Abstract
Purpose
The expansion of micrometastatic tumors to macrometastatic ones is thought to be tightly regulated by several microenvironmental factors. The aim of this study was to elucidate the morphological and phenotypical differences between micrometastatic and macrometastatic tumors.
Method
We first examined the morphological characteristics of 66 lymph node (LN) micrometastatic tumors (less than 2 mm in size) and 51 macrometastatic tumors (more than 10 mm in size) in 42 lung adenocarcinoma cases. Then, we evaluated the expression level of E-cadherin, S100A4, ALDH1, and Geminin in cancer cells and the number of smooth muscle actin (SMA), CD34, and CD204 (+) stromal cells in the primary tumors, matched micrometastatic tumors, and macrometastatic tumors (n = 34, each).
Results
Tumor budding reflects the process of EMT, and stromal reactions were observed more frequently in macrometastatic tumors (P < 0.001). E-cadherin staining score for the micrometastatic tumors was significantly higher than that for the primary tumors (P < 0.001). In contrast, the E-cadherin staining score for the macrometastatic tumors was significantly lower than that for the micrometastatic tumors (P = 0.017). As for the stromal cells, the numbers of SMA (+) fibroblasts, CD34 (+) microvessels, and CD204 (+) macrophages were significantly higher for the macrometastatic tumors and primary tumors than for the micrometastatic tumors (P < 0.001, all).
Conclusion
The present study clearly showed that dynamic microenvironmental changes (e.g., EMT-related changes in cancer cells and structural changes in stromal cells) occur during the growth of micrometastases into macrometastases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metastasis is considered to be a complex and multistep process that ultimately results in the formation of a secondary mature tumor (Luzzi et al. 1998; Mehlen and Puisieux 2006; Tsai and Yang 2013). During this step, effective and dynamic molecular changes of cancer cells, such as the epithelial-mesenchymal transition (EMT) and the mesenchymal-epithelial transition (MET), play important roles (Thiery 2002; Yao et al. 2011; Samatov et al. 2013). Within the primary tumor, cancer cells induce local dissemination, possibly through an EMT, and intravasate into the lymphatics or blood vessels, allowing their passive transport to distant organs (Condeelis and Segall 2003; Ksiazkiewicz et al. 2012). Extravasated cancer cells form small tumor nest(s) at metastatic sites (more specifically known as “colonization of cancer cells” or “micrometastasis”) and continue growing, forming macroscopic metastases (Luzzi et al. 1998; Ksiazkiewicz et al. 2012).
Advanced detailed analyses of the molecular mechanisms of the metastatic process have revealed that during the early phase of metastasis, the majority of extravasated cancer cells undergo apoptosis or survive in a dormant state (Luzzi et al. 1998; Mehlen and Puisieux 2006; Wong et al. 2001). However, a small population of cancer cells becomes resistant to apoptosis or adapts to the foreign tissue microenvironment and proliferates to reach a size not much more than a few millimeters in diameter without developing a vascular network (Luzzi et al. 1998; Mehlen and Puisieux 2006). Recent reports have shown that the initial proliferation of cancer cells after colonization at a metastatic site is governed, in part, by common regulatory mechanism involving the formation of filopodium-like protrusions and the resulting assembly of mature adhesion plaques (Shibue and Weinberg 2009; Shibue et al. 2012, 2013). To continue growing, the colonized cancer cells induce angiogenesis, and several extrinsic factors in the microenvironment also contribute to determining the eventual successful formation of macroscopic metastatic tumors (Fidler 2003). Therefore, it should be kept in mind that the tumor microenvironment of early-phase metastases (micrometastasis) and that of late-phase metastases (macrometastasis) are obviously different.
