Abstract
Mycoplasma pneumoniae (MP) is an important cause of community-acquired pneumonia in children and young adolescents. Despite macrolide antibiotics effectiveness as a first-line therapy, persistence of fever and/or clinical deterioration sometimes may complicate treatment and may even lead to severe systemic disease. To date, there is no consensus on alternative treatment options, optimal dosage, and duration for treating severe, progressive, and systemic MP pneumonia after macrolide treatment failure. Macrolide-resistant MP pneumonia and refractory MP pneumonia are the two major complex conditions that are clinically encountered. Currently, the vast majority of MP isolates are resistant to macrolides in East Asia, especially China, whereas in Europe and North America, whereas in Europe and North America prevalence is substantially lower than in Asia, varying across countries. The severity of pneumonia and extrapulmonary presentations may reflect the intensity of the host’s immune reaction or the dissemination of bacterial infection. Children infected with macrolide-resistant MP strains who receive macrolide treatment experience persistent fever with extended antibiotic therapy and minimal decrease in MP-DNA load. Alternative second-line agents such as tetracyclines (doxycycline or minocycline) and fluoroquinolones (ciprofloxacin or levofloxacin) may lead to clinical improvement after macrolide treatment failure in children. Refractory MP pneumonia reflects a deterioration of clinical and radiological findings due to excessive immune response against the infection. Immunomodulators such as corticosteroids and intravenous immunoglobulin (IVIG) have shown promising results in treatment of refractory MP pneumonia, particularly when combined with appropriate antimicrobials. Corticosteroid-resistant hyperinflammatory MP pneumonia represents a persistent or recrudescent fever despite corticosteroid therapy with intravenous methylprednisolone at standard dosage.
Conclusion: This report summarizes the clinical significance of macrolide-resistant and refractory MP pneumonia and discusses the efficacy and safety of alternative drugs, with a stepwise approach to the management of MP pneumonia recommended from the viewpoint of clinical practice.
What is Known: • Although MP pneumonia is usually a benign self-limited infection with response macrolides as first line therapy, severe life-threatening cases may develop if additional treatment strategies are not effectively implemented. • Macrolide-resistant and refractory MP pneumonia are two conditions that may complicate the clinical course of MP pneumonia, increasing the risk for exacerbation and even death. | |
What is New: • This report summarizes the clinical relevance of macrolide-resistant and refractory MP pneumonia and discusses the efficacy and safety of alternative drug therapies. • A practical stepwise approach to the management of MP pneumonia is developed based on a comprehensive analysis of existing evidence and expert opinion. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mycoplasma pneumoniae (MP), a cell-wall-deficient prokaryote, is one of the most common infectious pathogens, accounting for up to 40% of cases of community-acquired pneumonia in children, especially in school-aged children and adolescents. MP infections tend to be endemic, interspersed by epidemics at 4–7-year intervals. The estimated annual incidence of sporadic MP pneumonia in children aged 5–14 years ranges from 3/1000 to 4/1000 which may increase several folds during epidemics [1, 2]. Younger children appear to have milder or subclinical infections, whereas older children, over 5 years of age, are more likely to develop pneumonia. Although pneumonia due to MP is usually characterized by a benign self-limited infection generally associated with a favorable prognosis, severe life-threatening cases of pneumonia with extrapulmonary manifestations involving almost every organ system may develop if early and accurate diagnosis and appropriate treatment strategies are not implemented [3, 4]. The severity of pneumonia and extrapulmonary presentations may represent the intensity of the host’s immune reaction or the spread of bacterial infection [5]. Macrolide-resistant MP infection and refractory MP pneumonia are the two conditions frequently proposed, especially in East Asia, which may complicate the clinical management of MP pneumonia, increasing the risk for exacerbation and even death. There has been no consensus on alternative treatment options and optimal dosage and duration for treating severe, progressive, and systemic MP pneumonia after macrolide treatment failure. Therefore, this report summarizes the clinical relevance of macrolide-resistant and refractory MP pneumonia and discusses the efficacy and safety of alternative drug therapies. Ultimately, we propose a practical stepwise approach to the management of MP pneumonia developed on a comprehensive analysis of existing evidence and expert opinion.
