Abstract
Purpose
Childhood cancer survivors (CCS) treated with cranial radiation therapy (CRT) are at risk of developing meningiomas. The aim of this study was to evaluate the cumulative incidence of meningiomas in a cohort of CCS who previously underwent CRT.
Methods
We considered all CCS who received CRT and were followed up at the “Transition Unit for Childhood Cancer Survivors” in Turin. Even though asymptomatic, they had at least one brain computed tomography or magnetic resonance imaging performed at a minimum interval of 10 years after treatment for pediatric cancer.
Results
We identified 90 patients (median follow-up 24.6 years). Fifteen patients developed meningioma (median time from pediatric cancer, 22.5 years). In four patients, it was suspected on the basis of neurological symptoms (i.e., headache or seizures), whereas all other cases, including five giant meningiomas, were discovered in otherwise asymptomatic patients. Multiple meningiomas were discovered in four CCS. Ten patients underwent surgical resection. An atypical meningioma (grade II WHO) was reported in four patients. One patient with multiple meningiomas died for a rapid growth of the intracranial lesions. A second neoplasm (SN) other than meningioma was diagnosed in five out of the 15 patients with meningioma and in ten out of the 75 CCS without meningioma. Cox multivariate analysis showed that the occurrence of meningioma was associated with the development of other SNs, whereas age, sex, or CRT dose had no influence.
Conclusions
CCS at risk of the development of meningioma deserve close clinical follow-up, especially those affected by other SNs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of childhood cancer survivors (CCS) worldwide is increasing, due to the improvement in treatment modalities (Jemal et al. 2006). However, these children are at increased risk of developing a wide range of late effects, including second neoplasms (SNs), due to the anti-cancer therapies received in pediatric age (Olsen et al. 2009; Friedman et al. 2010). The occurrence of a SN has a dramatic impact on the duration and quality of life of cancer survivors. Second tumors have been reported to develop in about 10 % of survivors by 30 years from their initial cancer diagnosis and are considered the most frequent cause of mortality in patients who survive for more than 20 years (Lawless et al. 2007; Armstrong et al. 2009).
Even though since early 70 s pediatric chemotherapy and radiotherapy have been tailored to reduce toxicity while maximizing efficacy, the risk of developing a SN is real and several authors recommend continuous and stringent follow-up (Kaatsch et al. 2009; Friedman et al. 2010; Nathan et al. 2010; Reulen et al. 2011).
Ionizing radiation causes damage to DNA and to the DNA-repairing systems, thus promoting cancer. Neural tissue is mostly sensitive to the radiation effect. It is well known that exposure to ionizing radiation increases the risk of meningioma. It was reported (Unmansky et al. 2008) that this risk is higher and has a shorter latency after high doses of radiation, but even low doses significantly increase the risk of meningioma. The location of the SN is related to the previous site of radiation exposure. Furthermore, radiation-induced meningiomas differ from others meningiomas in patient age at presentation and in the multiplicity, aggressiveness, and rate of tumor recurrence (Sadetzki et al. 2002).
In 2010, Taylor and coworkers of The British Childhood Cancer Survivor Study reported, within a national, population-based, cohort study of 17,980 individuals surviving at least 5 years after diagnosis of childhood cancer, that the risk of meningioma was significantly increased (Taylor et al. 2010). More recently, Sabin and coworkers (Sabin et al. 2014) performed a non-contrast MRI of the brain as part of a broad assessment of neurocognitive status on 219 childhood cancer survivors in the St. Jude Lifetime Cohort. They report an incidental detection of secondary intracranial neoplasms in survivors who received cranial radiotherapy; all but one of the neoplasms had imaging features suggestive for meningioma.
The aim of this study was to evaluate the cumulative incidence of meningioma in a cohort of CCS previously treated with cranial radiotherapy and followed up at the “Transition Unit for Childhood Cancer Survivors” of the University Hospital of Turin. We also aimed to identify factors that could increase the risk of second meningioma development in CCS who underwent cranial radiotherapy.
