Abstract
The objective of this study was to examine the utility of the acceleration index observed in an electrocardiogram (ECG) for the prediction of the effectiveness of orthostatic training in pediatric patients diagnosed with postural orthostatic tachycardia syndrome (POTS). This investigation focused on children diagnosed with POTS and undergoing orthostatic training at the Department of Pediatrics of Peking University First Hospital from January 2012 to October 2022. Specifically, patients hospitalized from January 2012 to December 2019 were included in the training set (54 cases), while those hospitalized from January 2020 to October 2022 were included in the external validation set (37 cases). All children received a 3-month orthostatic training, and the baseline symptom score (SS) was calculated in agreement with the pretreatment orthostatic intolerance symptom frequency. Additionally, we determined post-treatment SS during follow-up via telephone after the 3-month treatment. Children with a decrease in post-treatment SS by ≥ 50% of the baseline were considered as responders; otherwise, they were considered as non-responders. Demographic data (age, sex, and body mass index), hemodynamic parameters (supine blood pressure, time to achieve a positive standing test, maximum increase in heart rate during the standing test, maximal heart rate reached during the standing test, and blood pressure at the point of maximal heart rate during the standing test), and electrocardiographic parameters (RR interval in the supine position, shortest RR interval in the upright position, and acceleration index) were collected from all the children prior to treatment. Univariate and multivariate regression analysis were conducted to investigate factors associated with the efficacy of orthostatic training. The predictive value of these indicators for the therapeutic effectiveness of orthostatic training in children with POTS was evaluated using receiver operating characteristic (ROC) analysis, and the indicators were validated using the validation set. Among the 54 children in the training set, 28 responded to orthostatic training, and 26 were nonresponsive. Compared with the non-responders, the responders demonstrated a significant reduction in acceleration index (P < 0.01). The ROC curve for the predictive value of the acceleration index exhibited an area under the curve = 0.81 (95% confidence interval: 0.685–0.926). With the acceleration index threshold < 27.93%, the sensitivity and specificity in the prediction of orthostatic training efficacy among children with POTS were 85.7% and 69.2%, respectively. The external validation results demonstrated that using acceleration index < 27.93% as the threshold, the sensitivity, specificity, and accuracy of predicting orthostatic training efficacy among children with POTS were 89.5%, 77.8%, and 83.8%, respectively.
Conclusions: Electrocardiographic acceleration index can be used to predict the effectiveness of orthostatic training in treating children with POTS.
What is Known: • Postural orthostatic tachycardia syndrome (POTS) is a chronic orthostatic intolerance involving multiple mechanisms. Autonomic dysfunction is one of the main mechanisms of POTS in children and could be treated with orthostatic training. • In order to improve the efficacy of orthostatic training in children with POTS, it is particularly important to identify the patients with autonomic dysfunction as the main mechanism before the treatment. What is New: • We found acceleration index of the electrocardiogram (ECG) can be used as a satisfactory index to predict the efficacy of orthostatic training in the treatment of POTS in children. • Using the acceleration index to predict the efficacy of orthostatic training on POTS in children is easy to be popularized in hospitals at all levels because it is non-invasive, convenient, and not expensive. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postural orthostatic tachycardia syndrome (POTS) is a chronic syndrome characterized by orthostatic intolerance and significant tachycardia in an upright position, with patients often experiencing symptoms, such as dizziness, palpitations, chest tightness, and gastrointestinal symptoms, after standing for a long time or changing from a sitting to a standing position, which significantly affects their quality of life [1,2,3]. The reported prevalence of POTS in children is approximately 6.8% [4]. Currently, the main treatment options for children with POTS include orthostatic training, fluid loading, oral rehydration salts, β-adrenoceptor blockers, α-adrenoceptor agonists, fludrocortisone, pyridostigmine, and ivabradine. However, observational studies showed unsatisfactory outcomes in children with POTS who received unselective treatment [5]. Therefore, there is an urgent need to further improve the therapeutic efficacy for children with POTS.
