Abstract
Fidgety movements provide early information about a potential development of cerebral palsy in preterm neonates. The aim was to assess differences in the combined outcome of mortality and fidgety movements defined as normal or pathological in very preterm neonates according to the group allocation in the randomised-controlled multicentre COSGOD III trial. Preterm neonates of two centres participating in the COSGOD III trial, whose fidgety movements were assessed as normal or pathological at six to 20 weeks of corrected age, were analysed. In the COSGOD III trial cerebral oxygen saturation (crSO2) was measured by near-infrared spectroscopy (NIRS) during postnatal transition and guided resuscitation in preterm neonates randomised to the NIRS-group, whereby medical support was according routine, as it was also in the control group. Fidgety movements were classified in normal or abnormal/absent at six to 20 weeks of corrected age. Mortality and fidgety movements of preterm neonates allocated to the NIRS-group were compared to the control-group. Normal outcome was defined as survival with normal fidgety movements. One-hundred-seventy-one preterm neonates were included (NIRS-group n = 82; control-group n = 89) with a median gestational age of 29.4 (27.4–30.4) and 28.7 (26.7–31.0) weeks in the NIRS-group and the control-group, respectively. There were no differences in the combined outcome between the two groups: 90.2% of the neonates in the NIRS-group and 89.9% in the control-group survived with normal outcome (relative risk [95% CI]; 0.96 [0.31–2.62]).
Conclusions: In the present cohort of preterm neonates, monitoring of crSO2 and dedicated interventions in addition to routine care during transition period after birth did not show an impact on mortality and fidgety movements defined as normal or pathological at six to 20 weeks corrected age.
What is Known • Fidgety movements display early spontaneous motoric pattern and may provide early information about a potential development of cerebral palsy in preterm neonates. |
What is New • This retrospective observational study of the randomised-controlled multicentre COSGOD III trial is the first study investigating the potential influence of cerebral oxygenation guided resuscitation during postnatal transition period on combined outcome of mortality and fidgety movements up to 20 weeks of corrected age in very preterm neonates. • This study adds to the growing interest of assessing cerebral oxygenation, that monitoring of cerebral oxygen saturation and dedicated interventions during postnatal transition period according to the COSGOD III trial has no significant influence on mortality and fidgety movements defined as normal or pathological in very preterm neonates. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Very premature birth is associated with a higher risk of impaired neurodevelopmental outcome following a higher rate of neonatal morbidities occurring during the neonatal period [1,2,3,4]. Early identification of abnormal neurological behaviour may have a positive impact on long-term neurodevelopmental outcome due to optimising neuroplasticity of the immature brain by accompanying therapies [5]. The Prechtl General Movement Assessment (GMA) performed until 20 weeks of corrected age is of increasing interest to provide predictive information about neonates concerning their neurodevelopment, including impaired neurodevelopmental outcome, cerebral palsy, minor neurological deficits or cognitive impairment [6,7,8,9,10]. General Movements (GMs) are spontaneous and complex movements that can be detected from early fetal life until four to five months of post-term age [5, 7, 11]. The repertoire of spontaneous movement patterns is described in the “global GMA” according to their age: writhing movements (after birth until six to nine weeks of corrected age) and fidgety movements (six to nine weeks until 14 to 20 weeks of corrected age).
Between six and nine weeks after birth, the writhing movements undergo a transformation into fidgety movements. Fidgety movements are characterised by circular movements with a small amplitude, moderate speed, and variable acceleration involving the neck, trunk and limbs, and can be observed until 15 to 20 weeks of corrected age. Fidgety movements can be classified as either normal or pathological. Pathological fidgety movements are further divided into mainly two categories: absence of fidgety movements (no observation of fidgety movements between six to 20 weeks of corrected age), or abnormal fidgety movements, which resemble normal fidgety movements but present themselves with irregular amplitude, speed and jerkiness [8, 12].
The impact of cerebral oxygenation using near-infrared spectroscopy (NIRS) during immediate transition after birth on neurological outcome has been described in two small observational studies analysing cerebral ultrasound findings [13] and GMA [14]. Analysing general movement optimality score (GMOS) as a semi-quantitative method showed impaired GMs, expressed by a lower GMOS, in association with increased burden of cerebral hypoxia within the first 15 min after birth [14].