Some previous reports have described differences in the molecular expression patterns of primary tumors and metastatic lymph node (LN) tumors. In breast cancer or colorectal cancer, tumor cells express EMT features in metastatic LN, compared with in the primary tumors (Elzagheid et al. 2006; Markiewicz et al. 2012). However, these studies did not take into account the size of the metastatic LNs. Examining the differences in cancer cell phenotypes and the stromal reaction between micrometastatic and macrometastatic, tumors may provide very important information regarding the dynamism of the tumor metastatic process. In the current study, we compared the morphological features, cancer cell immunophenotypes, and stromal cell features of micrometastatic (2 mm or less) and macrometastatic (10 mm or more) LN tumors.
Materials and methods
Patient selection
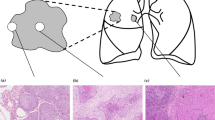
To examine the morphological differences between micrometastatic and macrometastatic tumors, a total of 440 consecutive patients with primary lung adenocarcinoma who underwent complete surgical resections at our hospital between January 2013 and April 2014 were enrolled in the present study. We defined LN metastasis of 2 mm or less as a micrometastatic tumor according to the TNM classification of breast cancer. LN metastasis of 10 mm or more was defined as a macrometastatic tumor in this study, since the clinical diagnosis of an LN with a minor axis of 1 cm or more on a CT image is diagnosed as a positive metastasis. We excluded patients who did not undergo a standard operation or who received preoperative treatment. LN metastasis was detected in 57 cases. The histological subtype, stromal reaction, and existence of budding cells were microscopically analyzed in 66 micrometastatic LN tumors and 51 macrometastatic LN tumors (Fig. 1a).
To investigate the phenotypic differences between micrometastatic and macrometastatic tumors, we selected adenocarcinoma cases with both micrometastatic and macrometastatic LN tumors. Among a total of 1025 consecutive surgically resected adenocarcinoma cases (between January 2008 and December 2013), 34 cases met this criterion (Fig. 1b). We examined the immunohistochemical phenotype of 1) the primary tumor, 2) the micrometastatic LN tumor, and 3) the macrometastatic LN tumor of these 34 cases. All the surgical specimens were collected and analyzed after receiving the approval of the Institutional Review Board of the National Cancer Center Hospital East.
Histological studies
The surgical specimens were fixed in 10 % formalin, embedded in paraffin, and serially sectioned at 4-mm intervals. The sections were stained using the hematoxylin and eosin (HE) method. All the histological materials included in this series were reviewed by two pathologists (N.A. and G.I.). The pathological stage was determined based on the TNM classification of the International Union Against Cancer (UICC), 7th edition. Histological typing of the primary tumors was performed based on the World Health Organization classification of cell types, 3rd edition. Histological subtyping of the primary tumors was based on the IASLC/ATS/ERS International Multidisciplinary Lung Adenocarcinoma Classification published in 2011.
Histological studies of the metastatic LN tumors
After extravasation from the lymphatic vessels, a small number of cancer cells form micrometastatic tumors in the LN and subsequently grow into macrometastatic tumors (Fig. 2a). We divided the metastatic LN tumors into the following two groups according to their size: 1) micrometastatic tumors (2 mm or less) (Fig. 2b, d) and 2) macrometastatic tumors (10 mm or more) (Fig. 2c, e). To examine their morphological components, we investigated their predominant subtypes (papillary, acinar, and solid pattern). For small metastatic tumors, isolated and small clusters of tumor cells that lacked a clear differentiation into a papillary or acinar pattern were classified as having a “solid subtype”.