Classical treatment of MP pneumonia: macrolides
Owing to the lack of a cell wall, MP is inherently resistant to beta-lactams and to all antimicrobials targeting the cell wall, leaving relatively few therapeutic alternatives for this organism. Macrolides are the most potent and first-line agents against macrolide-susceptible MP infections, probably owing to both the antimicrobial activities and the anti-inflammatory properties mediated through cytokine inhibition, with minimal inhibitory concentrations (MIC50s) typically being ≤ 0.001 µg/mL [6]. In addition, macrolides have a favorable toxicity profile and are easy to administer in a short (3 days) once daily dosing schedule. However, plasma concentration of ordinary dosage of macrolide treatment often cannot reach the effective MIC for macrolide-resistant MP infections. For example, mutations at positions A2063G and A2064G confer high-level resistance to macrolides, and MIC50 of erythromycin for those strains is 16 µg/mL, which is almost 10,000 times higher than that for susceptible strains [7]. Despite the fact that modern molecular methods capable of highlighting mutations in identified MP-DNA are available, these tests are not routinely used in clinical practice due to costs and feasibility [8]. Comparative clinical studies on macrolide-resistant and macrolide-susceptible patients have shown that 71–88% of patients with sensitive MP pneumonia experienced abatement of fever within 48 h after initiation of macrolide treatment. In resistant MP pneumonia patients, fever persisted in 52–73% more than 48 h and in 30% of patients for 72 h after initiation of macrolide treatment [9,10,11]. Thus, the clinical response of MP infections either susceptible or resistant to macrolides can be promptly assessed through the defervescence within 48–72 h after treatment is started [12, 13] (Table 1).
Overt macrolide treatment failure has also been reported, highlighting a need for alternative antibiotic treatment options for infections caused by resistant strains. Two main classes of second-line agents including tetracyclines and fluoroquinolones have been proposed and shown clinical improvement after macrolide treatment failure in children [14,15,16,17]. Drugs in the fluoroquinolone and tetracycline classes are not routinely used in pediatric populations because of their toxicity profile, but they continue to be prescribed for selected cases with difficult-to-treat infections where the benefit of therapy may outweigh the risk of toxicity. To date, naturally occurring acquired resistance to tetracyclines or fluoroquinolones in MP clinical isolates have not been reported in the literature.
Macrolide-resistant MP pneumonia
Since macrolide-resistant MP was first reported in Japan in the early 2000s, it has subsequently spread through Asia and eventually to Europe and North America, attributable, at least in part, to the widespread usage of macrolides globally. Almost 90% of MP isolates are now resistant to macrolides in East Asia including China and Japan, followed by Europe (approximately 1–30%) and North America (12 to 13%) [18]. Macrolides block protein synthesis by binding to specific nucleotides in domains II and/or V of 23S rRNA in the 50S bacterial ribosomal subunit, and mutations at the positions 2063 (A2063G/C), 2064 (A2064G/C), and 2617 (C2617G/A) in the V domain of the 23S rRNA of MP are the primary mechanisms of macrolide resistance [19]. To date, no plasmids or genes that mediate ribosomal modification, or any enzymes that break down macrolides, have been described for MP [20]. Mutations at position 2617 tend to produce lower levels of resistance, while those at positions 2063 and 2064 lead to high-level macrolide resistance. In some regions of China, the transition mutation A2063G has been reported to be the most common one, accounting for almost 90% of point mutations [21,22,23]. Although the presence of macrolide-resistant MP renders a longer febrile period and extended antibiotic therapy, its clinical relevance has been debated because whether resistant strains can cause more severe disease remains controversial and macrolides appear clinically effective in some patients infected by macrolide-resistant strains [9, 15, 17]. This phenomenon is probably attributable to the fact that MP infections are often self-limited diseases and that the anti-inflammatory effects of macrolides may improve clinical symptoms. Several studies have reported no differences in the clinical or radiographic presentation of pneumonia between macrolide-resistant and macrolide-susceptible MP in children [9, 24]. However, others reported that children infected with macrolide-resistant MP treated with azithromycin experienced more serious radiological findings and significantly more extrapulmonary complications than those infected with macrolide-susceptible strains [25]. These authors suggested that ineffective treatment of resistant MP pneumonia led to a stronger host reaction with increased cytokine-mediated inflammation, particularly interleukin-8 (IL-8) and IL-18, which may be associated with severity of illness in children. However, Matsuda et al. observed no differences in IL-8 and IL-18 in children with resistant MP infection but did observe higher levels of IL-10, interferon gamma (IFN-γ), and interferon gamma-induced protein 10 (IP-10) [26]. The consensus is that the acquisition of resistance does not render increased pathogen virulence but leads to more difficult treatment and complications with ineffective antimicrobial therapy [18].