Autonomic dysfunction is the main mechanism of POTS in children, and orthostatic training is a nonpharmacological therapy for pediatric POTS, which is believed to improve autonomic function [6,7,8,9]. Prior research has indicated that corrected QT interval dispersion may serve as a marker of autonomic function and could potentially predict efficacy. Lu et al. demonstrated that pretreatment-corrected QT dispersion aided in forecasting the outcome of autonomic exercise in children with POTS [10]. Nonetheless, numerous factors may impact QT dispersion, given that its specificity was only 60%. Therefore, identifying more sensitive and specific indicators for predicting the effectiveness of orthostatic training continues to be a challenge in treating children with POTS. The acceleration index in an electrocardiogram (ECG) reflects the immediate alteration of the heart rate when the patient changes from a supine to an upright position [11, 12]. Several studies found that the acceleration index was positively correlated to sympathetic activity [13,14,15]. However, whether the acceleration index can be used as a marker for predicting the effectiveness of orthostatic training on pediatric POTS treatment remains unclear.
This study explores whether the acceleration index is applicable as a predictive factor for the orthostatic training efficacy in children with POTS, thereby helping us to make a relatively accurate judgment of the main pathogenesis of the patient before treatment and to provide evidence for individualized treatment of POTS in children.
Materials and methods
Patients
Children with a confirmed diagnosis of POTS and those who received orthostatic training in the Department of Pediatrics of Peking University First Hospital between January 2012 and October 2022 were enrolled in this retrospective study. Specifically, children hospitalized from January 2012 to December 2019 and those from January 2020 to October 2022 were included in the training and validation sets, respectively. This study was conducted in accordance with the Declaration of Helsinki’s principles and approved by the Ethics Committee of Peking University First Hospital (2022 [496]).
The diagnostic criteria for POTS [16, 17] consist of the following: (1) presence of symptoms exacerbated by predisposing factors like prolonged standing; (2) symptoms of orthostatic intolerance, including headache, dizziness, blurred vision, fatigue, palpitations, chest tightness, exercise intolerance, hand tremors, or syncope, typically experienced in an upright position; (3) a positive response suggestive of POTS during head-up tilt test (HUTT); and (4) exclusion of cardiovascular, neurological, and endocrine and metabolic disorders that could lead to symptoms of orthostatic intolerance.
The criteria for diagnosing POTS during a HUTT [18] include the following: (1) there is a sustained increase in heart rate of ≥ 40 beats per minute (bpm) and/or attainment of a maximal heart rate within 10 min after tilting during HUTT, meeting the specified thresholds of ≥ 130 bpm for children aged 6–12 years and ≥ 125 bpm for those aged 12–18 years; and (2) a decrease in systolic blood pressure by < 20 mmHg (1 mmHg = 0.133 kPa) and a decrease in diastolic blood pressure by < 10 mmHg within 10 min after tilting during HUTT.
The inclusion criteria for the participants were as follows: (1) children who were hospitalized at the Peking University First Hospital between January 2012 and October 2022 with a confirmed diagnosis of POTS; (2) those at the age range of 5–18 years; (3) those receiving standardized assessments, including the record of the complete medical history, physical and neurological examinations, 12-lead ECG, HUTT, and standing tests; and (4) those treated with health education and orthostatic training for at least 3 months.
The exclusion criteria for the participants were as follows: (1) children with cardiovascular disease, cerebrovascular disease, endocrine diseases such as hyperthyroidism and Addison’s disease, hypovitaminosis D, or iron deficiency; (2) those with non-sinus rhythm; (3) those with incomplete baseline medical records; and (4) those, in addition to orthostatic training, were receiving pharmacological therapy, such as metoprolol, midodrine, or oral rehydration salts.
Data collection
The data of all children were obtained from the Medical Records Management Digital System (Kaihua, Beijing, China). The study encompassed demographic data (age, sex, and body mass index [BMI]), hemodynamic parameters (supine blood pressure, maximal increase in heart rate during the standing test, maximal heart rate reached during the standing test, and blood pressure at the point of maximal heart rate during the standing test), and electrocardiographic parameters (the period between the two adjacet R waves (RR interval) in the supine position, the shortest RR interval in the upright position, and acceleration index).
Symptom score
Symptom score (SS) was assessed in all the children. First, children were asked individually about the frequencies of occurrence of the respective 10 main orthostatic intolerance symptoms, including headache, syncope, dizziness, palpitations, chest tightness, sweating, hand tremors, blurred vision or amaurosis, gastrointestinal symptoms, and difficulty of concentrating. Then, each symptom was scored according to the frequencies of occurrence as follows (Supplemental Table 1): 0 point represented absence of symptoms; 1 point stood for symptoms once a month; 2 points for symptoms 2–4 times monthly; 3 points for symptoms 2–7 times weekly; and 4 points for symptoms more than once daily. The scores of all 10 orthostatic intolerance symptoms were determined and recorded, respectively. Finally, the SS for each child was calculated by adding up the scores of the 10 symptoms recorded above [19,20,21]. Using the calculation method above, we assessed the pre-treatment and the post-treatment SS for each child.