a Schema of LN metastasis pathway of primary lung cancer. b, d Micrometastatic tumor (2 mm or less) of lung adenocarcinoma, composed of a solid pattern, without stromal reaction; c, e macrometastatic tumor (10 mm or more) of lung adenocarcinoma, mainly showing an acinar pattern, with stromal reaction and budding cells
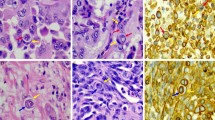
Comparison of immunohistochemical staining scores of cancer cells in primary tumors and micrometastatic and macrometastatic LN tumors. a E-cadherin, b S100A4, c Vimentin, d ALDH1 and e Geminin. The expression levels of each antibody in each tumor were examined using the Wilcoxon signed-rank test. Representative immunohistochemical staining results for primary tumor tissue and micrometastatic and macrometastatic LN tumors. Left column (f, i), primary tumors. Middle column (g, j), micrometastatic LN tumors. Right column (h, k), macrometastatic LN tumors. Top row (f, g, h), E-cadherin. Bottom row (i, j, k), ALDH1
Comparison of immunohistochemical staining results of stromal cells in primary tumors and micrometastatic and macrometastatic LN tumors. a Smooth muscle actin (SMA)-positive myofibroblasts, b CD34-positive vessels, and c CD204-positive macrophages. The expression levels of each antibody in each tumor were examined using the Wilcoxon signed-rank test. Representative immunohistochemical staining results for primary, micrometastatic, and macrometastatic tumors. Left column (d, g, j), primary tumors. Middle column (e, h, k), micrometastatic tumors. Right column (f, i, l), macrometastatic tumors. First row (d, e, f), SMA. Second row (g, h, i), CD34. Last row (j, k, l), CD204
Hypothetical molecular machinery for the development of LN micrometastases to macrometastases. Tumor cells forming a micrometastasis show an increase in the expression of E-cadherin and a subsequent decrease during the evolution to a macrometastasis. A drastic stromal reaction is also induced during this process
A stromal reaction has been defined as an organic change including fibrosis surrounding the tumor cells (Ronnov-Jessen et al. 1996; Troester et al. 2009; Otranto et al. 2012). The definition of “positive” was a stromal component accounting for more than 10 % of the entire tumor. Isolated single cancer cells and clusters composed of less than five cancer cells were defined as tumor budding (Yamaguchi et al. 2010).
Antibodies and immunohistochemical staining
Immunohistochemical staining was performed according to a previously reported method (Kirita et al. 2013). The slides were deparaffinized in xylene and dehydrated in a graded ethanol series, and endogenous peroxidase was blocked with 3 % hydrogen peroxide in absolute methyl alcohol. After epitope retrieval, the slides were washed with phosphate-buffered saline and were incubated overnight at 4 °C using primary antibodies at their final dilution in the blocking buffer. The primary antibodies used in this study are summarized in Supplemental Table 1.
The slides were washed again and incubated with EnVision (Dako, Glostrup, Denmark) for 1 h at room temperature. The reaction products were stained with diaminobenzidine; lastly, the slides were counterstained with Meyer hematoxylin.
Immunohistochemical scoring
All the stained tissue sections were semiquantitatively scored and evaluated independently under a light microscope by two pathologists (N.A. and G.I.). The immunostaining scores of E-cadherin, S100A4, Vimentin, and ALDH1 were evaluated based on the staining intensity and the percentage of cancer cells that showed positive staining. The following scoring system was used: 0 (negative staining, defined as no immunoreactivity); 1+ (weak staining intensity); and 2+ (strong staining intensity). We also evaluated the extent of staining in a lesion corresponding to every ten percentages (0–100 %). The staining scores were calculated by multiplying the percentage values by the staining intensity, with the scores ranging from 0 to 200. The percentage score for Geminin was determined by calculating the number of positive tumor cells per all tumor cells counted in three high-power microscopic fields (×400; 0.0625 mm2). The positive CD204 and CD34 cells were counted in three high-power microscopic fields, and the averages were determined. To investigate the SMA score, we performed a computer-assisted analysis using WinROOF image processing software (Mitani Corp., Tokyo, Japan). We set the macroinstruction, which was composed of algorithms for color identification based on red-green-blue and the hue-luminosity-saturation parameters, to identify the immunostained area and calculated the ratio (Hatanaka et al. 2003; Kojima et al. 2014). We confirmed that the positive control tissues were stained with each antibody, and we also performed negative control studies without the primary antigen for all the antibodies. When the evaluation results differed, the final report was determined based on a consensus reached between the two pathologists who evaluated the slides together under a conference microscope.