The reduction of burden of cerebral hypoxia during the immediate transition period, that might improve neurological outcome by cerebral oxygen saturation (crSO2) monitoring guided resuscitation of preterm neonates, has been demonstrated by Pichler et al. 2016 in a randomised pilot feasibility trial (Cerebral Oxygen Saturation to Guide Oxygen Delivery—COSGOD II trial) [15]. In the COSGOD III trial [16], the aim was to increase survival without cerebral injury by crSO2 guided resuscitation. In this randomised-controlled trial, there was a non-significant difference of 4.3% in survival without cerebral injury between the intervention group and the control group.
Beside cerebral injury, various factors including perinatal asphyxia, chorioamnionitis, moderate-to-severe bronchopulmonary dysplasia (BPD) and/or prolonged invasive respiratory support, may contribute to poor neurodevelopmental outcome [17,18,19].
The aim of the present study was to assess combined outcome defined by mortality until 20 weeks of corrected age and fidgety movements between six to 20 weeks of corrected age in preterm neonates included in the COSGOD III trial. We hypothesised that crSO2 guided transition in addition to routine care during immediate fetal-to-neonatal transition in preterm neonates reduces mortality and/or leads to a more frequent appearance of normal fidgety movements, compared to preterm neonates treated according routine alone.
Methods
Study design
In the present retrospective observational study neonates included in the prospective randomised-controlled COSGOD III multicentre trial, conducted between October 2017 and February 2022, were eligible. The protocol [20] and the primary outcome [16] of the COSGOD III trial have already been published elsewhere.
For this retrospective observational study combined outcome, defined as mortality until 20 weeks of corrected age and fidgety movements performed between six to 20 weeks of corrected age were analysed and compared between the intervention (NIRS) group and control group of the COSGOD III trial. Out of eleven participating centres in the COSGOD III trial two centres performed GMA routinely and were therefore eligible for this analysis: Division of Neonatology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Austria and Department of Pediatrics II, Medical University of Innsbruck, Austria. The present study, as an ancillary study to the COSGOD III trial, has been approved by the Regional Committee on Biomedical Ethics of the Medical University of Graz (EC number: 35–438 ex 22/23) and Medical University of Innsbruck (EC number: 1264/2023) and was conducted in accordance with the Declaration of Helsinki. The prospective randomised-controlled COSGOD III multicentre trial was registered at Clinical Trials (Number: NCT06105333).
COSGOD III trial
Preterm neonates < 32 weeks of gestational age, were included in the COSGOD III trial and obtained continuous measurement of cerebral oxygenation during the first 15 min after birth. Before birth, preterm neonates were randomised either to the NIRS group or to the control group. In the intervention group (NIRS group) resuscitation was conducted in accordance with local guidelines and/or with the latest “Resuscitation Consensus Guidelines” [21, 22]. CrSO2 monitoring was visible to the clinical team. Provided that SpO2 was within targets, medical support was changed if crSO2 was less than the 10th centile or above the 90th centile. In the control group, crSO2 values were not visible to the clinical team and resuscitation was performed according to routine. The methods of the COSGOD III trial have been described in more detail elsewhere [16, 20].
Inclusion and exclusion criteria for the present study
Centres that participated in the COSGOD III trial with available data on mortality and routine assessments of fidgety movements between six to 20 weeks of corrected age were included.
Centres with no available data on fidgety movements were excluded from analyses a priori.
Demographic data and neonatal morbidities
Demographic data and neonatal morbidities including cerebral injury and all-cause mortality assessed in the COSGOD III trial were analysed in the present study. Cerebral injury was defined as intraventricular haemorrhage (IVH grade I-IV), or cystic periventricular leukomalacia (cystic PVL grade II-III). Further documented morbidities of the COSGOD III trial were respiratory distress syndrome (IRDS grade I-IV), culture proven sepsis, necrotizing enterocolitis (NEC), BPD defined as oxygen dependency or need of respiratory support at 36 weeks corrected age, retinopathy of prematurity (ROP ≥ grade II) and persistent ductus arteriosus (PDA) with medical and/or surgical intervention.