Tetracyclines
Doxycycline and minocycline are broad-spectrum, second-generation semisynthetic bacteriostatic antibiotics belonging to the tetracycline family, which block protein synthesis by binding to the bacterial ribosome at high- (30S) and low-affinity (50S) sites and by inhibiting the attachment of transfer RNA to an acceptor site on the messenger RNA ribosomal complex. Doxycycline and minocycline each have good activity against both macrolide-susceptible MP strains (MIC90 0.125–0.5 µg/mL and 0.125–2 µg/mL, respectively) and resistant strains (MIC90 0.5 and 2 µg/mL, respectively) with comparable concentrations [27]. Several studies have shown that in children with macrolide-resistant MP pneumonia, tetracyclines (doxycycline, minocycline) exhibited excellent clinical and microbial efficacy on achieving defervescence and decreasing MP-DNA load after macrolide treatment failure [28,29,30] or compared with macrolide treatment [14, 30]. The remarkable side effect of tetracyclines is incorporation into mineralizing tissues such as teeth, cartilage, and bone that are calcifying at the time of their administration, resulting in discoloration of both the primary and permanent dentitions [31]. Consequently, the application of tetracyclines in pregnant women and children less than 8 years of age was discouraged. However, recent studies on doxycycline use have reported no or only negligible teeth staining even in young children aged 2–8 years, possibly attributed to a low affinity for calcium binding [32, 33]. The development of teeth discoloration by tetracyclines appears to be dependent on the dose and the duration of treatment. Short cycles with limited courses (less than six courses, approximately 6 days per course) were reported to cause negligible tooth discoloration in children in the first 5 years of life [34]. Although adverse reactions are more commonly reported for minocycline, both doxycycline and minocycline are generally well tolerated. It is unlikely that children with macrolide-resistant MP pneumonia would receive a total dosage of tetracyclines that would result in cosmetically significant staining of the teeth. Considering the above, we recommend doxycycline as first alternative antibiotic for macrolide-resistant MP pneumonia at a dose of 4 mg/kg/day orally administered twice daily (Table 2).
Fluoroquinolones
Fluoroquinolones act against topoisomerases, thus inhibiting DNA synthesis and replication, with high effectiveness against MP in vitro [35]. In vitro activity against macrolide-resistant PM isolates has been reported for some fluoroquinolones, with MIC90s being 0.0008–0.125 mg/mL for moxifloxacin, 0.25–0.5 mg/mL for levofloxacin and tosufloxacin, and 0.5–4.0 mg/mL for ciprofloxacin [27]. Levofloxacin is known as a respiratory fluoroquinolone due to strong activity against many of the respiratory pathogens, such as Streptococcus pneumoniae and MP, while retaining activity against many of the Gram-negative pathogens. A newer fluoroquinolone, tosufloxacin, developed by Toyama Chemical Co. Ltd. (Tokyo, Japan), has been approved only in Japan for pediatric pneumonia when a resistant bacterial infection is suspected, and ordinary antimicrobial agents are expected to be ineffective [36]. Despite not being as effective as other fluoroquinolones, ciprofloxacin is the only fluoroquinolone to be included on the list of “Essential Medicines for Children” prepared by the WHO, probably due to its lowest cartilage toxicity [37]. Various case reports have shown that replacing macrolides with fluoroquinolones in children with resistant MP infections (levofloxacin, ciprofloxacin, and tosufloxacin) resulted in clinical improvement with prompt resolution of fever and cough [10, 15, 38]. Although early observations of fluoroquinolone-associated joint/cartilage toxicity in juvenile animals led to contraindication of use in pediatric population, fluoroquinolones have been generally considered to be well tolerated, and they continue to be prescribed for selected pediatric patients with difficult-to-treat infections [39, 40]. Among children included in randomized, prospective, comparative studies treated with levofloxacin or a comparator, safety monitoring focused on musculoskeletal disorders including arthralgia, arthritis, tendinopathy, and gait abnormality. Higher incidences of disorders involving weight-bearing joints were reported at 2 months (2.1% vs. 0.9%) and 12 months (3.4% vs. 1.8%) after starting levofloxacin versus nonfluoroquinolone antibiotics, but no clinically detectable differences were observed between levofloxacin- and comparator-treated children for 1–5 years posttreatment assessment [41, 42]. These findings suggest that if long-term injury occurs with levofloxacin, the rate of these events is low; if injury occurs, it appears to be reversible within 1–5 years.