Statistical analysis
Differences in the metastatic LN characteristics between the micrometastatic and macrometastatic tumors were compared using the Fisher exact test. The Wilcoxon signed-rank test was performed to calculate the statistical significance of the differences. All the P values were two-sided, and the significance level was set at <0.05. All the statistical analyses were performed using JMP 10 statistical software (version 10.0.2., 64-bit edition; SAS Institute Inc., Cary, NC) and GraphPad Prism (Prism for Windows, version 5.02; GraphPad Software, Inc., La Jolla, CA).
Results
Morphological features of micrometastatic and macrometastatic LN tumors
The results of pathological analyses of 66 micrometastatic LN tumors and 51 macrometastatic tumors are summarized in Table 1. The number of metastatic tumors with a predominantly papillary and acinar pattern was significantly smaller among the micrometastatic tumors than among the macrometastatic tumors (P = 0.024). Macrometastatic tumors exhibited a stromal reaction more frequently than micrometastatic tumors (85 vs. 32 %, P < 0.001). The number of metastatic tumors displaying budding cells, which is an EMT morphology of cancer cells, was significantly higher among the macrometastatic tumors than among the micrometastatic tumors (59 vs. 11 %, P < 0.001).
Immunophenotype of tumor cells in primary tumors and micrometastatic and macrometastatic LN tumors (Fig. 3)
We selected 36 lung adenocarcinoma patients who had both micrometastatic and macrometastatic LN tumors (Supplemental Table 2) and stained the specimens using 7 molecular markers, then compared the staining score for each region. The number of metastatic tumors predominantly composed of a papillary and acinar pattern tended to be lower among the micrometastatic tumors than among the macrometastatic tumors (P = 0.061). The macrometastatic tumors exhibited a higher frequency of stromal reaction than the micrometastatic tumors (94 vs. 29 %, P < 0.001). Budding cells were observed significantly more frequently in the macrometastatic tumors than in the micrometastatic tumors (56 vs. 9 %, P < 0.001) (Supplemental Table 3).
EMT-related molecules
The median staining scores (ranges) for E-cadherin in the primary, micrometastatic, and macrometastatic tumors were 50 (0–160), 85 (10–200), and 75 (10–170), respectively. The median staining scores (ranges) for S100A4 in the primary, micrometastatic and macrometastatic tumors were 0 (0–90), 15 (0–100), and 10 (0–90), respectively. The median staining scores (ranges) for Vimentin in the primary, micrometastatic, and macrometastatic tumors were 0 (0–15), 0 (0–30), and 0 (0–45), respectively. The E-cadherin expression level in the macrometastatic tumors was significantly lower than that in the micrometastatic tumors (Wilcoxon signed-rank test P = 0.017). On the other hand, the E-cadherin expression level in the micrometastatic tumors was significantly higher than that in the primary tumors (Wilcoxon signed-rank test P < 0.001). There were no significant differences in S100A4 and Vimentin expression among the primary tumors, the micrometastatic tumors, and the macrometastatic tumors.
Cancer stem cell-related molecules
The median staining scores (ranges) for ALDH1 in the primary, micrometastatic, and macrometastatic tumors were 20 (0–120), 0 (0–100), and 5 (0–100), respectively. When examined using the Wilcoxon signed-rank test, the ALDH1 expression level in the micrometastatic tumors was significantly lower than that in the primary tumors (P = 0.008) or in the macrometastatic tumors (P = 0.024).
Cell proliferation
The median staining scores (ranges) for Geminin in the primary, micrometastatic, and macrometastatic tumors were 9 % (1–28 %), 10 % (0–40 %), and 10 % (2–30 %), respectively. No significant differences among the tumor types were observed when examined using a Wilcoxon signed-rank test.
