the progression of intestinal tumorigenesis
In Apc Min/+ mice, all intestinal polyps were histologically identified as adenocarcinoma, which demonstrates an early stage of colonic tumorigenesis. With size growth, these intestinal polyps might gradually progress from benign adenoma to adenocarcinoma with high-grade dysplasia and develop intramucosal carcinoma. Small intestinal polyps showed the crowded pencil-shaped hyperchromatic nuclei with preserved polarity and diminished mucin (Fig. 2a). Larger polyps were seen in colons, showing intramucosal adenocarcinoma with focal high-grade dysplasia and nuclear pleomorphism, lack of nuclear polarity, frequent mitoses and architectural distortion (Fig. 2b). Naringin blocked the progression of intestinal polyps. The number of dysplastic cells obviously decreased, and the degree of high-grade dysplasia was significantly reduced in small intestinal (Fig. 2c) and colonic polyps (Fig. 2d). Normal intestinal villi were seen in most small and colonic intestines in the naringin-fed mice.
H&E-stained intestinal sections from Apc Min/+ mice. a Control mice with crater-shaped adenomatous polyps in small intestine (×100). a (Inset) adenomatous epithelium shows the enlarged, hyperchromatic, elongated and crowded dysplastic nuclei (×400). b Advanced adenomatous polyps with focal high-grade dysplasia in colonic section of control mice (×100). b (Inset) crypt architecture appears more complex, and the nuclei are more pleomorphic with frequent mitoses. There is a lack of nuclear polarization in glands (×400). c Small intestine in the naringin-fed mice (×100). c (Inset) crypt architecture is essentially normal with unremarkable epithelial nuclei (×400). d Colon from naringin-fed mice (×100). d (Inset) structure and nuclei of epithelium are basically normal (×400)
Naringin inhibited the proliferation and induced apoptosis in the intestinal polyps
Excessive proliferation mostly contributed to the formation and progression of adenocarcinoma polyps. We examined the expression levels of β-catenin targets, cyclin D1, PCNA and c-Myc, which are considered as the major markers of tumorigenesis. Naringin selectively inhibited the proliferation of intestinal adenocarcinomas. Naringin did not significantly affect the crypt architecture and the proliferation of epithelia on the normal intestines (data not shown). IHC analysis showed a reduction of cyclin D1 staining cells by 61.2 % (p < 0.01 vs. vehicle control) and 72.9 % (p < 0.01 vs. vehicle control) (Fig. 3a) and a reduction of PCNA staining cells by 57.8 % (p < 0.01 vs. vehicle control) and 70.4 % (p < 0.01 vs. vehicle control) (Fig. 3b), respectively, in small intestinal polyps and colonic polyps. Further, Western blotting showed the decrease in cyclin D1 by 49.6 % (p < 0.05 vs. vehicle control) and 42.2 % (p < 0.05 vs. vehicle control) (Fig. 3c), PCNA by 76.6 % (p < 0.01 vs. vehicle control) and 59.3 % (p < 0.05 vs. vehicle control) and c-Myc by 39.1 % (p < 0.05 vs. vehicle control) and 40.7 % (p < 0.05 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps.
Naringin inhibited expression of cyclin D1 and PCNA in epithelia of intestinal polyps. IHC showed the expressions of cyclin D1 (a) and PCNA-positive cells (b) in colonic polyps (×400). The stained adenomatous cells were compared with total number cells at five selected fields from each polyp. Results were denoted as a percentage of stained cells in each figure. c Western blotting showed the expressions of cyclin D1, PCNA and c-Myc in small intestinal polyps and colonic polyps. Densitometric bands were adjusted with β-actin and compared with vehicle control. Bars represent mean ± SD of triplicate experiments. *p < 0.05, **p < 0.01 versus vehicle control
Intestinal adenocarcinoma is mostly characterized by insufficient apoptosis. Naringin selectively induced adenocarcinoma cells to apoptosis. TUNEL assay showed the increase in apoptotic cells by 49.4 % (p < 0.05 vs. vehicle control) in small intestinal polyps (Fig. 4a) and 154.1 % (p < 0.01 vs. vehicle control) in colonic polyps (Fig. 4b). Western blotting showed the modulation of proapoptotic Bax, anti-apoptotic Bcl-xL and apoptotic executor c-caspase-3 in naringin-treated intestinal polyps (Fig. 4c). Naringin treatment resulted in an increase in c-caspase-3 by 193.5 % (p < 0.01 vs. vehicle control) and 246.3 % (p < 0.01 vs. vehicle control) and an increase in Bax by 89.6 % (p < 0.01 vs. vehicle control) and 149.5 % (p < 0.01 vs. vehicle control), respectively, in small intestinal and colonic polyps. Naringin reduced the level of Bcl-2 by 79.3 % (p < 0.01 vs. vehicle control) and 60.3 % (p < 0.05 vs. vehicle control) and Bcl-xL by 87.8 % (p < 0.01 vs. vehicle control) and 89.3 % (p < 0.01 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps.
