Abstract
Purpose
Distant metastasis (DM) of head and neck squamous cell carcinoma (HNSCC) is not common but remains a substantial problem. Here, we evaluated factors predictive of long-term survival in HNSCC patients presenting with DM after initial definitive treatment.
Methods
The medical records of patients with HNSCC who underwent definitive treatment between 2006 and 2011 were reviewed. Univariate and multivariate analyses were performed to identify clinicopathological factors associated with long-term survival after DM.
Results
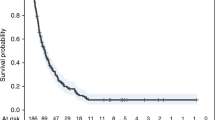
Of 779 HNSCC patients, 98 (12.6 %) had DM after completion of definitive treatment, with a median time to DM of 15 months (range 1–87 months). Overall survival (OS) rates at 1 and 2 years after DM were 43.1 and 20.5 %, respectively. In multivariate analysis, hypoalbuminemia (P < 0.001, hazard ratio [HR] 3.45, 95 % confidence interval [CI] 2.01–5.92), prior or simultaneous locoregional failure events (P < 0.001, HR 2.36, 95 % CI 1.47–3.79), multisite DM (P = 0.001, HR 2.30, 95 % CI 1.42–3.72), and no salvage treatment for DM (P = 0.003, HR 2.19, 95 % CI 1.32–3.64) were independent predictors of OS after the development of DM. Seventeen (18 %) patients survived >2 years. Patients who did not have any of these risk factors had the most favorable outcomes, with a 2-year survival of 100 %.
Conclusions
In the absence of risk factors, long-term survival can be achieved despite the development of DM after definitive treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Head and neck cancer is a group of tumors arising in the oral cavity, pharynx, larynx, nasal cavity, and paranasal sinus, and squamous cell carcinoma is the predominant histological type. Head and neck squamous cell carcinoma (HNSCC) is the eighth most common cancer worldwide, with more than half a million patients diagnosed each year (Jemal et al. 2011). Approximately 55,070 estimated new HNSCC cases and 12,000 estimated HNSCC-related deaths were reported in the USA in 2014 (Siegel et al. 2014). At the time of diagnosis, approximately two-thirds of HNSCC patients have advanced-stage disease, including regional lymph node metastasis, and they are commonly treated by a multidisciplinary approach involving surgery, radiotherapy (RT), and chemotherapy (Argiris et al. 2008; Haddad and Shin 2008). Despite the aggressive multimodal approach, the locoregional recurrence rate remains high in up to 54 % of patients with advanced HNSCC, and distant metastasis (DM), which is less frequent, occurs at a rate of 10 % at 3 years and 13 % at 5 years (Ang et al. 2001; Kramer et al. 1986; Xu et al. 2011).
Many studies have investigated the risk factors of DM development. Advanced tumor (T) and lymph node involvement (N) stages are independent factors predictive of DM (Chen et al. 2013; Dragovic et al. 2013; Leon et al. 2000; Yao et al. 2012). Patients with contralateral neck metastasis or locoregional failure (LRF) have reduced DM-free survival (Dragovic et al. 2013). DM is difficult to detect early because of the lack of specific symptoms or systemic markers; however, regular imaging surveillance in patients with locoregionally advanced HNSCC has improved the detection of DM (Kim et al. 2013; Yi et al. 2012). Despite improvements in the early diagnosis of DM through the identification of prognostic factors and advances in imaging surveillance, DM from HNSCC remains highly lethal, with most patients dying within 3–6 months after the diagnosis of DM (Liao et al. 2007; Vermorken et al. 2008).
A number of studies have prospectively evaluated prognostic factors in patients with recurrent HNSCC, including those with both locoregional and distant failure. A few studies have focused on examining clinical outcomes after distant failure in patients with DM (Huang et al. 2013; McBride et al. 2014). In a recent study that investigated the factors predictive of long-term survival in patients with DM after definitive treatment for oropharyngeal cancer, Karnofsky performance status score and limited, single-organ disease were predictors of increased survival (McBride et al. 2014). However, the outcomes of post-treatment DM in patients with HNSCC arising in other anatomical sites have not been analyzed in detail. Therefore, in the present study, we evaluated the factors predictive of long-term survival in HNSCC patients who developed DM after initial definitive treatment.