Immunophenotype of stromal cells in primary tumors and micrometastatic and macrometastatic LN tumors (Fig. 4)
Myofibroblast density
The average density areas of SMA-positive myofibroblasts in the primary, micrometastatic, and macrometastatic tumors were 7.2, 3.1, and 8.0 %, respectively. When examined using the Wilcoxon signed-rank test, the myofibroblast densities of the macrometastatic tumors and the primary tumors were significantly higher than that of the micrometastatic tumors (P < 0.001 and P < 0.001, respectively).
Microvessel density
The average numbers of vessels formed by CD34-positive endothelial cells in the primary, micrometastatic, and macrometastatic tumors were 13.7, 5.9, and 10.7, respectively. When examined using the Wilcoxon signed-rank test, the microvessel densities in the macrometastatic tumors and the primary tumors were significantly higher than that in the micrometastatic tumors (P < 0.001 and P < 0.001, respectively).
M2 macrophage density
The average numbers of CD204-positive TAMs in the primary, micrometastatic, and macrometastatic tumors were 29.3, 9.3, and 29.4, respectively. The CD204-positive TAM densities of the macrometastatic tumors and the primary tumors were significantly higher than that of the micrometastatic tumors (P < 0.001 and P < 0.001, respectively).
Discussion
It has been generally accepted that the process leading to the formation of macrometastases requires several microenvironment factors, including both cancer cell-derived intrinsic factors and stromal cell-derived extrinsic factors (Gao et al. 2012; Wan et al. 2013). In experimental models, extrinsic factors can reportedly induce MET in cancer cells through the reexpression of E-cadherin within a metastatic microenvironment (Chao et al. 2010). However, immunostaining studies using surgically resected specimens have also reported that metastasized cancer cells can display an EMT phenotype (Ksiazkiewicz et al. 2012; Brabletz et al. 2001; Chaffer et al. 2006; Hudson et al. 2008). This discrepancy suggests that both MET and EMT occur in the same metastatic organs but at different time points. Actually, how and when the EMT and/or MET occurs in metastatic organs is not fully understood. Moreover, it is also not clear how and when the stromal cells are recruited. To clarify this matter, we investigated the microenvironment features of micrometastatic and macrometastatic tumors focusing on both cancer cell and stromal cell phenotypes.
In the current study, E-cadherin expression was significantly higher in micrometastatic tumors than in primary tumors. This phenomenon was consistent, in part, with previous experimental results showing that subsequent colonization in distant organs requires the reversion of EMT and/or the activation of the MET program to establish secondary tumors (Tsai and Yang 2013; Tsai et al. 2012). On the other hand, in macrometastatic tumors, E-cadherin expression was significantly reduced, compared with that in micrometastatic tumors. This result was interpreted as indicating that EMT occurred during the process of macrometastatic tumor formation, and this process was confirmed by the morphological finding that a budding cell appearance was more frequent in macrometastatic tumors. Taken together, the current results suggested that during the formation of macrometastatic tumors, cancer cells undergo dynamic phenotypic changes associated with MET and a subsequent EMT. We found that micrometastatic tumors exhibited fewer stromal reactions than macrometastases using both morphological and immunohistochemical analyses. The lower microvessel density in micrometastatic tumors was consistent with previous concepts that angiogenesis hardly occurs in micrometastatic tumors (MacDonald et al. 2002; Goubran et al. 2014). On the other hand, the recruitment of CAFs and TAMs in micrometastatic tumors has not been reported to date. Our reports revealed, for the first time, that CAFs and TAMs are also recruited during macrometastatic formation. These findings may indicate that colonized cancer cells do not require stromal cells for survival during the early phase of metastasis, but need them for survival and proliferation during the later phase. Alternatively, extrinsic factors from stromal cells might be less important for the generation or maintenance of micrometastatic tumors.