Fidgety movements
Assessment of fidgety movements was performed between six to 20 weeks of corrected age by video recording of sequences of at least three minutes. The neonates were recorded after feeding, during periods of active wakefulness and lied in a supine position. The assessment had to be restarted when the neonates started crying, fussing or they were in suckling periods. Fidgety movements were documented by clinical staff trained and certified for GMA, who were blinded for the allocation of the neonate in the COSGOD III trial. Fidgety movements were stratified as either normal or pathological, whereby pathological fidgety movements were further divided into two categories: absent (no observation of fidgety movements between six to 20 weeks of corrected age) or abnormal (resembling normal fidgety movements but with irregular amplitude, speed and jerkiness) [8, 12].
Primary outcome
The primary outcome of the present study was the combined outcome of mortality before 20 weeks of corrected age and the appearance of fidgety movements between six to 20 weeks of corrected age. Normal outcome was defined as survival with normal fidgety movements. Poor outcome was defined as mortality or absent/abnormal fidgety movements.
Secondary outcomes
Survival without cerebral injury (IVH, cystic PVL), mortality, IRDS, sepsis, NEC, BPD, ROP and PDA were defined as secondary outcome parameters and were compared between the NIRS group and the control group in the present cohort.
Statistics
Baseline characteristics of neonates are given with median and interquartile range for continuous data and numbers and percentages for categorical data. Comparison of baseline characteristics were done using Mann Whitney U-test for continuous data and Chi-square test or Fisher’s exact test for categorical data. To answer the hypothesis whether combined outcome (mortality and fidgety movements) differ between neonates of the NIRS group and neonates of the control group Chi-square test was used. Relative Risk (RR) with 95% confidence interval (95% CI) were calculated. Secondary parameters were compared between the two groups using Mann Whitney U-test for continuous data and Chi-square test or Fisher’s exact test for categorical data. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
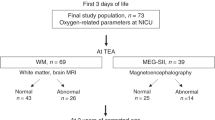
In the two centres, 178 preterm neonates (Graz n = 112; Innsbruck n = 66) were included into the COSGOD III trial and were therefore eligible for the present study. Seven preterm neonates were excluded as there was no assessment of fidgety movements performed between six to 20 weeks of corrected age (Graz n = 4, Innsbruck n = 3). Thus, for the final analysis 82 preterm neonates were included in the NIRS group (Graz n = 52; Innsbruck n = 30) and 89 in the control group (Graz n = 56; Innsbruck n = 33) (Fig. 1. Study flow chart).
Demographic data of the included neonates are presented in Table 1. The median gestational age was 29.4 (27.4–30.4) weeks in the NIRS group and 28.7 (26.7–31.0) weeks in the control group. The median birth weight was 1190 (900–1450) grams and 1135 (900–1420) grams in the NIRS group and in the control group, respectively. There were no significant differences in gestational age (p = 0.372) and birth weight (p = 0.344).
In the NIRS group 89.0% (n = 73) and in the control group 94.4% (n = 84) (p = 0.202) were delivered by Caesarean section and 11.0% (n = 9) and 5.6% (n = 5) in the NIRS group and in the control group were delivered spontaneously, respectively. The time until cord clamping did not differ between the groups (p = 0.708). Cord clamping was performed at less than 30 s in 80.8% (n = 63) in the NIRS group and in 75.3% (n = 61) in the control group. The cord was clamped after 30 to 60 s in 3.9% (n = 3) in the NIRS group and in 4.9% (n = 4) in the control group, whereby cord clamping was delayed more than 60 s in 15.4% (n = 12) in the NIRS group and in 19.8% (n = 16) in the control group. Maternal and fetal causes for preterm birth are displayed in Table 1.
Provided respiratory support and medications during the first 15 min after birth and within the first 24 h after birth are presented in Table 2.
Primary outcome
Fidgety movements were analysed at 12.1 (11.9–12.7) weeks of corrected age in the NIRS group and at 12.1 (12.0–12.6) weeks in the control group (p = 0.819). No difference in the combined primary outcome (survival and normal fidgety movements between six to 20 weeks of corrected age) was observed comparing the NIRS group to the control group (RR [95%CI] 0.96 [0.31–2.62], p = 0.938) (Table 3).