HUTT
Basic HUTT procedures [22,23,24,25]
The participants discontinued any diet, including the consumption of tea and coffee, and medications that affect autonomic function for over five half-lives before this trial. The participants were requested to fast and avoid liquids for at least 4 h before the test. The test was performed between 8:00 am and 11:00 am. The test room environment was quiet, warm, and relatively dim. The tiltable table (SHUT-100A, Standley, Jiangsu) was employed for HUTT, and ECG and heart rate were continuously monitored with the multi-lead ECG monitor (GE, NY, USA). The changes in blood pressure were recorded using a noninvasive continuous blood pressure monitor Finapres Medical system-FMS (FinometerPRO, FMS company, Amsterdam, Netherlands). Initially, the participants quietly lay for a 10–30 min period in the supine position, and blood pressure and basic ECG were continuously monitored. When stable heart rate, blood pressure, and ECG were achieved, the participants were tilted at 60°, and these three variables were constantly recorded until a positive reaction occurred or 45 min of the test was completed.
Twelve-lead ECG examination
ECGs were recorded using an electrocardiography machine (FX-7402, Fukuda, Japan). All subjects underwent standard 12-lead surface electrocardiography in both supine and upright positions. The procedure was conducted in a quiet and temperature-controlled dedicated room. Participants were instructed to maintain quiet breathing throughout the examination. The ECG during the supine and upright positions was recorded on thermal paper under the following recording conditions: voltage, 1 mV/cm; paper speed, 25 mm/s. A good quality ECG should include complete lead marks, paper speed, and voltage marks, and the 12-lead ECG pattern should have a stable baseline and clear waveforms.
Acceleration index measurement
Standing testing was conducted on all the participants for acceleration index calculation.
Standing test procedure [22]: The participants were exposed to a quiet, dimly lit environment at a suitable temperature. The participants were requested to rest for a 10–20 min period in the supine position. After the stabilization of the heart rate, the supine heart rate and blood pressure were simultaneously recorded. Then, the participants were advised to stand for another 10 min without support. The ECG, heart rate, and blood pressure were monitored with the Dash 2000 monitor (General Electric Company, NY, USA) during the resting and 10-min standing period. The participant’s complaints of discomfort were recorded throughout the test. When the participant was intolerant to the test, it was terminated at once, and the participant was assisted to return to the supine position.
Calculation of acceleration index [26]: Eligible ECGs were digitized using a scanner. The digitized ECG patterns were magnified threefold on a high-resolution (3840 × 2160) computer screen, and ECG parameters were measured by a trained researcher using Image-Pro Plus version 6.0.0.260 image analysis software. The average RR interval within the first 15 s before the change in body position and the minimal RR interval within 15 s following the body position change were measured by the same researcher in both the training and validation sets. Standard ECG RR intervals were measured in lead II, with each RR interval measured three times and averaged. The acceleration index was calculated according to previous studies [11, 12] using the formula: acceleration index = [(A − B)/A] × 100%, where A represented the average RR interval within 15 s before the change in position, and B represented the minimal RR interval within 15 s following the change in position.
Before the commencement of the study, the repeatability and stability of the measurement method were evaluated. In the repeatability test, electrocardiographic parameters were measured independently by two researchers, including those who later conducted the actual data measurements, in 15 children. Subsequently, the results obtained by these two researchers were compared for consistency. In the stability test, the same researcher repeated measurements of the ECG parameters in 15 children every week and compared the results of both measurements.
Treatment and follow-up
All participants received orthostatic training following the diagnosis of POTS. The protocol for orthostatic training was as follows: the child stood approximately 15–20 cm from the wall, with their back leaning against the wall for 3 to 5 min each time. The duration of each training session was gradually increased up to 30 min based on the child’s tolerance level. If symptoms of orthostatic intolerance occurred during training, it was immediately discontinued. Orthostatic training sessions were repeated once to thrice daily for a total of 3 months. Additionally, health education was provided to the children, including guidance on ensuring adequate sleep, avoiding triggers, and preparing for emergency measures in the event of orthostatic intolerance symptoms.