Naringin induced apoptosis of intestinal polyps. Naringin increased the TUNEL-positive cells in the small (a) and colonic intestinal polyps (b) (×200). TUNEL-positive cells with dark brown nuclear staining were compared with vehicle control. c Western blotting showed the expression of c-caspase-3, Bax, Bcl-2 and Bcl-xL in small intestinal polyps and colonic polyps. Densitometric bands were adjusted with β-actin and compared with vehicle control. Bars represent mean ± SD of triplicate experiments. *p < 0.05, **p < 0.01 versus vehicle control
Naringin modulated GSK-3β activity and inhibited β-catenin expression in adenomatous cells
In colonic cancer cells, loss of APC function leads to dysfunction of GSK-3β and results in an accumulation of β-catenin. Naringin modulated the activity of GSK-3β and inhibited β-catenin expression in intestinal adenomatous cells. IHC assay showed an increase in active GSK-3β staining cells by 63.4 % (p < 0.05 vs. vehicle control) and 125.6 % (p < 0.01 vs. vehicle control), respectively, in small intestine and colon (Fig. 5a). Naringin decreased the expression of β-catenin staining cells by 68.1 % (p < 0.01 vs. vehicle control) and 66.9 % (p < 0.01 vs. vehicle control), respectively, in small intestine and colon (Fig. 5b). Further analysis by Western blotting assay showed an increase in active GSK-3β by 46.8 % (p < 0.05 vs. vehicle control) and 42.7 % (p < 0.05 vs. vehicle control), while phosphorylated GSK-3β was decreased by 80.2 % (p < 0.01 vs. vehicle control) and 85.8 % (p < 0.01 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps (Fig. 5c). The expression of β-catenin significantly reduced by 68.3 % (p < 0.01 vs. vehicle control) and 79.2 % (p < 0.01 vs. vehicle control) and phosphorylated β-catenin by 77.8 % (p < 0.01 vs. vehicle control) and 80.4 % (p < 0.01 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps (Fig. 5c).
Naringin modulated the expressions of GSK-3β and β-catenin in intestinal adenomatous cells. a IHC showed the expression of GSK-3β and p-GSK-3β in small and colonic intestinal polyps (×400). b IHC showed the expression of β-catenin in small and colonic intestinal polyps (×400). c Western blotting analyzed the expression of GSK3β and p-β-catenin (Ser 37) in small and colonic intestinal polyps. Densitometric bands were adjusted with β-actin and compared with vehicle control. *p < 0.05, **p < 0.01 versus vehicle control. Bars represent mean ± SD of triplicate experiments
Naringin reduced inflammatory cytokines in intestinal polyps and plasma
Chronic inflammation stimulates intestinal tumorigenesis by altering the microenvironment in ways that stimulate stroma and epithelial cells. High levels of Cox-2, NF-κB, TNF-α, PGE2 and IL-6 are considered to be the most valuable malignant indicators in adenomatous cells in Apc Min/+ mice. Naringin possessed the activity of suppressing chronic inflammatory cytokines in both intestinal adenomatous polyps and plasma. IHC analysis showed that naringin reduced the level of Cox-2 by 56.6 % (p < 0.01 vs. vehicle control) and 67.8 % (Fig. 6a, p < 0.01 vs. vehicle control) and p-NF-κB by 83.5 % (p < 0.01 vs. vehicle control) and 86.7 % (Fig. 6b, p < 0.01 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps. Further, Western blotting showed the inhibition Cox-2 by 40.4 % (p < 0.05 vs. vehicle control) and 50.8 % (p < 0.05 vs. vehicle control), p-NF-κB by 90.2 % (p < 0.01 vs. vehicle control) and 78.6 % (p < 0.01 vs. vehicle control) and TNF-α by 75.6 % (p < 0.01 vs. vehicle control) and 86.3 % (p < 0.01 vs. vehicle control), respectively, in small intestinal polyps and colonic polyps (Fig. 6c). The levels of PGE2 and IL-6 in serum were assayed by ELISA. High levels of PGE2 and IL-6 were determined in Apc Min/+ mice. Naringin reduced the concentration of PGE2 from 4898.1 ± 222.1 pg/mL to 2927.8 pg/mL ± 270.3 pg/mL (Fig. 6d, p < 0.05 vs. vehicle control). The level of serum IL-6 reduced from 19.5 ± 1.4 pg/mL to 11.7 ± 1.2 pg/mL (Fig. 6e, p < 0.05 vs. vehicle control).