There have been no prospective randomized controlled trials designed to directly compare the clinical efficacy between tetracyclines and fluoroquinolones in macrolide-resistant MP pneumonia. Data from several observational studies suggested that doxycycline or minocycline was more effective than tosufloxacin in achieving defervescence within 24–48 h and in decreasing the number of MP-DNA copies assessed by real-time PCR at 2–4 days compared with the number of DNA copies present at admission [14, 29]. Both doxycycline and minocycline showed a higher-peak serum concentration and longer half-life compared with tosufloxacin, which may explain these differences in clinical efficacy. For example, the advantage of minocycline was attributable to a relatively high blood concentration (2.3 µg/mL after giving 4 mg/kg) and to a very long half-life of 10 h. However, when tosufloxacin was administered at 6 mg/kg, the maximum blood concentration was 1.0 µg/mL, and the half-life was 3.8 h [14]. In a study comparing the killing kinetics of various antibiotics against macrolide-resistant MP strains, minocycline, doxycycline, and tosufloxacin showed excellent bactericidal activity. However, post-antibiotic effect on resistant strains was stronger for minocycline and doxycycline compared with tosufloxacin [43]. The findings should be interpreted with caution, and definitive conclusions cannot be drawn regarding the benefit of other fluoroquinolones. That is because most reports comparing the response of fluoroquinolones to tetracyclines are limited to tosufloxacin, and those reports were observational studies rather than prospective randomized controlled trials.
Refractory MP pneumonia
In addition to macrolide-resistant MP pneumonia, refractory MP pneumonia is another condition that may complicate the clinical management of MP pneumonia. Refractory MP pneumonia is defined as prolonged fever and/or deterioration of clinical and radiological findings despite administration of appropriate antibiotics, including macrolides, for 7 days or more [44,45,46]. Children with refractory MP pneumonia not only have longer duration of fever and hospitalization than those with non-refractory MP pneumonia but also have more severe pneumonia and higher incidence of extrapulmonary complications [47, 48]. The clinical relevance of macrolide-resistant strains in refractory MP pneumonia has been debated due to lack of sufficient evidence. Macrolide-resistant strains are in general not associated with the development of refractory MP pneumonia because the acquisition of resistance does not render increased virulence of the pathogen or increased severity of pneumonia. Several studies have shown that resistance rates were comparable between refractory and non-refractory MP pneumonia groups [49, 50]. However, a possible relationship between the dramatic increase in macrolide-resistant MP strains and frequently reported refractory MP pneumonia in East Asia warrants further investigation.
The precise underlying pathological mechanisms of refractory PM pneumonia remains unclear. The immune-mediated inflammatory response has been considered to be the primary cause [49, 50]. MP infections could stimulate macrophages via toll-like receptors (TLR) to release immunomodulatory and inflammatory cytokines and chemokines, which in turn cause excessive immune responses, resulting in severe and life-threatening pneumonias and a variety of other extrapulmonary complications [51]. The critical importance of pulmonary macrophages in controlling MP infection and defining the extent of lung infection was demonstrated in several rodent models suggesting that macrophage depletion, but not neutrophils, impairs organism clearance [52]. Macrophage activation and subsequent clearance of MP are dependent on TLR, a mechanism further confirmed by studies in rodents deficient in the essential TLR signaling protein MyD88 [52]. Macrophage activation and cytokine production activate the antiviral/antibacterial immunity but can also trigger the so-called cytokine storm and normal tissue damage through immunological hypersensitivity [53]. For example, macrophage-derived proinflammatory cytokines such as IL-18 and IL-8 were closely associated with the severity of MP pneumonia [54]. Therefore, the severity of pulmonary injuries and the extent of extrapulmonary complications appear to be related to the characteristics and the magnitude of host immune response to infection. In other words, the more vigorous the cytokine stimulation and cell-mediated response is, the more severe pulmonary injury becomes. In addition, the presence of MP in blood, cerebrospinal fluid, and pericardial fluid has been documented by PCR and/or culture, suggesting that direct invasion and damage may participate, at least in part, to an autoimmune process in the pathogenesis of extrapulmonary manifestations [55, 56]. Among various inflammatory biomarkers, lactate dehydrogenase (LDH) is substantially upregulated in this condition, being reported as the most reliable predictor for refractory pneumonia, with cut-off values ranging from 379 to 480 IU/L [49, 57, 58]. Immunosuppressive therapy has shown a beneficial effect by reducing the immune-mediated pulmonary injuries and extrapulmonary complications in refractory MP pneumonia with hyperactive immune-mediated response.