Secondary outcome
Eight preterm neonates died before 20 weeks of corrected age (n = 4 NIRS group; n = 4 control group). Survival without cerebral injury was 90.2% in the NIRS group and 80.9% in the control group (RR [95% CI] 1.12 [0.99–1.26], p = 0.084). Secondary outcomes including IVH, cystic PVL, IRDS, EOS, NEC, BPD, ROP and PDA with interventions are displayed in Table 3.
Discussion
This is the first study, investigating the potential influence of cerebral oxygenation guided resuscitation during immediate fetal-to-neonatal transition period on combined outcome of mortality and fidgety movements defined as normal or pathological in very preterm neonates. We did not observe substantial impact on mortality and fidgety movements in the NIRS group when compared to the control group.
Pathological fidgety movements, especially their absence, have been described as a predictive value for a later development of cerebral palsy [23, 24]. Whereby, a combination of both, cerebral injury and absence of fidgety movements have the highest predictive value. Abnormal GMs or absence of fidgety movements in preterm neonates with cerebral morbidities, especially PVL, are explained by disruptions of the corticospinal projection as a consequence of brain lesions [25, 26]. In our present cohort, the proportion of analysed preterm neonates with cerebral injuries was quite low. Therefore, the overall risk for the development of a cerebral palsy in our observed cohort is very low, and it may be speculated that the results of the present study may be more pronounced in cases of a higher proportion of cerebral injuries.
Beside the high predictive value for cerebral palsy in case of absent fidgety movements, no significant association of abnormal/absent fidgety movements and Bayley Scales of Infant and Toddler Development-Third Edition (BSID-III) has been described [27]. The potential influence of crSO2 on long-term outcome at a corrected age of two years, assessed by BSID-III has been observed by Wolfsberger et al. [28]. They stated that preterm neonates with a very low gestational age and birth weight with poor long-term outcome (mortality, testing not possible due to cognitive impairment and/or BSID-III ≤ 70), showed significant lower crSO2 values during immediate fetal-to-neonatal transition period, when being compared to preterm neonates with favourable outcome.
The results of fidgety movements between six to 20 weeks of corrected age can be influenced by different perinatal factors and morbidities. The predictive value of fidgety movements also depends on the postnatal age / corrected age of the neonate at time point of assessment [25]. The mean corrected age at time point of assessment of fidgety movements of the included preterm neonates in our cohort was 12 weeks. According to literature, the optimal period for evaluating fidgety movements is generally regarded between ten to 12 weeks post-term [8]. Based on that, results of fidgety movements demonstrated in the present study were assessed in the ideal period concerning evaluation of neurobehavioral repertoire. Beside postnatal age, severe infections during early neonatal period may have an impact on fidgety movements, with a higher proportion of abnormal/absent fidgety movements being observed in neonates with infections [29]. In the present study no difference in culture proven sepsis was described between the two groups. Therefore, the similar distribution of this influencing factor can be reassuring for interpretation of the present results. Furthermore, a retrospective study described a higher percentage of abnormal fidgety movements in very preterm neonates who received systemic corticosteroids compared to neonates of the non-corticosteroid group [30]. In our present study, unfortunately no information about therapy with systemic corticosteroids were available. Gestational age and severity of illnesses, however, were similar between the two groups suggesting also similar corticosteroid application.
It has been described that serial GMAs provide a more accurate information about the early neurodevelopmental state of the analysed neonate [31, 32]. Changes in the quality of the GMs, can be influenced by different factors, including provided medications or interventions, as described above. Multiple GMAs at different time points might help to rule out these potential influencing factors [31, 32]. In our present cohort of neonates, serial GMAs have only been performed in a few preterm neonates, therefore serial GMA analysis was not possible, which might be a limiting factor.