Several papers have emphasized that stromal cells promote the EMT of tumor cells. CAFs and TAMs in primary tumors secret pro-inflammatory/pro-angiogenic cytokines and matrix metalloproteinases, leading to the invasion of cancer cells into the surrounding tissue through the EMT (Pollard 2004; Taddei et al. 2013). The present study suggests that the EMT might occur concurrently with an increased stromal reaction in metastatic organs as well. The molecular mechanisms of these dynamic changes in the cancer cells and stroma cells of micrometastatic tumors will require further investigation.
The current results indicated that the expression of ALDH1 was significantly reduced in micrometastatic tumors. This phenomenon may be, in part, explained by the followings. The low expression of the cancer stem cell marker ALDH1 is reportedly correlated with a poor prognosis in patients with NSCLC (Dimou et al. 2012). We previously reported that intralymphatic cancer cells with low ALDH1 expression levels have a critical impact on LN metastasis, suggesting that intralymphatic cancer cells expressing less ALDH1 have a higher tumorigenic capacity (Kirita et al. 2013). In addition, the forced expression of ALDH1A1 markedly reduced clonogenicity and prolonged the doubling time in a NSCLC cell line (Okudela et al. 2013). Further studies will be needed to clarify the possibility whether ALDH1-negative cancer cells act as cancer stem/initiating cells in lung adenocarcinoma.
We previously found that during lung metastasis, extravasated cancer cells from lymphatics first displayed an EMT phenotype (decreased E-cadherin expression and a “budding” feature) in the broncho-vascular bundle (Aokage et al. 2011). In contrast, few budding cells are seen in micrometastatic LN tumors, and E-cadherin expression was increased in the cancer cells. Thus, the biological natures of micrometastasized cancer cells are quite different between those that form within the lung and those that form within the LN. These findings suggest that the organ-dependent microenvironment might cause different biological features in micrometastasized cancer cells.
In conclusion, the present study suggested that during macroscopic LN tumor formation, cancer cells undergo dynamic epithelial phenotypic changes. Moreover, these changes appear in parallel with dynamic stromal changes (Fig. 5). Thus, the tumor microenvironment is quite different between early and mature metastatic LN tumors. The elucidation of the biological mechanisms responsible for these dynamic changes in tumor cells as well as stromal cells during the evolution of LN macrometastasis will be a future research goal for understanding the molecular machinery of cancer metastasis.
Abbreviations
- LN:
-
Lymph node
- EMT:
-
Epithelial-mesenchymal transition
- MET:
-
Mesenchymal-epithelial transition
- PT:
-
Primary tumor
- Mic:
-
Micrometastatic tumor
- Mac:
-
Macrometastatic tumor
- CAF:
-
Cancer-associated fibroblast
- TAM:
-
Tumor-associated macrophage
- NSCLC:
-
Non-small cell lung cancer
- ALDH1:
-
Aldehyde dehydrogenase 1
- SMA:
-
Smooth muscle actin
References
Aokage K, Ishii G, Ohtaki Y, Yamaguchi Y, Hishida T et al (2011) Dynamic molecular changes associated with epithelial-mesenchymal transition and subsequent mesenchymal-epithelial transition in the early phase of metastatic tumor formation. Int J Cancer 128:1585–1595
Brabletz T, Jung A, Reu S, Porzner M, Hlubek F et al (2001) Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 98:10356–10361
Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW et al (2006) Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res 66:11271–11278
Chao YL, Shepard CR, Wells A (2010) Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer 9:179
Condeelis J, Segall JE (2003) Intravital imaging of cell movement in tumours. Nat Rev Cancer 3:921–930
Dimou A, Neumeister V, Agarwal S, Anagnostou V, Syrigos K et al (2012) Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer. Am J Pathol 181:1436–1442
Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R et al (2006) E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol 12:4304–4309
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458
Gao D, Vahdat LT, Wong S, Chang JC, Mittal V (2012) Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res 72:4883–4889
Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T (2014) Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis 7:9–18