SS was calculated based on the frequency of orthostatic intolerance symptoms [19,20,21]. The frequency of symptoms before orthostatic training was recorded as the baseline SS. Children with POTS were followed up through telephone communication. Trained researchers conducted the follow-up, assessing treatment compliance and symptom frequency to calculate the post-treatment SS. Participants were classified as responders if their SS decreased by at least 50% compared to baseline; otherwise, they were classified as non-responders [22].
Statistical analysis
This study utilized Microsoft Excel (Microsoft, Redmond, WA, USA) for entering patient information and SPSS version 26.0 (IBM, New York, USA) for statistical analysis. The graphs were plotted using the GraphPad Prism version 9.5.0 software (GraphPad Software, San Diego, USA). The repeatability and stability of ECG measurements were assessed using paired sample t-tests or paired sample nonparametric tests.
Univariate analysis
Normally distributed data were presented as mean ± standard deviation, and the Student’s t-test was used for comparison. Non-normally distributed data were represented as median and quartiles, and the Mann–Whitney U test was conducted for comparison. The enumeration data were represented as frequency and percentage (%), with the χ2 test used for comparison.
Multivariate analysis
The significant factors obtained from the univariate regression analysis in responders compared with non-responders and the demographic characteristics indicators in the training set were subjected to binary logistic regression analysis to determine the odds ratios (ORs) and 95% confidence intervals (CIs). The predictive value of the acceleration index for orthostatic training treatment efficacy in children with POTS was assessed through receiver operating characteristic (ROC) analysis, followed by determining the area under the curve (AUC) values. Consistent with the maximum Youden index, the acceleration index threshold with preferable sensitivity and specificity in predicting orthostatic training effectiveness on children with POTS from the training set was determined.
External validation
According to the acceleration index threshold obtained from the training set, we classified participants from the validation set as responders or non-responders to determine the sensitivity, specificity, and accuracy of the acceleration index in predicting the efficacy of orthostatic training treatment in the validation set.
Results
Patient’s basic features
All POTS patients in this study met the diagnostic standard that there was a sustained increase in heart rate of ≥ 40 beats per min (bpm) within 10 min after tilting during HUTT. A total of 60 cases were included in the training set, of which 6 (10.0%) were lost to follow-up. Of the remaining 54 cases (28 males and 26 females, aged 6–17 years), 28 (51.9%) and 26 (48.1%) were responders and non-responders to orthostatic training, respectively.
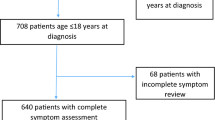
A total of 39 cases were included in the validation set, of which 2 were lost to follow-up with a rate of 5.1%, and finally, 37 cases were included in the study. Of these (14 males and 23 females, aged 8–16 years), 19 (51.4%) and 18 (48.6%) were responders and non-responders, respectively. The flow chart of the inclusion procedure for this study is shown in Fig. 1.
Repeatability and stability of ECG measurement
In the repeatability test, mean RR intervals within 15 s before position change (A) and minimal RR interval within 15 s following body position change (B) were not significantly different between the two values of each of the parameters measured by the two independent researchers (P > 0.05) (Table 1), indicating the reproducibility of the method.
In the stability test, no significant difference in A and B between the two values measured at an interval of 1 week by the same researcher was observed (P > 0.05) (Table 1), indicating the stability of the method.
Univariate analysis
All patients underwent standing tests in our study. A total of 13 variables were included for univariate regression analysis, comprising demographic characteristics (sex, age, BMI, and pretreatment SS), hemodynamic parameters (supine systolic blood pressure, supine diastolic blood pressure, maximal increase in heart rate during the standing test, maximal heart rate reached during the standing test, systolic blood pressure at the point of maximal heart rate during the standing test and diastolic blood pressure at the point of maximal heart rate during the standing test), and electrocardiographic parameters (RR interval in the supine position, shortest RR interval in the upright position, and acceleration index). Compared with the non-responders in the training set, responders demonstrated a significant reduction in acceleration index (P < 0.01). No statistically significant differences were observed for the other variables in both groups (P > 0.05, Tables 2 and 3).