Naringin suppressed inflammatory cytokines in intestinal polyps. a IHC showed the expression of Cox-2 in the small and colonic intestinal polyps (×400). b IHC showed the expression of p-NF-κB in the small and colonic intestinal polyps (×400). c Western blotting showed the inhibition of Cox-2, p-NF-κB and TNF-α in the small and colonic intestinal polyps. Densitometric bands were adjusted with β-actin and compared with vehicle control. ELISA analyzed the level of IL-6 (d) and PGE2 (e) in serum. Bars represent mean ± SD of triplicate experiments in six mice. *p < 0.05, **p < 0.01 versus vehicle control
Discussion
Apc min/+ mouse model represents the phenotypes of familial adenomatous polyposis (FAP) in humans with spontaneous intestinal tumorigenesis. In this model, tumors in small intestine are composed of dysplastic crypts surrounded by hyperplastic villi and crypts. Colonic tumors are peduncular, forming a spherical mass of dysplastic cells supported by a stromal stalk and displaying a characteristic ‘rose’ shape in larger polyps (McAlpine et al. 2006). This model is advantageous for evaluating the chemopreventive agents against the ‘adenoma–carcinoma’ sequence in intestines. In this study, Apc Min/+ mice were fed with naringin from 6 weeks of age and administrated for 12 consecutive weeks. At 18 weeks of age, mice with naringin developed less and smaller polyps with lower degree of cytological dysplasia in intestines. Molecular analysis showed that naringin selectively inhibited proliferation and induced intestinal adenoma to apoptosis. The effects of treatments were associated with naringin’s activities of blocking the progression of tumorigenesis and reducing the proliferation of adenomatous polyps, leading to a chemoprevention in colonic cancer. Further, naringin possessed the activity of anti-proinflammatory cytokines, such as Cox-2, NF-κB, TNF-α, PGE2 and IL-6 in the adenomatous polyps and plasma. Naringin inhibited intestinal tumorigenesis without adverse effects to mice. These properties of naturally food component characterized the basic requirements for a chemopreventive agent. We therefore suggested that naringin might develop as a potential agent for reducing the risk of colonic cancers.
Chemopreventive effect of naringin was accompanied by its anti-proliferative and proapoptotic mechanisms. It has been widely considered that excessive cell proliferation and insufficient apoptosis are associated with tumorigenesis (Rigby et al. 2007; Wang et al. 2014). In Apc min/+ mouse model, different polyp sizes represent different stages of tumorigenesis. An early stage of intestinal tumorigenesis is the benign adenoma that could develop to adenoma with high-grade dysplasia (Cheung et al. 2014; Kwong and Dove 2009; Shih et al. 2001). Intramucosal carcinomas with ulceration frequently occurred in the larger polyps >3 mm (Liu et al. 2013; Rajamanickam et al. 2010). We analyzed the inhibitory effects of naringin on the intestinal polyps of different sizes. Naringin was found to reduce larger polyps (>2 mm) more significantly than smaller-sized polyps. Pathology analysis showed the reduction of dysplastic cells and dysplasia degree in intestinal polyps. These effects of naringin might be associated with its selective anti-proliferation and induction of apoptosis in adenomatous cells, because naringin did not significantly inhibit the proliferation of normal cells or induce adjacent normal tissue to apoptosis (data not shown).