Materials and methods
Patients
A total of 779 patients who underwent definitive treatment for HNSCC at our tertiary referral center between 2006 and 2011 were analyzed. The inclusion criteria were previously untreated HNSCC without DM, definitive treatment, and adequate clinical follow-up of >1 year (Fig. 1). Finally, 779 patients were included in analysis. All patients were treated with curative intent at initial diagnosis with either surgery alone (n = 272), surgery plus postoperative radiotherapy (PORT)/concurrent chemoradiation therapy (CRT, n = 268), radiotherapy (RT) alone (n = 93), or definitive CRT (n = 141). Fifty-nine patients received salvage surgery after RT or CRT. Tumor stage was determined according to the American Joint Committee on Cancer (AJCC) tumor node metastasis (TNM) staging system (7th ed. Edge et al. 2010). This study was reviewed and approved by the Institutional Review Board of our institute, and the requirement for informed consent from each patient was waived.
The flowchart showed the inclusion and exclusion of patients, and the patients with development of post-treatment DM and their survival outcomes (Fig. 1). At the time of initial diagnosis, the characteristics of patients with and without DM are shown in Supplementary Table S1. The 779 study patients diagnosed included 641 men and 138 women with a median age of 60 years (range 20–88 years) and a median Karnofsky performance score of 90 (range 70–100). The most common primary site was the larynx (n = 273, 35.0 %) followed by the oral cavity (n = 199, 22.5 %), oropharynx (n = 143, 18.3 %), hypopharynx (n = 87, 11.2 %), and others (n = 77, 9 %). Of 779 patients, 245 (32.6 %) were in advanced T3–4 classification; 359 were in N1–3 classification; and 450 (55.8 %) were in advanced overall III–IV stage.
Variables and assessments
The data obtained from the medical records included patient age and gender, the site and TNM stage of the primary tumor, the underlying comorbidities and Karnofsky performance score, smoking status, alcohol consumption, body mass index (BMI, kg/m2) at the time of diagnosis of DM, educational level and marital status, serum albumin and hemoglobin concentrations, histological differentiation, TNM stage, prior or simultaneous events of local, regional or locoregional failure, metastatic sites and extents, and the modalities of definitive and failure treatments. Co-existing morbidities were categorized according to the Charlson comorbidity index (CCI) (Charlson et al. 1987).
The primary end-point was overall survival (OS) from DM after completion of definitive treatment. Time to DM was defined as the time from completion of definitive treatment to the first radiographic evidence of distant metastatic disease (McBride et al. 2014). OS from DM was defined as the time from the first radiographic evidence of distant failure to death or the last follow-up. Evidence of DM was obtained by radiological studies and confirmed by biopsy or serial imaging follow-up. All images showing evidence of DM were re-reviewed for the purposes of this study.
Statistical analyses
The Kaplan–Meier method was used for cumulative OS curve estimation. Prognostic factors for survival outcomes were analyzed, including the categorized variables mentioned above. The log-rank test and Cox-proportional hazards model were used to examine the significance of differences in survival outcomes from DM according to the categorized values of the tested variables. Variables with P values <0.1 on univariate analysis were analyzed by multivariate Cox-proportional hazard regression with backward elimination. In addition, when the long-term survival after the development of DM was defined as 2 years (Huang et al. 2013; McBride et al. 2014), the Chi-square test was used to compare patients who survived ≥2 and <2 years after detection of post-treatment DM. A two-sided P value of <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 21.0 (IBM, Armonk, NY, USA).
Results
Characteristics of patients with post-treatment distant metastasis
Of 779 patients, 98 (12.6 %) had no DM at initial diagnosis, but developed DM after completion of definitive treatment (Fig. 1). The characteristics of patients with DM are shown in Table 1. The 98 patients diagnosed with post-treatment DM included 81 men and 17 women with a median age of 62 years (range 30–79 years) and a median Karnofsky performance score of 70 (range 20–90). The most common primary site was the oral cavity (31 %) followed by the hypopharynx (26 %), larynx (19 %), oropharynx (15 %), and others (9 %). At the time of DM detection, anemia (hemoglobin <11.0 g/dL) and hypoalbuminemia (<3.5 g/dL) were found in 51 (52 %) and 59 (60 %) patients, respectively.
During a median follow-up of 56 months (range 5–149 months), the 3- and 5-year actuarial OS rates of all 779 study patients were 79.1 and 69.6 %, respectively, and the 3- and 5-year DM-free survival rates for all patients were 88.0 and 85.9 %, respectively. The median follow-up period of the 98 patients with post-treatment DM was 26 months (range 5–99 months). The median time to DM after completion of definitive treatment was 15 months (range 1–87 months). The 3- and 5-year DM rates of all study patients were 12.0 and 14.1 %, respectively. Eighty of 98 (81 %) patients developed DM within 2 years after definitive treatment.