Multivariate analysis
The acceleration index was subjected to multivariate regression. This study also conducted binary logistic regression analysis to explore factors related to therapeutic efficacy, baseline acceleration index, and demographic characteristics (sex, age, and BMI), which were included as independent variables, probably affecting the autonomic function of the children (Table 4). The results showed that the baseline acceleration index was an independent factor related to orthostatic training efficacy among children with POTS. The AUC of ROC curve for baseline acceleration index in the prediction of orthostatic training effectiveness in children with POTS was 0.81 (95% CI: 0.685–0.926) (Fig. 2). Considering a cutoff value of < 27.93% according to the maximum Youden index, the sensitivity and specificity in the prediction of orthostatic training effectiveness were 85.7% and 69.2%, respectively.
ROC curve of ECG acceleration index in training set to predict the efficacy of orthostatic training in children with POTS. The X-axis represents 1-specificity for prediction of the efficacy of orthostatic training treatment, and the Y-axis represents sensitivity for prediction of the efficacy of orthostatic training treatment. The 45-degree reference line of this figure indicates equal sensitivity and specificity. The blue curve represents the ROC curve for the predictive value of ECG acceleration index for the efficacy of orthostatic training treatment, with an area under the curve of 0.81 and a 95% confidence interval of 0.685–0.926. POTS, postural orthostatic tachycardia syndrome
External validation
For external validation, children with electrocardiographic acceleration index < 27.93% and ≥ 27.93% in the validation set were predicted to respond and not respond to orthostatic training, respectively. Compared with the actual follow-up results, the sensitivity, specificity, and accuracy of the acceleration index were 89.5%, 77.8%, and 83.8%, respectively, for predicting orthostatic training efficacy (Table 5).
Discussion
We found that, compared with non-responders, children with POTS who responded well to orthostatic training demonstrated remarkably decreased electrocardiographic acceleration index. Thus, the acceleration index could be used as a predictor for orthostatic training effectiveness in children with POTS. When the acceleration index < 27.93% was used as a threshold condition, the sensitivity and specificity in the prediction of orthostatic training effectiveness were 85.7% and 69.2%, respectively. External validation showed that electrocardiographic acceleration index predicted orthostatic training effectiveness in children with POTS, and its sensitivity, specificity, and accuracy were 89.5%, 77.8%, and 83.8%, respectively.
The current study revealed that orthostatic training was more effective in children with POTS who had lower electrocardiographic acceleration index. This finding aligns with a previous study by Tao et al., which demonstrated that children with vasovagal syncope (another form of orthostatic intolerance) and decreased mean acceleration index responded more favorably to orthostatic training [26]. Ector et al. [27] showed that patients with autonomic dysfunction experienced clinical improvement solely through orthostatic training, without requiring medical intervention. They concluded that repeated orthostatic stress exposure may treat the cardiovascular reflex impairment, which can be adjusted and restored with long-term orthostatic training treatment. Abe et al. revealed that orthostatic self-training once or twice a day could prevent neurally mediated syncope during 1–11 months of follow-up [28, 29].
Electrocardiographic acceleration index reflects the immediate change in heart rate as the patient switches from the supine to an upright position, which reflects sympathetic activity according to the previous studies [30]. Sundkvist et al. noted a positive correlation between electrocardiographic acceleration index and plasma epinephrine content during the initial 1 min post-standing (r = 0.59, P < 0.05) [13]. Additionally, several studies have indicated that patients with diabetes and autonomic neuropathy who exhibit lower electrocardiographic acceleration index also display reduced adrenergic responses [31, 32]. Another previous study showed a reduction in maximum heart rate during HUTT and a significant rise in systemic vascular resistance after 6 weeks of orthostatic training [6]. Consequently, according to these studies and the results in our study, we hypothesized that individuals with POTS, showing relatively lower acceleration index, might experience inadequate orthostatic sympathetic activation, and, thus, might derive greater benefits from orthostatic training. Although it is known that a considerable portion of patients with POTS demonstrate increased sympathetic nervous system tone (known as hyperadrenergic POTS), however, another subtype with partial peripheral sympathetic denervation (known as neuropathic POTS) also has been reported [33]. Under normal conditions, postural stress induces compensatory vasoconstriction through sympathetic activation triggered by baroreceptors to maintain normal orthostatic blood pressure and cerebral perfusion. However, the compensatory physiological regulation mechanism in the patients with neuropathic POTS is damaged, and the venous return is continuously insufficient; thus, the heart rate in standing position abnormally increases, and orthostatic intolerance occurs [34]. Therefore, the therapeutic effect of upright training therapy may be the result of improving impaired sympathetic activity and reducing the sensitivity of cardiopulmonary receptors, so it could increase peripheral vascular resistance [6, 7, 22]. We speculated that POTS patients with lower acceleration index would obtain more benefits from orthostatic training since those patients might have a damaged sympathetic system-controlled vasoconstrictor reserve [35]. However, whether children with lower acceleration index are the “neuropathic POTS” subtype should be identified in further studies. And the specific mechanisms by which orthostatic training improves the symptoms of POTS should be clarified in the future.