In normal cells, GSK-3β phosphorylates β-catenin and therefore induces its ubiquitination and degradation by proteasome system (Schneikert et al. 2013). GSK-3β destabilizes β-catenin by phosphorylating it at Ser33, 37 and Thr41 (Boo et al. 2009). However, in intestinal adenomatous cells, because of Apc mutation, these phosphorylation sites are mutated and therefore fail to phosphorylate β-catenin, thereby leading to its accumulation in the cytoplasm. It then translocates to the nucleus and engages the Tcf/Lef transcription factor complex to activate the transcription of a large number of target genes, such as cyclinD1, PCNA, c-Myc and CRDBP (van Noort et al. 2002; Fujisawa et al. 2008; Tarapore et al. 2012). Intestinal adenomatous cells in Apc min/+ mice have low level of active non-phosphorylated GSK-3β and high level of phosphorylated GSK-3β (Kumar et al. 2012; Lee et al. 2012). Our results showed an increase in phosphorylated β-catenin at the site of Ser37. Because naringin increased the active non-phosphorylated GSK-3β and decreased its non-function phosphorylated form, we accordingly suggested that naringin might inhibit intestinal tumorigenesis through modulation of GSK-3β to block the accumulation of β-catenin in the nuclei of intestinal adenomatous cells.
Chronic inflammation contributes to amplifying and sustaining ongoing tumorigenesis in Apc min/+ mice. Increased inflammatory stress stimulates the growth of adenomatous cells (Pierre et al. 2015; Jamieson et al. 2012). Important inflammatory stimuli include cytokines COX-2, NF-κB, PGEs, IL-6, TNF-α, lipoxygenase (LOX) and lipopolysaccharide (LPS) (Liu et al. 2013). In the progression of tumorigenesis, NF-κB-COX-2 interaction might play a pivotal role in the stimulation of intestinal adenomatous polyps (Diab et al. 2015). NF-κB acts as ‘first responder’ to various cellular stresses, such as free radicals, proinflammatory cytokines and bacterial components or LPS, and thereafter intercellular communication takes place leading to tumorigenesis. COX-2 is an immediate-early growth response gene product (Greene et al. 2011). TNF-α induces COX-2 and cyclin D1 in adenomatous cells in which these molecules have the NF-κB binding site in their promoters. The activated NF-κB further induces COX-2 expression in response to cellular stresses (Liu et al. 2013). Accumulation of these cellular stresses further stimulates the APC/β-catenin pathway, thereby leading to the expansion of intestinal polyps.
Mountains of studies showed that high level of PGE2, IL-6, IL-1β and TNF-α is associated with the expansion of intestinal polyps size (Khare et al. 2015). PGE2 is a potential stimulator to accelerate the growth of adenomatous cells through activation of prostaglandin receptor EP2-mediated cellular events (O’Callaghan et al. 2008). In Apc Min/+ mice, high level of IL-6, TNF-α and PGE2 was found to respond with rapid increase in intestinal polyps at earlier age (O’Callaghan et al. 2008; Khare et al. 2015). Further increase in these cytokines with age was found to associate with increased polyp size (O’Callaghan et al. 2008; Khare et al. 2015). These proinflammatory cytokines were also found to provoke the phosphorylated GSK3β and activate nuclear β-catenin, thereby stimulating the expansion of intestinal polyps (Klampfer 2011; Kumar et al. 2012). Naringin possessed the activity of reducing chronic inflammation factors IL-6 and PGE2 both in the adenomatous polyps and in the plasma. We therefore suggested that the naringin’s effect was also associated with its biological activity of anti-chronic inflammation.
Conclusion
Naringin possessed the activity of chemoprevention malignant progression of intestinal adenomatous polyps in Apc Min/+ mice. The underlying mechanisms of chemopreventive effects were associated with its multiple biological activities including anti-proliferation, induction of apoptosis and anti-inflammation. Naringin feeding did not produce considerable adverse effects to mice. Naringin is a potential chemopreventive agent for reducing the risk of colonic cancers.