Patterns and treatments of distant metastasis
Of these 98 patients, 68 (69 %) had single, limited DM at the time of detection and 30 (31 %) had metastases to multiple organs. Forty-nine (50 %) patients had prior or synchronous locoregional recurrences. The first sites of single DM were the lung in 50 (76 %), bone in six (8 %), mediastinum in three (4 %), skin in three (4 %), liver in two (3 %), brain in two (3 %), pleura in one (1 %), and heart in one (1 %) patient (Supplementary Table S2). Of the 30 patients with multiple distant failure, 24 (80 %) had lung metastasis and 17 (57 %) had bone metastasis.
Seventy-five (76 %) patients underwent treatment for DM, including chemotherapy (n = 49), metastasectomy plus chemotherapy (n = 8), or metastasectomy or radiotherapy alone (n = 18). The last status of patients was no evidence of disease in five (5 %), alive with diseases in 12 (12 %), and died of disease in 81 (83 %) patients (Table 1). Surgical intervention included wedge resection for pulmonary metastases in 13 patients, and pericardiectomy in one patient.
Risk factors for development of distant metastasis
The comparison of characteristics between patients with (n = 98) and without (n = 681) DM is shown in Supplementary Table S1. Distribution of anemia, tumor location, T and N classification, overall TNM stage, median follow-up, last status, and LRF between patients with and without DM significantly differ (P < 0.05). The univariate and multivariate analyses of risk factors for the development of DM are shown in Supplementary Table S3. On univariate analysis, anemia (P = 0.004), Karnofsky performance score (P = 0.046), tumor location (P < 0.001), T and N classification (P < 0.001), overall TNM stage (P < 0.001), chemotherapy (P = 0.004), and prior or simultaneous events of LRF (P < 0.001) were significant risk factors for DM-free survival in the 779 patients analyzed. On multivariate analysis, tumor location (P = 0.001), overall TNM stage (P < 0.001), and prior or simultaneous LRF events (P < 0.001, hazard ratio [HR] 3.24, 95 % confidence interval [CI] 2.16–5.86) were independent risk factors for DM-free survival. Of tumor location, the oral cavity (P = 0.039, HR 1.97, 95 % CI 1.03–3.74) and hypopharyngeal cancer (P = 0.001, HR 3.03, 95 % CI 1.59–5.79) showed higher risk of the development of DM than oropharyngeal cancer. Patients with overall stage III (P = 0.033, HR 2.26, 95 % CI 1.07–4.79) and IV (P < 0.001, HR 3.90, 95 % CI 2.16–7.05) tumors had more post-treatment DM than those with stage I–II tumors.
Survival after distant metastasis
The median OS after DM was 14 months (range, 1–70 months). Survival at 1 and 2 years after distant failure was 43.1 and 20.5 %, respectively. Of 98 patients with DM, six were survivors who were followed up for <2 years. After exclusion of these patients, 17 of 92 (18 %) patients survived >2 years. At last follow-up, five (5 %) patients were alive without disease; 12 (12 %) patients were alive with disease; and the remaining 81 (83 %) patients were died of disease. In univariate analysis, BMI (P = 0.008), hemoglobin (P < 0.001), hypoalbuminemia (P < 0.001), performance status (P = 0.001), tumor location (P = 0.005), prior LRF events (P < 0.001), median time to DM (P = 0.084), sites of DM (P = 0.001), and no salvage treatment for DM (P = 0.004) were significant predictors of long-term survival (Table 2). Multivariate Cox-proportional hazard regression analysis showed that hypoalbuminemia (P < 0.001, hazard ratio [HR] 3.45, 95 % confidence interval [CI] 2.01–5.92), prior LRF events (P < 0.001, HR 2.36, 95 % CI 1.47–3.79), sites of DM (P = 0.001, HR 2.30, 95 % CI 1.42–3.72), and no salvage treatment for DM (P = 0.003, HR 2.188, 95 % CI 1.32–3.64) were independent predictors of OS after the development of DM (Fig. 2). When the dichotomized variables were compared between patients who survived ≥2 and <2 years after the detection of post-treatment DM, BMI, anemia, hypoalbuminemia, performance status, tumor location, and prior or simultaneous LRF events were significant factors (P < 0.05) (Table 3).