POTS is a complex disorder affecting multiple systems, characterized by persistently elevated heart rate and symptoms of orthostatic intolerance following postural changes, significantly impacting the daily life of patients and their families [32, 36]. Orthostatic training therapy is recommended for children with POTS as it enhances autonomic function and is convenient to implement [37]. However, the pathogenesis of POTS varies among individuals. As mentioned above, orthostatic training may mainly improve impaired sympathetic activity; therefore, unselective use of orthostatic training in all children with POTS may result in limited efficacy. As shown in this study, among the 91 children with a confirmed diagnosis of POTS and who received orthostatic training, the total effective rate was only 51.6%. Thus, if children with POTS characterized by autonomic dysfunction are predicted before treatment and individualized orthostatic training treatment is provided, a significant improvement is noted in the orthostatic training effectiveness in children with POTS [38].
A previous study [10] demonstrated that, compared with normal controls, supine corrected QT dispersion in children with POTS markedly increased, whereas, compared with non-responders, responders to orthostatic training demonstrated increased supine corrected QT dispersion before treatment. When the cutoff value is > 43.0 ms, the sensitivity and specificity of effective treatment with autonomic functional exercise are 90% and 60%, respectively. Therefore, in children with POTS, pretreatment-corrected QT dispersion helps to predict the efficacy of autonomic exercise treatments such as orthostatic training. QT interval dispersion requires measurement of a 12-lead QT interval and corrected QT interval involves a more complex formula for calculation. Although the corrected QT interval dispersion demonstrated high sensitivity, the specificity was only 60%. Lu et al. [10] demonstrated that children treated with orthostatic training also received other methods of autonomic function training such as rubbing the skin of the medial extremities with a dry towel. Therefore, further studies are warranted to enhance the prediction of orthostatic training effectiveness in children with POTS.
This study explored the biomarkers from ECG to predict the effectiveness of orthostatic training in treating POTS in children. Electrocardiographic acceleration index is highly sensitive and specific in the prediction of orthostatic training effectiveness in children with POTS, and the result has been confirmed using a validation set. Meanwhile, the acceleration index can be easily promoted in hospitals at all levels because it is noninvasive, convenient, and inexpensive.
The main limitations of this study include the retrospective study design and the relatively small sample size. Moreover, there was a limitation about the inclusion of patients. The patients were recruited from a single medical center. Prospective, large-sample size multicenter studies should be conducted in the future to further explore the predictive value of electrocardiographic acceleration index for long-term outcomes in children with POTS after orthostatic training. Another limitation is the absence of a healthy control group showing normal acceleration index levels and a control group with orthostatic intolerance but without POTS, such as neurocardiogenic syncope/pre-syncope. Additionally, comorbidities like joint hypermobility and mast cell activation syndrome were not evaluated in all children with POTS in our study. Due to the nature of the retrospective study and the limited number of cases, no subgroup assessment was performed. It would be interesting to explore how the acceleration index changes when some comorbidities are combined in the future. It is also worth noting that the accelerative response of heart rate within the first minute after standing is also seen in initial orthostatic hypotension [39], which is can be seen in children with POTS. It will be interesting to investigate whether there is a difference in acceleration index between POTS patients with and without initial orthostatic hypotension.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AUC:
-
Area under curve
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- ECG:
-