Taking the results of the present study into account it may be assumed that crSO2 monitoring with dedicated interventions according to the COSGOD III trial cannot influence mortality up to 20 weeks and the appearance of fidgety movements between six to 20 weeks of corrected age in very preterm neonates. This is in contrast to already published data observing the potential influence of burden of cerebral hypoxia within the first 15 min after birth on GMA, and GMOS performed between 36 and 40 weeks postmenstrual age [14]. These results suggest that cerebral oxygenation during immediate fetal-to-neonatal transition period affect GMs at term age. Pansy et al. [14] suggested burden of hypoxia defined by crSO2 values below the 10th percentile [33] might have an influence on cerebral injury and thus on neonatal neurodevelopmental outcome. Different assumptions may explain the discrepancy between our present findings to already published data describing an influence of crSO2 on short-term neurological outcome. Writhing movements, as investigated in the study of Pansy et al. [14], and fidgety movements cannot be equated. Souza et al. [34] observed potential correlation between writhing movements and fidgety movements in context to assess the time point when writhing movements are predictive for fidgety movements. They described that abnormal writhing movements at the neonatal intensive care unit or up to five weeks of corrected age, were only in 85% in accordance to absent fidgety movements nine to 20 weeks of corrected age.
The major strength of the study is the amount of overall included preterm neonates with available fidgety movements (n = 163) of a multicentre study. The main limitation of this study was the overall number of analysed preterm neonates with abnormal/absent fidgety movements. This, however, may be explained by the overall low proportion of preterm neonates with a cerebral injury, especially with a diagnosis of cystic PVL.
Conclusion
Cerebral oxygen saturation monitoring combined with dedicated treatment guidelines in accordance to the protocol of the COSGOD III trial during immediate fetal-to-neonatal transition period was not associated with an improvement on combined outcome, death and/or pathological fidgety movements between six to 20 weeks of corrected age.
Data availability
Data are available on reasonable request.
Abbreviations
- BSID-III :
-
Bayley Scales of Infant and Toddler Development-Third Edition
- BPD:
-
Bronchopulmonary dysplasia
- crSO2 :
-
Cerebral oxygen saturation
- GMA:
-
General Movement Assessment
- GMOS:
-
General movement optimality score
- GMs :
-
General Movements
- IRDS :
-
Infant respiratory distress syndrome
- IVH :
-
Intraventricular haemorrhage
- NIRS:
-
Near-infrared spectroscopy
- NEC :
-
Necrotizing enterocolitis
- PVL :
-
Periventricular leukomalacia
- PDA:
-
Persistent ductus arteriosus
- ROP:
-
Retinopathy of prematurity
References
Pascal A, Govaert P, Oostra A, Naulaers G, Ortibus E, Van den Broeck C (2018) Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: a meta-analytic review. Dev Med Child Neurol 60(4):342–355. https://doi.org/10.1111/dmcn.13675
Rogers EE, Hintz SR (2016) Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol 40(8):497–509. https://doi.org/10.1053/j.semperi.2016.09.002
Platt MJ (2014) Outcomes in preterm infants. Public Health 128(5):399–403. https://doi.org/10.1016/j.puhe.2014.03.010
Asztalos EV, Church PT, Riley P, Fajardo C, Shah PS, Network CN, Investigators C-U (2017) Neonatal Factors Associated with a Good Neurodevelopmental Outcome in Very Preterm Infants. Am J Perinatol 34(4):388–396. https://doi.org/10.1055/s-0036-1592129
Glass HC, Li Y, Gardner M, Barkovich AJ, Novak I, McCulloch CE, Rogers EE (2021) Early Identification of Cerebral Palsy Using Neonatal MRI and General Movements Assessment in a Cohort of High-Risk Term Neonates. Pediatr Neurol 118:20–25. https://doi.org/10.1016/j.pediatrneurol.2021.02.003