Kaplan–Meier estimates of overall survival in patients with distant metastasis after definitive treatment according to the dichotomized variables of serum albumin concentration (g/dL, a), prior or simultaneous locoregional failure events (b), distant metastatic sites (c), and treatment for distant metastasis (d). The log-rank test, P < 0.005. Overall survival from distant metastasis was defined as the time from the first radiographic evidence of distant failure to death or the last follow-up
Discussion
HNSCC has a high propensity for DM, which occurs with an incidence ranging from 6.1 to 16.3 % and is becoming the leading cause of treatment failure and death in patients with HNSCC.6 In the present study, the 3- and 5-year DM rates were 12.0 and 14.1 %, respectively, which is within the range reported in previous studies (Huang et al. 2013; McBride et al. 2014; Vermorken et al. 2008). In addition, we examined the risk factors for DM arising in different anatomical sites of the upper aerodigestive tract after definitive treatment for HNSCC. Tumor location, overall TNM stage, and prior or simultaneous LRF events were independent risk factors for DM-free survival. These findings are consistent with the results of previous studies that included patients with HNSCCs in overall or specific anatomical sites who underwent RT- or surgery-based definitive treatment with or without systemic chemotherapy (Liao et al. 2007; Vermorken et al. 2008). This implies that the improvement in locoregional control might lead to a reduced incidence of DM, as suggested by a Halstedian approach to patterns of failure (Hellman 1994). However, the results of the present study suggest that chemotherapy does not play a significant role in preventing post-treatment DM in locoregionally limited HNSCCs, which differs from prior findings (Adelstein and Leblanc 2006). This discrepancy could be attributed to the limitations of our retrospective design. Advancements in modern treatment modalities for HNSCC have resulted in improved locoregional control, and DM has become a frequently recognized cause of treatment failure. Therefore, it might be useful to explore the clinical or pathological risk factors for DM after curative treatment for locoregionally limited HNSCCs.
The present study focused on the survival of patients who developed DM after the completion of definitive treatment. Most patients died of distant failure, and few patients (18 %) survived longer than 2 years after the diagnosis of DM. The independent predictors of OS in patients with post-treatment DM were hypoalbuminemia, prior or simultaneous LRF events, multisite metastases, and no salvage treatment for DM (P < 0.005). A recent study describing the natural course of DM following RT or CRT in oropharyngeal cancer reported that the survival rates at 2 years after the development of DM were 11 % in human papilloma virus (HPV)-positive tumors and 4 % in HPV-negative tumors (P = 0.002) (Huang et al. 2013). A small number of patients, e.g., five out of six patients with lung oligo-metastasis, were stably alive beyond 2 years after salvage procedure for DM. Another recent study that investigated the factors predictive of long-term survival in 25 (7.1 %) patients who developed post-treatment DM out of 353 patients who underwent definitive treatment for non-metastatic oropharyngeal cancer reported survival rates of 72.0 % at 1 year and 40.8 % at 2 years after the development of DM (McBride et al. 2014). In that study, the most significant factors associated with favorable outcomes were DM to limited sites and good Karnofsky performance scores, as patients with both risk factors survived for more than 2 years after the diagnosis of DM. Both studies included only patients with oropharyngeal cancer, suggesting that this type of HNSCC has a relatively favorable prognosis compared with other head and neck cancers.
In the present study, the predictive factors for DM were analyzed in a large patient cohort, including HNSCCs arising in four major anatomical sites. Our results showed that the oral cavity was associated with the worse prognosis regarding the survival of patients with post-treatment DM, although this was confirmed in univariate but not in multivariate analyses. Furthermore, patients who did not have the risk factors for hypoalbuminemia, prior or simultaneous LRF events, multisite metastases, and no salvage treatment had the most favorable outcomes, with a 2-year survival of 100 %. Studies have shown that resection of pulmonary metastases can prolong the survival of head and neck cancer patients (Shiono et al. 2009; Wedman et al. 1996; Winter et al. 2008). In accordance with previous results, patients who developed post-treatment DM survived longer than 2 years after aggressive local treatment of solitary metastasis in remote sites. These results indicate that aggressive treatment should be recommended in patients with metastasis at distant sites.
The present study had limitations inherent to its retrospective design. We included cases with HNSCCs arising at different anatomical sites, which could have different clinical behaviors and outcomes. However, our head and neck oncology specialists used a team approach, which included proper planning and multimodal treatments for each HNSCC patient. In addition, p16 and HPV status were obtained in a small fraction of patients, which led to difficulties in the analysis according to these biomarkers. The lack of subjective symptoms or makers, or false-negative imaging results can make early detection of post-treatment DM difficult, which might result in variation in the post-DM periods. However, our team attempted to adhere to institutional protocols in the follow-up of patients with head and neck cancer, which included regular follow-up periods and imaging check-ups (Kim et al. 2013). Another potential limitation is that solitary metastases can be second primary cancers, which may be associated with better outcomes than systemic metastases (McBride et al. 2014). This effect was minimized by the inclusion of supplementary diagnostic examinations and biopsies of suspicious lesions as part of the histopathological evaluation. Finally, these lesions were diagnosed by discussion of our institutional tumor board.