Corticosteroids
Progression to severe MP pneumonia may occur despite appropriate antibiotic therapy. The potential pathogenesis of unresponsiveness and progressive condition is generally considered to be related to vigorous immunological responses. Corticosteroids have received considerable attention and have shown promising results in the treatment of refractory MP pneumonia, either macrolide susceptible or resistant, particularly when combined with appropriate antimicrobials. For example, children with refractory MP pneumonia who received prednisolone 2 mg/kg/day combined with azithromycin for 5 days had clinical, laboratory, and radiological improvement faster than children who received azithromycin [59] alone. A combination of intravenous methylprednisolone at 2 mg/kg/day and ciprofloxacin for 7–12 days improved clinical, laboratory, and radiological abnormalities of refractory MP pneumonia among children aged 4–12 years. These patients had initially received macrolide and third-generation cephalosporin treatment for more than 7 days, but they showed a persistent fever with aggravated respiratory symptoms and signs, while their radiographic findings had progressed to lobar consolidation and even pleural effusion [60]. Moreover, the elevated inflammatory biomarkers such as LDH were also down-regulated following corticosteroid treatment [44, 59, 60]. The rapid response to corticosteroids in children illustrates the importance of hyperactive immune responses of the host rather than direct invasion of the organism in the pathogenesis of refractory MP pneumonia.
Corticosteroid-resistant hyperinflammatory MP pneumonia
Most pediatric patients with refractory MP pneumonia achieve defervescence within 48 h after starting of conventional corticosteroid regimens at 2 mg/kg/day, but fever may persist for more than 3 days after treatment is initiated in about 20% of children [61, 62]. Corticosteroid-resistant hyperinflammatory MP pneumonia is defined as a persistent or relapsed fever for more than 3 days after intravenous methylprednisolone therapy at standard dosage [61, 62]. In this condition, an increase of corticosteroid dosage (4–6 mg/kg/day) and/or intravenous immunoglobulin (IVIG) may be considered [62]. Moreover, combination treatment of high-dose methylprednisolone pulse therapy at 30 mg/kg/day for 3 days, and appropriate antimicrobials led to improvement in clinical condition and even a full recovery in children with refractory MP pneumonia [44, 45]. Despite rapid clinical improvement of refractory MP pneumonia in children after systemic corticosteroid administration, the optimal dosage regimen is still undetermined. Systematic studies are warranted to determine the benefits of combinations of immunomodulators and antimicrobial agents in the treatment of refractory MP infections, as well as the appropriate dosage and course, including the timing of pulse corticosteroid treatment.
Intravenous immunoglobulin (IVIG)
In addition to antimicrobials, IVIG therapy with a total dosage of 2 g/kg divided into 1 to 4 days has been reported to be another effective alternative approach in ameliorating symptoms of severe and systemic refractory MP infections, including fulminant MP pneumonia, pneumonia with neurological manifestations, and possibly the dermatological lesions associated with Stevens-Johnson syndrome [63,64,65]. Although IVIG has proved beneficial in a variety of autoimmune-mediated disorders, its effectiveness in MP-associated complications remains unclear. This rapid improvement may be related to the blockade of the immune-mediated response by the IVIG-induced provision of anti-idiotypic antibodies [65]. IVIG therapy has significant activity against MP, which could provide an additional protection against the disease, predominantly in those patients with suboptimal response to the current antibiotic therapy [66]. To date, there are no serious adverse events reported during or after corticosteroid and/or IVIG administration. Furthermore, clinical presentations and radiological findings did not deteriorate after discontinuation of corticosteroid treatment, and no patient has been reported with additional complications of MP infection.