Olsen JE, Allinson LG, Doyle LW, Brown NC, Lee KJ, Eeles AL, Cheong JLY, Spittle AJ (2018) Preterm and term-equivalent age general movements and 1-year neurodevelopmental outcomes for infants born before 30 weeks’ gestation. Dev Med Child Neurol 60(1):47–53. https://doi.org/10.1111/dmcn.13558
Aizawa CYP, Einspieler C, Genovesi FF, Ibidi SM, Hasue RH (2021) The general movement checklist: A guide to the assessment of general movements during preterm and term age. J Pediatr (Rio J) 97(4):445–452. https://doi.org/10.1016/j.jped.2020.09.006
Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D (1997) An early marker for neurological deficits after perinatal brain lesions. Lancet 349(9062):1361–1363. https://doi.org/10.1016/S0140-6736(96)10182-3
Bosanquet M, Copeland L, Ware R, Boyd R (2013) A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol 55(5):418–426. https://doi.org/10.1111/dmcn.12140
Bruggink JL, Einspieler C, Butcher PR, Van Braeckel KN, Prechtl HF, Bos AF (2008) The quality of the early motor repertoire in preterm infants predicts minor neurologic dysfunction at school age. J Pediatr 153(1):32–39. https://doi.org/10.1016/j.jpeds.2007.12.047
Prechtl HF (2001) General movement assessment as a method of developmental neurology: new paradigms and their consequences. The, 1999 Ronnie MacKeith lecture. Dev Med Child Neurol 43(12):836–842
Einspieler C, Prechtl HF, Ferrari F, Cioni G, Bos AF (1997) The qualitative assessment of general movements in preterm, term and young infants–review of the methodology. Early Hum Dev 50(1):47–60. https://doi.org/10.1016/s0378-3782(97)00092-3
Baik N, Urlesberger B, Schwaberger B, Schmölzer GM, Avian A, Pichler G (2015) Cerebral haemorrhage in preterm neonates: does cerebral regional oxygen saturation during the immediate transition matter? Arch Dis Child Fetal Neonatal Ed 100(5):F422–F427. https://doi.org/10.1136/archdischild-2014-307590
Pansy J, Baik N, Schwaberger B, Scheuchenegger A, Pichler-Stachl E, Avian A, Schmölzer GM, Urlesberger B, Pichler G (2017) Cerebral hypoxia during immediate transition after birth and short term neurological outcome. Early Hum Dev 110:13–15. https://doi.org/10.1016/j.earlhumdev.2017.04.009
Pichler G, Urlesberger B, Baik N, Schwaberger B, Binder-Heschl C, Avian A, Pansy J, Cheung PY, Schmölzer GM (2016) Cerebral Oxygen Saturation to Guide Oxygen Delivery in Preterm Neonates for the Immediate Transition after Birth: A 2-Center Randomized Controlled Pilot Feasibility Trial. J Pediatr 170:73–8.e1–4. https://doi.org/10.1016/j.jpeds.2015.11.053. Erratum in: (2017) J Pediatr 187:338. https://doi.org/10.1016/j.jpeds.2017.05.028.
Pichler G, Goeral K, Hammerl M et al (2023) Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): multicentre randomised phase 3 clinical trial. BMJ 380:e072313. https://doi.org/10.1136/bmj-2022-072313.Erratum.In:(2023)BMJ381:p1102
Hee Chung E, Chou J, Brown KA (2020) Neurodevelopmental outcomes of preterm infants: a recent literature review. Transl Pediatr 9(Suppl 1):S3–S8. https://doi.org/10.21037/tp.2019.09.10
Woythaler M (2019) Neurodevelopmental outcomes of the late preterm infant. Semin Fetal Neonatal Med 24(1):54–59. https://doi.org/10.1016/j.siny.2018.10.002
Hollanders JJ, Schaëfer N, van der Pal SM, Oosterlaan J, Rotteveel J, Finken MJJ, on behalf of the Dutch POPS-19 Collaborative Study Group (2019) Long-Term Neurodevelopmental and Functional Outcomes of Infants Born Very Preterm and/or with a Very Low Birth Weight. Neonatology 115(4):310–319. https://doi.org/10.1159/000495133
Pichler G, Baumgartner S, Biermayr M et al (2019) Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial. Trials 20(1):178. https://doi.org/10.1186/s13063-019-3258-y