Methods
Study population
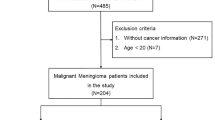
We considered all patients referred to the “Transition Unit for Childhood Cancer Survivors” (Turin, Italy) from November 2001 to March 2014. This is a specialized adult-focused follow-up clinic, located in a tertiary cancer center (and connected with a network of multidisciplinary specialists), to whom CCS are transitioned by the pediatric oncologist usually when they are over 18 years and off-therapy for at least 5 years (Brignardello et al. 2013).
For this study, we selected all subjects who satisfied the following criteria: (a) age at diagnosis under 18 years; (b) at least one visit after the 18th birthday; (c) previous treatment with cranial radiotherapy.
At our unit, CCS underwent periodical follow-up visits including physical examinations and laboratory/imaging investigations depending on the previous anticancer treatments. All the information about the cancer diagnosis, treatments, and the medical history (relapses, second tumors, late toxicities) were collected during visits and recorded in a specific database.
Until few years ago, in our practice and according to the Children’s Oncology Group guidelines (The Children’s Oncology Group Guidelines 2008), CCS previously treated with cranial radiotherapy underwent a brain MRI (or CT scan, in the presence of contraindication for MRI) in case of neurological symptoms only. However, after finding some large meningiomas in asymptomatic patients, more recently we started to routinely perform brain MRI in all CCS who received cranial irradiation. Therefore, at the time of the study, all included patients had at least one brain imaging evaluation performed within the previous 3 years.
MRI examinations were obtained on a 1.5 Tesla scanner. In all patients, pre-contrast sagittal T1 and axial T2 fluid attenuated invertion recovery (FLAIR), fast spin-echo (FSE) sagittal T1 and T2, and coronal FSE T2 and axial T1 sequences were performed. After contrast medium (Gadovist Schering, 0, 2 mmol/kg) administration, axial, sagittal, and coronal T1 sequences were obtained.
Statistical analysis
The characteristics of the patients were described using medians and ranges for continuous variables and percentage frequencies for categorical variables. Cox multivariable analysis was performed to identify potential risk factors for the development of meningioma.
Given the ratio event/variable, a propensity score was forced into Cox multivariable analysis, in order to obtain more accurate results (D’Ascenzo et al. 2012). Variables selected for multivariable analysis were chosen according to the clinical experience and literature.
Results
Ninety patients in our cohort were treated with cranial radiotherapy. All patients received whole-brain radiation therapy (from 18 to 54 Gy); some of them (n = 30) received additional spinal treatment (from 18 to 47 Gy). Demographic and clinical characteristics are detailed in Table 1. The median follow-up was 24.6 years (range: 13.2–36.8 years). During follow-up, 15 patients developed a meningioma (Table 2). The median time elapsed between the pediatric cancer and the diagnosis of meningioma was 22.5 years (range 12.2–34.3 years). In four patients, meningioma was suspected on the basis of neurological symptoms (i.e., headache or seizures), whereas all other cases were discovered by MRI in asymptomatic patients. Multiple meningeal tumors were discovered in four CCS. In five patients, of whom three had mild symptoms only, the neuroimaging showed a giant meningioma (Fig. 1).
a, b CNS MRI (T2 and T1 with gadolinium images) showing a frontal cystic meningioma (gr I WHO) in a 39-year-old patient treated with whole-brain radiation therapy (25 Gy) for ALL when he was 13 months old. c, d Large frontal atypical meningioma (gr II WHO) in a 38-year-old patient who received whole-brain radiation therapy (18 Gy) for ALL when he was 10 years old
Ten patients (66.6 %) diagnosed with “suspected meningioma” on CT/MRI underwent surgery due to the large volume with mass effect or progressive enlargement of the lesion, or due to the onset of neurological symptoms. One patient died during anesthesia induction, due to cardiac arrhythmia.