Electrocardiogram
- HUTT:
-
Head-up tilt test
- OR:
-
Odds ratio
- ORS:
-
Oral rehydration salts
- POTS:
-
Postural orthostatic tachycardia syndrome
- ROC:
-
Receiver operating characteristics
- SS:
-
Symptom score
References
Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD (2019) Postural orthostatic tachycardia syndrome: JACC focus seminar. J Am Coll Cardiol 73(10):1207–1228
Vernino S, Bourne KM, Stiles LE et al (2021) Postural orthostatic tachycardia syndrome (POTS): state of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - part 1. Auton Neurosci 235:102828
Wang Y, Du J, Jin H (2020) Differential diagnosis of vasovagal syncope and postural tachycardia syndrome in children. World J Pediatr 16(6):549–552
Wooley CF (1976) Where are the diseases of yesteryear? DaCosta’s syndrome, soldiers heart, the effort syndrome, neurocirculatory asthenia–and the mitral valve prolapse syndrome. Circulation 53(5):749–751
Xu B, Zhang Q, Li X, Tang C, Du J, Liu X, Jin H (2023) A predictive model of response to metoprolol in children and adolescents with postural tachycardia syndrome. World J Pediatr 19(4):390–400
Verheyden B, Ector H, Aubert AE, Reybrouck T (2008) Tilt training increases the vasoconstrictor reserve in patients with neurally mediated syncope evoked by head-up tilt testing. Eur Heart J 29(12):1523–1530
Tan MP, Newton JL, Chadwick TJ, Gray J, Nath S, Parry SW (2010) Home orthostatic training in vasovagal syncope modifies autonomic tone: results of a randomized, placebo-controlled pilot study. Europace 12(2):240–246
Gajek J, Zyśko D, Halawa B, Mazureket W (2006) Influence of tilt training on activation of the autonomic nervous system in patients with vasovagal syncope. Acta Cardiol 61(2):123–128
Gajek J, Zyśko D, Krzemińska S, Mazureket W (2009) The influence of a tilt training programme on the renin-angiotensin-aldosterone system activity in patients with vasovagal syncope. Acta Cardiol 64(4):505–509
Lu W, Yan H, Wu S, Chen S, Xu W, Jin H, Du J (2016) Electrocardiography-derived predictors for therapeutic response to treatment in children with postural tachycardia syndrome. J Pediatr 176:128–133
Cybulski G, Niewiadomski W (2003) Influence of age on the immediate heart rate response to the active orthostatic test. J Physiol Pharmacol 54(1):65–80
Ohlsson B, Ekberg O, Sundkvist G (2004) Achalasia: a vagal disease. Scand J Gastroenterol 39(6):527–530
Sundkvist G, Lilja B, Manhem P, Almér LO (2009) Responses of plasma catecholamines to tilt in patients with diabetes mellitus. Acta Med Scand 216(2):223–227
Ewing DJ, Hume L, Campbell IW, Murray AW, Neilson JM, Clarke BF (1980) Autonomic mechanisms in the initial heart rate response to standing. J Appl Physiol Respir Environ Exerc Physiol 49(5):809–814
Korner PI (1971) Integrative neural cardiovascular control. Physiol Rev 51(2):312–367
Wang C, Du J, Li Y et al (2018) 2018 Chinese Pediatric Cardiology Society (CPCS) guideline for diagnosis and treatment of syncope in children and adolescents. Sci Bull 63(23):1558–1564
Brignole M, Moya A, de Lange FJ et al (2018) 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J 39(21):1883–1948
Zhao J, Han Z, Zhang X et al (2015) A cross-sectional study on upright heart rate and BP changing characteristics: basic data for establishing diagnosis of postural orthostatic tachycardia syndrome and orthostatic hypertension. BMJ Open 5(6):e007356
Winker R, Barth A, Dorner W, Mayr O, Pilger A, Ivancsits S, Ponocny I, Heider A, Wolf C, Rüdiger HW (2003) Diagnostic management of orthostatic intolerance in the workplace. Int Arch Occup Environ Health 76(2):143–150
Wang Y, Sun Y, Zhang Q, Zhang C, Liu P, Tang C, Jin H, Du J (2021) Baseline corrected QT interval dispersion is useful to predict the effectiveness of metoprolol on pediatric postural tachycardia syndrome. Front Cardiovasc Med 8:808512
Yozgat Y, Temur HO, Coban S, Öner T, Karaarslan U, Yozgat Y, Karadeniz C, Ergor SN, Erenberk U (2020) Short-term efficacy of ORS formulation and propranolol regimen in children with POTS. Arch Pediatr 27(6):328–332
Tao C, Li H, Li X, Tang C, Jin H, Du J (2019) Hemodynamic changes in standing-up test of children and adolescents with postural tachycardia syndrome. Beijing Da Xue Xue Bao Yi Xue Ban 51(3):414–421