References
Adeniyi AO, Olubolade AO, Owira PM (2015) Naringin mitigates cardiac hypertrophy by reducing oxidative stress and inactivating c-Jun Nuclear Kinase (JNK-1) protein in type I diabetes. J Cardiovasc Pharmacol. doi:10.1097/FJC.0000000000000325
Adil M, Kandhare AD, Visnagri A, Bodhankar SL (2015) Naringin ameliorates sodium arsenite-induced renal and hepatic toxicity in rats: decisive role of KIM-1, Caspase-3, TGF-β, and TNF-α. Ren Fail 37:1396–1407
Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L (2011) Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case–control study in Western Australia. J Am Diet Assoc 111:1479–1490
Arvelo F, Sojo F, Cotte C (2015) Biology of colorectal cancer. Ecancermedicalscience 9:520
Banjerdpongchai R, Wudtiwai B, Khaw-On P, Rachakhom W, Duangnil N, Kongtawelert P (2015) Hesperidin from Citrus seed induces human hepatocellular carcinoma HepG2 cell apoptosis via both mitochondrial and death receptor pathways. Tumour Biol. doi:10.1007/s13277-015-3774-7
Boo JH, Song H, Kim JE, Kang DE, Mook-Jung I (2009) Accumulation of phosphorylated β-catenin enhances ROS-induced cell death in presenilin-deficient cells. PLoS ONE 4:e4172
Bosman F, Yan P (2014) Molecular pathology of colorectal cancer. Pol J Pathol 65:257–266
Cheung KL, Lee JH, Khor TO, Wu TY, Li GX, Chan J, Yang CS, Kong AN (2014) Nrf2 knockout enhances intestinal tumorigenesis in Apc(min/+) mice due to attenuation of anti-oxidative stress pathway while potentiates inflammation. Mol Carcinog 53:77–84
Chtourou Y, Aouey B, Kebieche M, Fetoui H (2015) Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem Biol Interact 239:76–86
Cordenonsi LM, Bromberger NG, Raffin RP, Scherman EE (2015) Simultaneous separation and sensitive detection of naringin and naringenin in nanoparticles by chromatographic method indicating stability and photodegradation kinetics. Biomed Chromatogr. doi:10.1002/bmc.3531
Diab S, Fidanzi C, Léger DY, Ghezali L, Millot M, Martin F, Azar R, Esseily F, Saab A, Sol V et al (2015) Berberis libanotica extract targets NF-κB/COX-2, PI3K/Akt and mitochondrial/caspase signalling to induce human erythroleukemia cell apoptosis. Int J Oncol 47:220–230
Fujisawa T, Sugiyama M, Tomimoto A, Wada K, Endo H, Takahashi H, Yoneda K, Yoneda M, Inamori M, Saito S (2008) Inhibition of peroxisome proliferator-activated receptor gamma promotes tumorigenesis through activation of the beta-catenin/T cell factor (TCF) pathway in the mouse intestine. J Pharmacol Sci 108:535–544
Greene ER, Huang S, Serhan CN, Panigrahy D (2011) Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat 96:27–36
Huang J, Zhang D, Xie F, Lin D (2015) The potential role of COX-2 in cancer stem cell-mediated canine mammary tumor initiation: an immunohistochemical study. J Vet Sci 16:225–231
Jamieson T, Clarke M, Steele CW, Samuel MS, Neumann J, Jung A, Huels D, Olson MF, Das S, Nibbs RJ et al (2012) Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. J Clin Invest 122:3127–3144
Khare V, Dammann K, Asboth M, Krnjic A, Jambrich M, Gasche C (2015) Overexpression of PAK1 promotes cell survival in inflammatory bowel diseases and colitis-associated cancer. Inflamm Bowel Dis 21:287–296
Klampfer L (2011) Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets 11:451–464
Kulasekaran G, Ganapasam S (2015) Neuroprotective efficacy of naringin on 3-nitropropionic acid-induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol Cell Biochem 409:199–211
Kumar A, Pandurangan AK, Lu F, Fyrst H, Zhang M, Byun HS, Bittman R, Saba JD (2012) Chemopreventive sphingadienes downregulate Wnt signaling via a PP2A/Akt/GSK3β pathway in colon cancer. Carcinogenesis 33:1726–1735
Kwong LN, Dove WF (2009) APC and its modifiers in colon cancer. Adv Exp Med Biol 656:85–106
Lee J, Kim JC, Lee SE, Quinley C, Kim H, Herdman S, Corr M, Raz E (2012) Signal transducer and activator of transcription 3 (STAT3) protein suppresses adenoma-to-carcinoma transition in Apcmin/+ mice via regulation of Snail-1 (SNAI) protein stability. J Biol Chem 287:18182–18189