The histological diagnosis was: meningioma grade I WHO in five of nine patients and atypical meningioma (grade II WHO) in four of nine.
After surgery, a recurrence or a residual disease was found in five of our patients. Two of them were re- operated, two were treated with stereotactic radiotherapy on the postoperative residual tumor, and one is still in follow-up (Table 2). One patient with multiple meningiomas, who previously underwent surgery and stereotactic radiotherapy, died because of the rapid growth of intracranial lesions. Non-operated meningiomas have been followed up with regular MRI scans, showing stable lesions without any neurological symptoms to date.
Five of 15 CCS with meningioma also developed a SN other than meningioma: thyroid cancer (three patients), melanoma (one patient), and basal cell carcinoma (one patient). Instead, a SN was diagnosed in ten out of the 75 CCS who did not develop a meningioma.
At multivariable analysis, the occurrence of SN other than meningioma was associated with the presence of meningioma, whereas no influence was demonstrated for age at pediatric cancer diagnosis, sex or cranial radiotherapy dose (Table 3).
Discussion
Our data confirm that CCS previously treated with cranial radiotherapy are at high risk of a subsequent development of meningioma. The prevalence of meningioma detected in our study (16.6 %, after a median follow-up of 24.6 years.) is quite similar to that reported in recently published series (Goshen et al. 2007; Banerjee et al. 2009; Sabin et al. 2014), but higher when compared to epidemiological studies based on cancer registries (Taylor et al. 2010).
It should be highlighted that none of our CCS developed high-grade CNS tumors. Accordingly to a previous report (Chowdhary et al. 2012), this is likely due to the young median age of our patients. Indeed, after brain exposure to ionizing radiation, younger patients are more likely to have lower-grade lesions (i.e., menigiomas) as a SN, while older patients show a higher risk to develop high-grade lesions.
In our series, meningiomas were suspected on the basis of clinical symptoms only in about 25 % of patients. Nevertheless, a significant proportion of the silent lesions were large, if not giant, meningiomas. In more than one half of our patients, a surgical resection was needed, due to initial tumor size, and/or progressive enlargement, and/or to the presence of neurological symptoms. We did not find any malignant menigioma; however, 26.6 % of our meningiomas showed atypical histology according to WHO classification (Louis et al. 2000), and more than 20 % of lesions were multiple. The results of our study confirm that radiation-induced meningiomas have a greater biological aggressiveness if compared with sporadic lesions (Sadetzki et al. 2002; Unmansky et al. 2008).
At multivariate analysis, RT dose did not impact on the risk of meningioma. This result, probably influenced by the small number of included patients, could also be explained considering that, in our cohort, brain irradiation doses were always >18 Gy. Indeed, it has been demonstrated that the risk of meningioma is stronger for doses higher than 9.9 Gy (Neglia et al. 2006; Taylor et al. 2010).
The causal relationship between ionizing radiation and meningioma is well known, as well as that between ionizing radiation and tumors at other sites (Sadetzki et al. 2002; Unmansky et al. 2008; Friedman et al. 2010; Nathan et al. 2010; Chowdhary et al. 2012); thus, a common mechanism initiating carcinogenesis at different sites (including extra-CNS sites) should be hypothesized. At least three different putative molecular mechanisms have been postulated in CCS. Recently, the telomere content was assessed in CCS who developed secondary breast cancer, thyroid cancer, or sarcoma, following radiotherapy; in these patients, a reduction in telomere content was reported. The authors suggest that shortening of telomeres could be a predisposing factor for the development of SNs but also that, taking into consideration further age-related telomere shortening, in the next 5–10 years, the number of CCS developing SNs could increase (Gramatges et al. 2014). Another recent study reported that higher basal and post-irradiation DNA double-strand break levels were associated with risk of SNs in CCS. It was suggested that CCS cells present a clear defect in double-strand breaks (DSB) repair that could predispose to the development of SNs (Haddy et al. 2014).