Zhang Q, Liao Y, Tang C, Jin H, Du J (2012) Twenty-four-hour urinary sodium excretion and postural orthostatic tachycardia syndrome. J Pediatr 161(2):281–284
Li H, Wang Y, Liu P, Chen Y, Feng X, Tang C, Du J, Jin H (2016) Body mass index (BMI) is associated with the therapeutic response to oral rehydration solution in children with postural tachycardia syndrome. Pediatr Cardiol 37(7):1313–1318
Plash WB, Diedrich A, Biaggioni I, Garland EM, Paranjape SY, Black BK, Dupont WD, Raj SR (2013) Diagnosing postural tachycardia syndrome: comparison of tilt testing compared with standing haemodynamics. Clin Sci (Lond) 124(2):109–114
Tao C, Li X, Tang C, Jin H, Du J (2019) Acceleration index predicts efficacy of orthostatic training on vasovagal syncope in children. J Pediatr 207:54–58
Ector H, Reybrouck T, Heidbuchel H, Gewillig M, Werf FV (1998) Tilt training: a new treatment for recurrent neurocardiogenic syncope and severe orthostatic intolerance. Pacing Clin Electrophysiol 21:193–196
Abe H, Sumiyoshi M, Kohshi K, Nakashima Y (2003) Effects of orthostatic self-training on head-up tilt testing for the prevention of tilt-induced neurocardiogenic syncope: Comparison of pharmacologic therapy. Clin Exp Hypertens 25:191–198
Abe H, Kohshi K, Nakashima Y (2003) Efficacy of orthostatic self-training in medically refractory neurocardiogenic syncope. Clin Exp Hypertens 25:487–493
Topcu B, Akalin F (2010) The autonomic nervous system dysregulation in response to orthostatic stress in children with neurocardiogenic syncope. Cardiol Young 20(2):165–172
Bergström B, Manhem P, Bramnert M, Lilja B, Sundkvist G (1989) Impaired responses of plasma catecholamines to exercise in diabetic patients with abnormal heart rate reactions to tilt. Clin Physiol 9(3):259–267
Hilsted J, Galbo H, Christensen NJ, Parving H, Benn J (1982) Haemodynamic changes during graded exercise in patients with diabetic autonomic neuropathy. Diabetologia 22(5):318–323
Boris JR, Moak JP (2022) Pediatric postural orthostatic tachycardia syndrome: where we stand. Pediatrics 150(1):e2021054945
Raj SR (2013) Postural tachycardia syndrome (POTS). Circulation 127(23):2336–2342
Fu Q, Witkowski S, Levine BD (2004) Vasoconstrictor reserve and sympathetic neural control of orthostasis. Circulation 110(18):2931–2937
Wang Y, Li X, Du J, Sun Y, Xu W, Wang Y, Liao Y, Jin H (2022) Impact of comorbidities on the prognosis of pediatric vasovagal syncope. World J Pediatr 18(9):624–628
Tao C, Jin H, Du J (2020) Management of orthostatic intolerance in children: the state of the art. World J Pediatr 16(6):543–548
Xu W, Du J, Jin H (2022) Can pediatric vasovagal syncope be individually managed? World J Pediatr 18(1):4–6
Stewart JM, Javaid S, Fialkoff T, Tuma-Marcella B, Visintainer P, Terilli C, Medow MS (2019) Initial orthostatic hypotension causes (transient) postural tachycardia. J Am Coll Cardiol 74(9):1271–1273
Funding
This work was supported by the National High-Level Hospital Clinical Research Funding (Multi-center Clinical Research Project of Peking University First Hospital) (2022CR59), Clinical Medicine Plus X—Young Scholars Project (PKU2022LCXQ028), and the Fundamental Research Funds for the Central Universities, China.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The authors’ contributions are as follows: B. X. and Y. G. had primary responsibility for the protocol development, patient enrollment, data collection and verification, and preliminary data analysis and wrote the draft. Q. Z. analyzed the data and revised important content. Y. L., J. D., and H. J. supervised the design and execution of the study, checked the data analysis, contributed to the writing of the manuscript, and had a final approval of the manuscript submitted. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This study was conducted in accordance with the Declaration of Helsinki's principles. This study has been reviewed and approved by the Ethics Committee of Peking University First Hospital (2022 [496]).
Consent to participate
Informed consent was obtained from the parents.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, B., Gao, Y., Zhang, Q. et al. Acceleration index predicts efficacy of orthostatic training on postural orthostatic tachycardia syndrome in children. Eur J Pediatr 183, 4029–4039 (2024). https://doi.org/10.1007/s00431-024-05664-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05664-7