Leoz ML, Carballal S, Moreira L, Ocaña T, Balaguer F (2015) The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl Clin Genet 8:95–107
Lewinska A, Siwak J, Rzeszutek I, Wnuk M (2015) Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol In Vitro 29:417–425
Li H, Yang B, Huang J, Xiang T, Yin X, Wan J, Luo F, Zhang L, Li H, Ren G (2013) Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol Lett 220:219–228
Liu HP, Gao ZH, Cui SX, Wang Y, Li BY, Lou HX, Qu XJ (2013) Chemoprevention of intestinal adenomatous polyposis by acetyl-11-keto-beta-boswellic acid in APC(Min/+) mice. Int J Cancer 132:2667–2681
McAlpine CA, Barak Y, Matise I, Cormier RT (2006) Intestinal-specific PPARgamma deficiency enhances tumorigenesis in ApcMin/+ mice. Int J Cancer 119:2339–2346
Meidenbauer JJ, Ta N, Seyfried TN (2014) Influence of a ketogenic diet, fish-oil, and calorie restriction on plasma metabolites and lipids in C57BL/6J mice. Nutr Metab (Lond) 11:23
Merritt AJ, Gould KA, Dove WF (1997) Polyclonal structure of intestinal adenomas in ApcMin/+ mice with concomitant loss of Apc+ from all tumor lineages. Proc Natl Acad Sci USA 94:13927–13931
O’Callaghan G, Kelly J, Shanahan F, Houston A (2008) Prostaglandin E2 stimulates Fas ligand expression via the EP1 receptor in colon cancer cells. Br J Cancer 99:502–512
Pierre CC, Longo J, Mavor M, Milosavljevic SB, Chaudhary R, Gilbreath E, Yates C, Daniel JM (2015) Kaiso overexpression promotes intestinal inflammation and potentiates intestinal tumorigenesis in ApcMin/+ mice. Biochim Biophys Acta 1852:1846–1855
Qi Z, Xu Y, Liang Z, Li S, Wang J, Wei Y, Dong B (2015) Naringin ameliorates cognitive deficits via oxidative stress, proinflammatory factors and the PPARγ signaling pathway in a type 2 diabetic rat model. Mol Med Rep. doi:10.3892/mmr.2015.4232
Raha S, Yumnam S, Hong GE, Lee HJ, Saralamma VV, Park HS, Heo JD, Lee SJ, Kim EH, Kim JA, Kim GS (2015) Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. Int J Oncol 47:1061–1069
Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R (2010) Chemoprevention of intestinal tumorigenesis in APCmin/+ mice by silibinin. Cancer Res 70:2368–2378
Rigby RJ, Simmons JG, Greenhalgh CJ, Alexander WS, Lund PK (2007) Suppressor of cytokine signaling 3 (SOCS3) limits damage-induced crypt hyper-proliferation and inflammation-associated tumorigenesis in the colon. Oncogene 26:4833–4841
Schneikert J, Vijaya Chandra SH, Ruppert JG, Ray S, Wenzel EM, Behrens J (2013) Functional comparison of human adenomatous polyposis coli (APC) and APC-like in targeting beta-catenin for degradation. PLoS ONE 8:e68072
Shih IM, Wang TL, Traverso G, Romans K, Hamilton SR, Ben-Sasson S, Kinzler KW, Vogelstein B (2001) Top-down morphogenesis of colorectal tumors. Proc Natl Acad Sci USA 98:2640–2645
Tarapore RS, Siddiqui IA, Mukhtar H (2012) Modulation of Wnt/β-catenin signaling pathway by bioactive food components. Carcinogenesis 33:483–491
van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H (2002) Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem 277:17901–17905
Wang R, Wang Y, Gao Z, Qu X (2014) The comparative study of acetyl-11-keto-beta-boswellic acid (AKBA) and aspirin in the prevention of intestinal adenomatous polyposis in APC(Min/+) mice. Drug Discov Ther 8:25–32
Acknowledgments
This project was supported by the Natural Science Foundation of China (91229113, 81173090, 81373435) and the Natural Science Foundation of Shandong (ZR2009CQ019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We confirm that there is no potential conflict of interest or financial dependence regarding this paper.
Rights and permissions
About this article
Cite this article
Zhang, YS., Li, Y., Wang, Y. et al. Naringin, a natural dietary compound, prevents intestinal tumorigenesis in Apc Min/+ mouse model. J Cancer Res Clin Oncol 142, 913–925 (2016). https://doi.org/10.1007/s00432-015-2097-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2097-9