J Wyllie J Bruinenberg CC Roehr M Rüdiger D Trevisanuto B Urlesberger 2015 European Resuscitation Council Guidelines for Resuscitation 2015: Section 7 Resuscitation and support of transition of babies at birth Resuscitation 95 249 263 https://doi.org/10.1016/j.resuscitation.2015.07.029
Madar J, Roehr CC, Ainsworth S et al (2021) European Resuscitation Council Guidelines 2021: Newborn resuscitation and support of transition of infants at birth. Resuscitation 161:291–326. https://doi.org/10.1016/j.resuscitation.2021.02.014
Prechtl HF (1990) Qualitative changes of spontaneous movements in fetus and preterm infant are a marker of neurological dysfunction. Early Hum Dev 23(3):151–158. https://doi.org/10.1016/0378-3782(90)90011-7
Spittle AJ, Spencer-Smith MM, Cheong JL, Eeles AL, Lee KJ, Anderson PJ, Doyle LW (2013) General movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics 132(2):e452–e458. https://doi.org/10.1542/peds.2013-0177
Ferrari F, Frassoldati R, Berardi A, Di Palma F, Ori L, Lucaccioni L, Bertoncelli N, Einspieler C (2016) The ontogeny of fidgety movements from 4 to 20weeks post-term age in healthy full-term infants. Early Hum Dev 103:219–224. https://doi.org/10.1016/j.earlhumdev.2016.10.004
Ferrari F, Cioni G, Einspieler C, Roversi MF, Bos AF, Paolicelli PB, Ranzi A, Prechtl HF (2002) Cramped synchronized general movements in preterm infants as an early marker for cerebral palsy. Arch Pediatr Adolesc Med 156(5):460–467. https://doi.org/10.1001/archpedi.156.5.460
Teschler U, Dathe AK, Heuser-Spura KM, Bialas J, Cordier LJ, Albayrak B, Felderhoff-Mueser U, Huening BM (2023) General Movements trajectories and outcome at 12 months in very preterm infants: An analysis of tendencies and pathological persistence. Sci Rep 13(1):21825. https://doi.org/10.1038/s41598-023-49037-w
Wolfsberger CH, Pichler-Stachl E, Höller N, Mileder LP, Schwaberger B, Avian A, Urlesberger B, Pichler G (2023) Cerebral oxygenation immediately after birth and long-term outcome in preterm neonates-a retrospective analysis. BMC Pediatr 23(1):145. https://doi.org/10.1186/s12887-023-03960-z
Skworc A, Marciniak S, Sławska H (2020) Influence of infections on the quality of general movements in premature infants. Early Hum Dev 148:105118. https://doi.org/10.1016/j.earlhumdev.2020.105118
Esterman E, Goyen TA, Jani P, Lowe G, Baird J, Maheshwari R, D’Cruz D, Luig M, Shah D (2023) Systemic postnatal corticosteroid use for the prevention of bronchopulmonary dysplasia and its relationship to early neurodevelopment in extremely preterm infants. World J Pediatr 19(6):586–594. https://doi.org/10.1007/s12519-023-00708-8
Olsen JE, Brown NC, Eeles AL, Lee KJ, Anderson PJ, Cheong JL, Doyle LW, Spittle AJ (2015) Trajectories of general movements from birth to term-equivalent age in infants born <30 weeks’ gestation. Early Hum Dev 91(12):683–688. https://doi.org/10.1016/j.earlhumdev.2015.09.009
Einspieler C, Prechtl HF (2005) Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev 11(1):61–67. https://doi.org/10.1002/mrdd.20051
Pichler G, Binder C, Avian A, Beckenbach E, Schmölzer GM, Urlesberger B (2013) Reference ranges for regional cerebral tissue oxygen saturation and fractional oxygen extraction in neonates during immediate transition after birth. J Pediatr 163(6):1558–1563. https://doi.org/10.1016/j.jpeds.2013.07.007
Souza F A, Nogueira C CL, Silva A J, Chagas P SC, Frônio J S (2021) Preterm and writhing movements: is it possible to predict fidgety movements in preterm infants? J Perinatol 41(10):2442–2448. https://doi.org/10.1038/s41372-021-01064-z
Acknowledgements
We would like to express our gratitude to the parents for giving permission to investigate their infants and to the team of midwives, nurses, laboratory staff, and physicians involved in their care.