It is known that several meningiomas present a complex pattern of gene copy number changes and rearrangements. In particular, the tumor suppressor NF2 gene is inactivated in approximately half of meningiomas, while in an additional 8 %, epigenetic alteration of NF2 is observed (Brastianos et al. 2013). Furthermore, epigenetic alteration, mainly hyper/hypomethylation of specific DNA regions (e.g., inactivation of tumor suppressor gene TIMP3, hypermethylation of TP73 gene interacting with cell cycle control), have been associated with meningioma development, progression, and recurrence (He et al. 2013).
One can speculate that CCS might harbor modifications of their DNA repair and/or of epigenetic mechanisms, both induced by radiotherapy, which initiate and maintain tumor development at different sites.
Regardless of the mechanism of radiation-induced carcinogenesis, the high prevalence of meningioma found in our cohort suggests the need for serial brain imaging studies in CCS who underwent cranial irradiation. Many factors must be considered when a screening program is proposed such as: prevalence and severity of the disease, potential reduction in morbidity and mortality associated with early detection, sensitivity, specificity, predictive value and cost of the screening program. On one hand, it should be taken into account that incidental detection of small and slowly growing lesions could induce anxiety in asymptomatic CCS (Sugden et al. 2014); on the other hand, a potential benefit of an early detection could be expected in terms of reduction of surgery-related morbidity, especially in case of giant and asymptomatic meningiomas. Our cohort is too small to draw conclusions regarding the need for a screening with MRI in CCS who were previously treated with cranial radiotherapy, and prospective studies defining the risk of meningioma in larger cohorts of CCS are warranted. Looking forward to definitive recommendations in this field, we would like to emphasize that CCS at risk of the development of meningioma, especially those affected by different SNs, deserve close clinical follow-up in order to promptly recognize the often subtle neurological symptoms and avoid delayed diagnosis of large lesions.
References
Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL et al (2009) Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol 27:2328–2338
Banerjee J, Pääkkö E, Harila M, Herva R, Tuominen J, Koivula A, Lanning M, Harila-Saari A (2009) Radiation-induced meningiomas: a shadow in the success story of childhood leukemia. Neuro Oncol 11:543–549
Brastianos PK, Horowitz PM, Santagata S, Jones RT, McKenna A, Getz G, Ligon KL, Palescandolo E, Van Hummelen P, Ducar MD, Raza A, Sunkavalli A, Macconaill LE, Stemmer-Rachamimov AO, Louis DN, Hahn WC, Dunn IF, Beroukhim R (2013) Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat Genet 45:285–289
Brignardello E, Felicetti F, Castiglione A, Chiabotto P, Corrias A, Fagioli F, Ciccone G, Boccuzzi G (2013) Endocrine health conditions in adult survivors of childhood cancer: the need for specialized adult-focused follow-up clinics. Eur J Endocrinol 168:465–472
Chowdhary A, Spence AM, Sales L, Rostomily RC, Rockhill JK, Silbergeld DL (2012) Radiation associated tumors following therapeutic cranial radiation. Neurol Int 3:48
D’Ascenzo F, Cavallero E, Biondi-Zoccai G, Moretti C, Omedè P, Bollati M, Castagno D, Modena MG, Gaita F, Sheiban I (2012) Use and misuse of multivariable approaches in interventional cardiology studies on drug-eluting stents: a systematic review. J Interv Cardiol 25:611–621
Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, Donaldson SS, Meadows AT, Robison LL, Neglia JP (2010) Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 102:1083–1095
Goshen Y, Stark B, Kornreich L, Michowiz S, Feinmesser M, Yaniv I (2007) High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer 49:294–297