Practical stepwise approach to the management of MP pneumonia
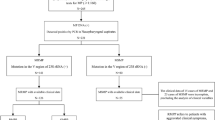
We propose a stepwise approach to the management of MP pneumonia which is summarized in Fig. 1. Although the diagnosis of MP infection based on clinical symptoms and signs alone is not reliable, there is a strong probability that MP infection occurs in children older than 3 years with community-acquired pneumonia who have fever and do not respond to beta-lactam antibiotics after more than 2–3 days of treatment [67, 68]. The diagnosis of acute MP respiratory infection is recommended by real-time PCR testing of sputum or nasopharyngeal specimens and/or paired serologic testing collected at least 2 weeks apart. Once MP infection is highly suspected or diagnosed, the class of macrolide antibiotics such as azithromycin (10 mg/kg/day, once daily for 3 consecutive days) should be prescribed as the first-line drug therapy, since some patients may benefit from macrolides even in the presence of resistance strains. Within 2–3 days after initiation of macrolides, almost all macrolide-susceptible patients show defervescence along with a rapid decrease in MP-DNA load, whereas the majority of macrolide-resistant patients exhibit persistent fever and a minimal decrease of DNA load [69]. The two main alternative second-line antibiotics in tetracycline and fluoroquinolone classes, doxycycline (4 mg/kg/day, twice daily for 10 days) and ciprofloxacin (20–40 mg/kg/day, twice daily for 7–14 days), are preferred as the main choice for the treatment of macrolide-resistant MP pneumonia. Refractory MP pneumonia is diagnosed when prolonged fever and/or deterioration of clinical and radiological findings have occurred despite administration of appropriate antibiotics for 7 days or more (e.g., initial azithromycin for 3 days, replaced with doxycycline for 4 days or more). Conventional corticosteroid therapy (2 mg/kg/day, twice daily for 3–7 days) combined with appropriate antibiotics would be prescribed for the treatment of refractory MP pneumonia. Corticosteroid-resistant hyperinflammatory MP pneumonia is characterized by persistent or recrudescent fever for more than 3 days despite intravenous methylprednisolone therapy at standard dosage. In this condition, initiation of methylprednisolone daily dose (4–6 mg/kg/day) or pulse therapy (30 mg/kg, once daily for 3 days), and/or IVIG (2 g/kg, divided into 1 to 4 days) may be considered. Finally, it is important that clinical benefit is evaluated at every stage of clinical decision-making and treatment regimen, and once significant clinical and/or radiological improvement is found, the next stage of treatment is not further required. The possibility of co-infection with other pathogens should be considered and included in the differential diagnosis at each stage of clinical decision-making. Fortunately, multiplexed PCR tests for bacterial and viral pathogens of respiratory infections have been widely used, and can be helpful to rule out the possibility of co-infection.
A practical stepwise approach to the clinical management of M. pneumoniae pneumonia. Clinical benefit should be evaluated at every stage of clinical decision-making and treatment regimen, and once significant clinical and/or radiological improvement is found, the next stage of treatment is not further required
Data availability
No datasets were generated or analyzed during the current study.
References
Atkinson TP, Waites KB (2014) Mycoplasma pneumoniae infections in Childhood. Pediatr Infect Dis J 33(1):92–94
Hammerschlag MR (2001) Mycoplasma pneumoniae infections. Curr Opin Infect Dis 14(2):181–186
Izumikawa K, Izumikawa K, Takazono T, Kosai K, Morinaga Y, Nakamura S, Kurihara S, Imamura Y, Miyazaki T, Tsukamoto M, Yanagihara K, Hara K, Kohno S (2014) Clinical features, risk factors and treatment of fulminant Mycoplasma pneumoniae pneumonia: a review of the Japanese literature. J Infect Chemother 20(3):181–185
Lee KY, Lee HS, Hong JH, Lee MH, Lee JS, Burgner D, Lee BC (2006) Role of prednisolone treatment in severe Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 41(3):263–268
Waites KB, Balish MF, Atkinson TP (2008) New insights into the pathogenesis and detection of Mycoplasma pneumoniae infections. Future Microbiol 3(6):635–648
Waites KB, Crabb DM, Duffy LB (2009) Comparative in vitro susceptibilities of human mycoplasmas and ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother 53(5):2139–2141
Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, Kang HM, Lee J, Ahn YM, Kang YH, Lee JH (2013) Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000–2011. Emerg Infect Dis 19(8):1281–1284
Principi N, Esposito S (2013) Macrolide-resistant Mycoplasma pneumoniae: its role in respiratory infection. J Antimicrob Chemother 68(3):506–511
Suzuki S, Yamazaki T, Narita M, Okazaki N, Suzuki I, Andoh T, Matsuoka M, Kenri T, Arakawa Y, Sasaki T (2006) Clinical evaluation of macrolide-resistant Mycoplasma pneumoniae. Antimicrob Agents Chemother 50(2):709–712
Kawai Y, Miyashita N, Kubo M, Akaike H, Kato A, Nishizawa Y, Saito A, Kondo E, Teranishi H, Ogita S, Tanaka T, Kawasaki K, Nakano T, Terada K, Ouchi K (2013) Therapeutic efficacy of macrolides, minocycline, and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae pneumonia in pediatric patients. Antimicrob Agents Chemother 57(5):2252–2258
Miyashita N, Akaike H, Teranishi H, Ouchi K, Okimoto N (2013) Macrolide-resistant Mycoplasma pneumoniae pneumonia in adolescents and adults: clinical findings, drug susceptibility, and therapeutic efficacy. Antimicrob Agents Chemother 57(10):5181–5185
Zuckerman JM, Qamar F, Bono BR (2009) Macrolides, ketolides, and glycylcyclines: azithromycin, clarithromycin, telithromycin, tigecycline. Infect Dis Clin North Am 23(4):997–1026 ix-x
Tsai TA, Tsai CK, Kuo KC, Yu HR Rational stepwise approach for Mycoplasma pneumoniae pneumonia in children. J Microbiol Immunol Infect. 2020:S1684-1182(20)30247-4.
Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, Chiba N, Iwata S, Ubukata K (2012) Rapid effectiveness of minocycline or doxycycline against macrolide-resistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis 55(12):1642–1649
Cardinale F, Chironna M, Chinellato I, Principi N, Esposito S (2013) Clinical relevance of Mycoplasma pneumoniae macrolide resistance in children. J Clin Microbiol 51(2):723–724
Morozumi M, Iwata S, Hasegawa K, Chiba N, Takayanagi R, Matsubara K, Nakayama E, Sunakawa K, Ubukata K, Acute Respiratory Diseases Study Group (2008) Increased macrolide resistance of Mycoplasma pneumoniae in pediatric patients with community-acquired pneumonia. Antimicrob Agents Chemother 52(1):348–350
Matsubara K, Morozumi M, Okada T, Matsushima T, Komiyama O, Shoji M, Ebihara T, Ubukata K, Sato Y, Akita H, Sunakawa K, Iwata S (2009) A comparative clinical study of macrolide-sensitive and macrolide-resistant Mycoplasma pneumoniae infections in pediatric patients. J Infect Chemother 15(6):380–383
Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP (2017) Mycoplasma pneumoniae from the respiratory tract and Beyond. Clin Microbiol Rev 30(3):747–809
Lucier TS, Heitzman K, Liu SK, Hu PC (1995) Transition mutations in the 23S rRNA of erythromycin-resistant isolates of Mycoplasma pneumoniae. Antimicrob Agents Chemother 39(12):2770–2773
Waites KB, Talkington DF (2004) Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev 17(4):697–728
Liu Y, Ye X, Zhang H, Xu X, Li W, Zhu D, Wang M (2009) Antimicrobial susceptibility of Mycoplasma pneumoniae isolates and molecular analysis of macrolide-resistant strains from Shanghai, China. Antimicrob Agents Chemother 53(5):2160–2162
Ma Z, Zheng Y, Deng J, Ma X, Liu H (2014) Characterization of macrolide resistance of Mycoplasma pneumoniae in children in Shenzhen, China. Pediatr Pulmonol 49(7):695–700
Xin D, Mi Z, Han X, Qin L, Li J, Wei T, Chen X, Ma S, Hou A, Li G, Shi D (2009) Molecular mechanisms of macrolide resistance in clinical isolates of Mycoplasma pneumoniae from China. Antimicrob Agents Chemother 53(5):2158–2159
Matsuoka M, Narita M, Okazaki N, Ohya H, Yamazaki T, Ouchi K, Suzuki I, Andoh T, Kenri T, Sasaki Y, Horino A, Shintani M, Arakawa Y, Sasaki T (2004) Characterization and molecular analysis of macrolide-resistant Mycoplasma pneumoniae clinical isolates obtained in Japan. Antimicrob Agents Chemother 48(12):4624–4630
Zhou Y, Zhang Y, Sheng Y, Zhang L, Shen Z, Chen Z (2014) More complications occur in macrolide-resistant than in macrolide-sensitive Mycoplasma pneumoniae pneumonia. Antimicrob Agents Chemother 58(2):1034–1038
Matsuda K, Narita M, Sera N, Maeda E, Yoshitomi H, Ohya H, Araki Y, Kakuma T, Fukuoh A, Matsumoto K (2013) Gene and cytokine profile analysis of macrolide-resistant Mycoplasma pneumoniae infection in Fukuoka, Japan. BMC Infect Dis 13:591
Cao B, Qu JX, Yin YD, Eldere JV (2017) Overview of antimicrobial options for Mycoplasma pneumoniae pneumonia: focus on macrolide resistance. Clin Respir J 11(4):419–429
Kawai Y, Miyashita N, Yamaguchi T, Saitoh A, Kondoh E, Fujimoto H, Teranishi H, Inoue M, Wakabayashi T, Akaike H, Ogita S, Kawasaki K, Terada K, Kishi F, Ouchi K (2012) Clinical efficacy of macrolide antibiotics against genetically determined macrolide-resistant Mycoplasma pneumoniae pneumonia in paediatric patients. Respirology 17(2):354–362