This study may guide clinicians to predict the patients who were at high risk of development of post-treatment DM. Further, this leads to select the patients who require aggressive salvage treatment and nutritional support, so as to increase their survivals after detection of DM. Future study will be required to eliminate the limitations of this retrospective study and avoid discrepancy between this and previous studies. The potential errors will be minimized by prospective studies including careful enrollments and follow-up of the patients according to different anatomical tumor sites and treatment modalities.
In conclusion, long-term survival of patients without the risk factors of hypoalbuminemia, LRF, and multisite metastases can be expected and achieved despite the development of DM after definitive treatment if these patients undergo adequate salvage procedures for post-treatment DM. Aggressive salvage treatment and proper nutritional support is therefore recommended in these patients.
References
Adelstein DJ, Leblanc M (2006) Does induction chemotherapy have a role in the management of locoregionally advanced squamous cell head and neck cancer? J Clin Oncol 24:2624–2628
Ang KK et al (2001) Randomized trial addressing risk features and time factors of surgery plus radiotherapy in advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 51:571–578
Argiris A, Karamouzis MV, Raben D, Ferris RL (2008) Head and neck cancer. Lancet 371:1695–1709
Charlson ME, Pompei P, Ales KL, Mackenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chr Dis 40:373–383
Chen TC et al (2013) The clinical predictive factors for subsequent distant metastasis in patients with locoregionally advanced oral squamous cell carcinoma. Oral Oncol 49(4):367–373
Dragovic AF, Caudell JJ, Spencer SA, Carroll WR, Nabell LA, Bonner JA (2013) Locoregional failure and the risk of distant metastasis after modern radiotherapy for head and neck cancer. Head Neck 35:381–387
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (2010) AJCC Cancer Staging Manual, 7th edn. NY, Springer, New York, pp 21–78
Haddad RI, Shin DM (2008) Recent advances in head and neck cancer. N Engl J Med 359:1143–1154
Hellman S (1994) Karnofsky Memorial Lecture. Natural history of small breast cancers. J Clin Oncol 12:2229–2234
Huang SH et al (2013) Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol 49:79–85
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Kim JW et al (2013) (18)F-FDG PET/CT surveillance at 3-6 and 12 months for detection of recurrence and second primary cancer in patients with head and neck squamous cell carcinoma. Br J Cancer 109:2973–2979
Kramer S, Marcial VA, Pajak TF, MacLean CJ, Davis LW (1986) Prognostic factors for loco/regional control and metastasis and the impact on survival. Int J Radiat Oncol Biol Phys 12:573–578
Leon X, Quer M, Orus C, Del Prado Venegas M, Lopez M (2000) Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck 22:680–686
Liao CT et al (2007) Analysis of risk factors for distant metastases in squamous cell carcinoma of the oral cavity. Cancer 110:1501–1508
McBride SM, Busse PM, Clark JR, Wirth LJ, Ancukiewicz M, Chan AW (2014) Long-term survival after distant metastasis in patients with oropharyngeal cancer. Oral Oncol 50:208–212
Shiono S et al (2009) Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg 88:856–860
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Vermorken JB et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Wedman J et al (1996) Value of resection of pulmonary metastases in head and neck cancer patients. Head Neck 18:311–316
Winter H et al (2008) Does surgical resection of pulmonary metastases of head and neck cancer improve survival? Ann Surg Oncol 15:2915–2926
Xu GZ, Guan DJ, He ZY (2011) (18)FDG-PET/CT for detecting distant metastases and second primary cancers in patients with head and neck cancer. A meta-analysis. Oral Oncol 47:560–565
Yao M et al (2012) Distant metastases in head-and-neck squamous cell carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 83:684–689
Yi JS et al (2012) 18F-FDG PET/CT for detecting distant metastases in patients with recurrent head and neck squamous cell carcinoma. J Surg Oncol 106:708–712
Acknowledgments
This study was supported by a Grant (No. 2014-0306) from the Asan Institute for Life Science and a Grant (HI14C23050000) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Seoul, Republic of Korea (J.-L. Roh).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interests to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kang, HS., Roh, JL., Kim, MJ. et al. Predictive factors for long-term survival in head and neck squamous cell carcinoma patients with distant metastasis after initial definitive treatment. J Cancer Res Clin Oncol 142, 295–304 (2016). https://doi.org/10.1007/s00432-015-2043-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-2043-x