Collaborators
Division of Neonatology, Department of Pediatrics, Medical University of Graz, Graz, Austria: Marlies Bruckner, Corinna Binder-Heschl, Daniel Pfurtscheller, Johann Martensen, Nina Höller, Evelyn Ziehenberger, Lukas Mileder;
Comprehensive Center for Pediatrics, Department of Pediatrics and Adolescent Medicine, Division of Neonatology, Intensive Care and Neuropediatrics, Medical University of Vienna, Vienna, Austria: Katharina Goeral, Julia Buchmayer, Katrin Klebermass-Schrehof, Angelika Berger (local principal investigator), Sigrid Baumgartner, Agnes Grill, Michaela Mayr, Judith Rittenschober-Boehm, Michael Schneider;
Department of Pediatrics II, Neonatology, Medical University of Innsbruck, Innsbruck, Austria: Christina Schreiner, Vera Neubauer, Peter Wöckinger, Anna Posod;
Neonatal Intensive Care Unit, Department for Perinatology, Division of Gynaecology and Obstetrics, University Medical Centre Ljubljana, Slovenia:
Tina Perme, Lilijana Kornhauser-Cerar, Anja Marolt, Ana Dimnik, Vlasta Lubej Kurtovič;
Infant Centre, University College Cork, Cork University Maternity Hospital, Cork, Ireland:
Eugene M Dempsey, Christoph E Schwarz, Garvey Aisling, Jurate Panaviene, David Healy, Nahla Ahmed, Ita Herlihy;
Department of Neonatology, University Children’s Hospital of Tübingen, Germany:
Laila Springer, Kerstin Gründler, Axel Franz;
Neonatologia e Terapia Intensiva Neonatale (TIN) Ospedale dei Bambini “V.Buzzi,” Milano, Italia: Gianluca Lista, Ilaria Stucchi, Francesca Castoldi, Francesco Cavigioli;
II Department of Neonatology, Neonatal Biophysical Monitoring and Cardiopulmonary Therapies Research Unit, Chair of Neonatology, Poznan University of Medical Sciences, Poznan, Poland:
Tomasz Szczapa, Lukasz Karpinski.
Ginekologiczno Położniczy Szpital Kliniczny Uniwersytetu Medycznego im. Karola Marcinkowskiego w Poznańiu, Poznań, Poland: Zuzanna Kozłowska, Marcin Minta, Zuzanna Owsiańska, Sonia Kahtan, Natalia Neumann-Klimasińska, Karolina Wróbel, Agata Kubiaczyk, Katarzyna Kosik, Katarzyna Olek, Michalina Bugiera, Julita Porwolik, Agnieszka Basiukajć, Elzbieta Czapla, Wojciech Łukaszuk, Katarzyna Gryczka, Dobrochna Naskręcka, Jan Mazela, Marta Szymankiewicz-Bręborowicz;
Division of Neonatology and Pediatric Intensive Care Medicine, Center for Pediatrics and Adolescent Medicine, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany:
Hans Fuchs, Daniel Klotz, Jana Baumgartner; Institute for Maternal and.
Child Health, “IRCCS Burlo Garofolo,” Trieste, Italy:
Jenny Bua, Jana Bembich, Laura Travan;
Department of Pediatrics, University of Alberta, Edmonton, Alberta, Canada:
Georg M Schmölzer, Brenda Law, Po-Yin Cheung.'
Funding
Open access funding provided by Medical University of Graz. No funding was received for the analysis of the data presented in the submitted manuscript, funders had no influence on analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
The COSGOD III trial was funded by the Austrian Science Fund (Fonds zur Förderung der wissenschaftlichen Forschung [FWF] Austria), through an unconditional and unrestricted grant (KLI 586-B31). HRB Clinical Research Facility at University College Cork supported the study at the Infant Centre, University College Cork, Cork University Maternity Hospital, Cork, Ireland. GMS was a recipient of the Heart and Stroke Foundation/University of Alberta Professorship of Neonatal Resuscitation, a National New Investigator of the Heart and Stroke Foundation Canada, and an Alberta New Investigator of the Heart and Stroke Foundation Alberta. This research was facilitated by the Women and Children’s Health Research Institute through the support of the Stollery Children’s Hospital Foundation.
Author information
Authors and Affiliations
Contributions
CHW and GP conceptualised and designed the ancillary study. GP and UKK coordinated this ancillary study at different sites. All authors contributed to the acquisition and preparation of study data. AV, CHW and GP performed the statistical analyses. All authors contributed to interpretation of the findings. CHW and GP drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript. GP obtained funding for the COSGOD III trial. The corresponding author GP attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wolfsberger, C.H., Schwaberger, B., Urlesberger, B. et al. Cerebral oxygenation during immediate fetal-to-neonatal transition and fidgety movements between six to 20 weeks of corrected age: An ancillary study to the COSGOD III trial. Eur J Pediatr 183, 4425–4433 (2024). https://doi.org/10.1007/s00431-024-05711-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05711-3