Gramatges MM, Liu Q, Yasui Y, Okcu MF, Neglia JP, Strong LC, Armstrong GT, Robison LL, Bhatia S (2014) Telomere content and risk of second malignant neoplasm in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Clin Cancer Res 20:904–911
Haddy N, Tartier L, Koscielny S, Adjadj E, Rubino C, Brugières L, Pacquement H, Diallo I, de Vathaire F, Averbeck D, Hall J, Benhamou S (2014) Repair of ionizing radiation-induced DNA damage and risk of second cancer in childhood cancer survivors. Carcinogenesis 35:1745–1749
He S, Pham MH, Pease M, Zada G, Giannotta SL, Wang K, Mack WJ (2013) A review of epigenetic and gene expression alterations associated with intracranial meningiomas. Neurosurg Focus 35:E5
Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ (2006) Cancer statistics. CA Cancer J Clin 56:106–130
Kaatsch P, Debling D, Blettner M, Spix C (2009) Second malignant neoplasms after childhood cancer in Germany—results from the long-term follow-up of the German Childhood Cancer Registry. Strahlenther Onkol 185:8–10
Lawless SC, Verma P, Green DM, Mahoney MC (2007) Mortality experiences among 15+ year survivors of childhood and adolescent cancers. Pediatr Blood Cancer 48:333–338
Louis DN, Scheithauer BW, Budka H, von Deimling A, Kepes JJ (2000) Meningiomas. World Health Organisation classification of tumours. In: Kleihues P, Cavanee WK (eds) Pathology and genetics: tumours of the nervous system. IARC Press, Lyon, pp 176–189
Nathan PC, Ness KK, Mahoney MC, Li Z, Hudson MM, Ford JS, Landier W, Stovall M, Armstrong GT, Henderson TO, Robison LL, Oeffinger KC (2010) Screening and surveillance for second malignant neoplasms in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study (CCSS). Ann Intern Med 153:442–451
Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, Yasui Y, Kasper CE, Mertens AC, Donaldson SS, Meadows AT, Inskip PD (2006) New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 98:1528–1537
Olsen JH, Moller T, Anderson H, Langmark F, Sankila R, Tryggvadottir L et al (2009) Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst 101:806–813
Reulen RC, Frobisher C, Winter DL, Kelly J, Lancashire ER, Stiller CA, Pritchard-Jones K, Jenkinson HC, Jenkinson HC, Hawkins MM, British Childhood Cancer Survivor Study Steering Group (2011) Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 305:2311–2319
Sabin ND, Santucci AK, Klimo P Jr, Hudson MM, Srivastava D, Zhang N, Kun LE, Krasin MJ, Pui CH, Patay Z, Reddick WE, Ogg RJ, Hillenbrand CM, Robison LL, Krull KR, Armstrong GT (2014) Incidental detection of late subsequent intracranial neoplasms with magnetic resonance imaging among adult survivors of childhood cancer. J Cancer Surviv 8:329–335
Sadetzki S, Flint-Richter P, Ben-Tal T, Nass D (2002) Radiation-induced meningioma: a descriptive study of 253 cases. J Neurosurg 97:1078–1082
Sugden E, Taylor A, Pretorius P, Kennedy C, Bhangoo R (2014) Meningiomas occurring during long-term survival after treatment for childhood cancer. JRSM Open 5:1–4
Taylor AJ, Little MP, Winter DL, Sugden E, Ellison DW, Stiller CA, Stovall M, Frobisher C, Lancashire ER, Reulen RC, Hawkins MM (2010) Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol 28:5287–5293
The Children’s Oncology Group (2008) Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult. Version 3.0 http://www.survivorshipguidelines.org. Accessed 15 March 2014
Unmansky F, Shoshag Y, Rosenthal G, Fraifeld S, Spektor S (2008) Radiation-induced meningioma. Neurosurg Focus 24:E7
Acknowledgments
The authors would like to acknowledge U.G.I. (Unione Genitori Italiani contro il tumore dei bambini), for the ongoing support to clinical and research activities at the Transition Unit for Childhood Cancer Survivors.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Felicetti, F., Fortunati, N., Garbossa, D. et al. Meningiomas after cranial radiotherapy for childhood cancer: a single institution experience. J Cancer Res Clin Oncol 141, 1277–1282 (2015). https://doi.org/10.1007/s00432-015-1920-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-015-1920-7