Hatanaka Y, Hashizume K, Nitta K, Kato T, Itoh I et al (2003) Cytometrical image analysis for immunohistochemical hormone receptor status in breast carcinomas. Pathol Int 53:693–699
Hudson LG, Zeineldin R, Stack MS (2008) Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis 25:643–655
Kirita K, Ishii G, Matsuwaki R, Matsumura Y, Umemura S et al (2013) Identification of biological properties of intralymphatic tumor related to the development of lymph node metastasis in lung adenocarcinoma. PLoS ONE 8:e83537
Kojima M, Higuchi Y, Yokota M, Ishii G, Saito N et al (2014) Human subperitoneal fibroblast and cancer cell interaction creates microenvironment that enhances tumor progression and metastasis. PLoS ONE 9:e88018
Ksiazkiewicz M, Markiewicz A, Zaczek AJ (2012) Epithelial-mesenchymal transition: a hallmark in metastasis formation linking circulating tumor cells and cancer stem cells. Pathobiology 79:195–208
Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873
MacDonald IC, Groom AC, Chambers AF (2002) Cancer spread and micrometastasis development: quantitative approaches for in vivo models. BioEssays 24:885–893
Markiewicz A, Ahrends T, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J et al (2012) Expression of epithelial to mesenchymal transition-related markers in lymph node metastases as a surrogate for primary tumor metastatic potential in breast cancer. J Transl Med 10:226
Mehlen P, Puisieux A (2006) Metastasis: a question of life or death. Nat Rev Cancer 6:449–458
Okudela K, Woo T, Mitsui H, Suzuki T, Tajiri M et al (2013) Downregulation of ALDH1A1 expression in non-small cell lung carcinomas–its clinicopathologic and biological significance. Int J Clin Exp Pathol 6:1–12
Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G et al (2012) The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr 6:203–219
Pollard JW (2004) Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 4:71–78
Ronnov-Jessen L, Petersen OW, Bissell MJ (1996) Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev 76:69–125
Samatov TR, Tonevitsky AG, Schumacher U (2013) Epithelial-mesenchymal transition: focus on metastatic cascade, alternative splicing, non-coding RNAs and modulating compounds. Mol Cancer 12:107
Shibue T, Weinberg RA (2009) Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A 106:10290–10295
Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA (2012) The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov 2:706–721
Shibue T, Brooks MW, Weinberg RA (2013) An integrin-linked machinery of cytoskeletal regulation that enables experimental tumor initiation and metastatic colonization. Cancer Cell 24:481–498
Taddei ML, Giannoni E, Comito G, Chiarugi P (2013) Microenvironment and tumor cell plasticity: an easy way out. Cancer Lett 341:80–96
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2:442–454
Troester MA, Lee MH, Carter M, Fan C, Cowan DW et al (2009) Activation of host wound responses in breast cancer microenvironment. Clin Cancer Res 15:7020–7028
Tsai JH, Yang J (2013) Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev 27:2192–2206
Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J (2012) Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell 22:725–736
Wan L, Pantel K, Kang Y (2013) Tumor metastasis: moving new biological insights into the clinic. Nat Med 19:1450–1464
Wong CW, Lee A, Shientag L, Yu J, Dong Y et al (2001) Apoptosis: an early event in metastatic inefficiency. Cancer Res 61:333–338
Yamaguchi Y, Ishii G, Kojima M, Yoh K, Otsuka H et al (2010) Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J Thorac Oncol 5:1361–1368
Yao D, Dai C, Peng S (2011) Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res 9:1608–1620
Acknowledgments
All work reported herein was performed at the National Cancer Center Hospital East, Kashiwa, Chiba, Japan. The research was approved by the Internal Review Board of the institution. No patient consent was required as the research is a retrospective chart review, and no personally identifiable information was included in the manuscript.
Conflict of interest
The authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aramaki, N., Ishii, G., Yamada, E. et al. Drastic morphological and molecular differences between lymph node micrometastatic tumors and macrometastatic tumors of lung adenocarcinoma. J Cancer Res Clin Oncol 142, 37–46 (2016). https://doi.org/10.1007/s00432-015-1996-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1996-0