Ishiguro N, Koseki N, Kaiho M, Ariga T, Kikuta H, Togashi T, Oba K, Morita K, Nagano N, Nakanishi M, Hara K, Hazama K, Watanabe T, Yamanaka T, Sasaki S, Furuyama H, Shibata M, Shida S, Ishizaka A, Tabata Y, Aoyagi H, Naito H, Yoshioka M, Horino A, Kenri T (2017) Hokkaido Pediatric Respiratory Infection Study Group. Therapeutic efficacy of azithromycin, clarithromycin, minocycline and tosufloxacin against macrolide-resistant and macrolide-sensitive Mycoplasma pneumoniae pneumonia in pediatric patients. PLoS ONE 12(3):e0173635
Lung DC, Yip EK, Lam DS, Que TL (2013) Rapid defervescence after doxycycline treatment of macrolide-resistant Mycoplasma pneumoniae-associated community-acquired pneumonia in children. Pediatr Infect Dis J 32(12):1396–1399
Smith K, Leyden JJ (2005) Safety of doxycycline and minocycline: a systematic review. Clin Ther 27(9):1329–1342
Volovitz B, Shkap R, Amir J, Calderon S, Varsano I, Nussinovitch M (2007) Absence of tooth staining with doxycycline treatment in young children. Clin Pediatr (Phila) 46(2):121–126
Todd SR, Dahlgren FS, Traeger MS, Beltrán-Aguilar ED, Marianos DW, Hamilton C, McQuiston JH, Regan JJ (2015) No visible dental staining in children treated with doxycycline for suspected Rocky Mountain spotted fever. J Pediatr 166(5):1246–1251
Abramson JS, Givner LB (1990) Should tetracycline be contraindicated for therapy of presumed Rocky Mountain spotted fever in children less than 9 years of age? Pediatrics 86(1):123–124
Principi N, Esposito S (2001) Emerging role of Mycoplasma pneumoniae and Chlamydia pneumoniae in paediatric respiratory-tract infections. Lancet Infect Dis 1(5):334–344
Uehara S, Sunakawa K, Eguchi H, Ouchi K, Okada K, Kurosaki T, Suzuki H, Tsutsumi H, Haruta T, Mitsuda T, Yamazaki T (2011) Japanese guidelines for the management of respiratory infectious diseases in Children 2007 with focus on pneumonia. Pediatr Int 53(2):264–276
The Selection and Use of Essential Medicines, Report of the WHO Expert Committee (2009) Available at: http://www.who.int/medicines/publications/TRS958June2010.pdf. Accessed September 14, 2010
Cardinale F, Chironna M, Dumke R, Binetti A, Daleno C, Sallustio A, Valzano A, Esposito S (2011) Macrolide-resistant Mycoplasma pneumoniae in paediatric pneumonia. Eur Respir J 37(6):1522–1524
Patterson DR (1991) Quinolone toxicity: methods of assessment. Am J Med 91(6A):35S–37S
Grady R (2003) Safety profile of quinolone antibiotics in the pediatric population. Pediatr Infect Dis J 22(12):1128–1132
Noel GJ, Bradley JS, Kauffman RE, Duffy CM, Gerbino PG, Arguedas A, Bagchi P, Balis DA, Blumer JL (2007) Comparative safety profile of levofloxacin in 2523 children with a focus on four specific musculoskeletal disorders. Pediatr Infect Dis J 26(10):879–891
Bradley JS, Kauffman RE, Balis DA, Duffy CM, Gerbino PG, Maldonado SD, Noel GJ (2014) Assessment of musculoskeletal toxicity 5 years after therapy with levofloxacin. Pediatrics 134(1):e146–e153
Morozumi M, Okada T, Tajima T, Ubukata K, Iwata S (2017) Killing kinetics of minocycline, doxycycline and tosufloxacin against macrolide-resistant Mycoplasma pneumoniae. Int J Antimicrob Agents 50(2):255–257
Tamura A, Matsubara K, Tanaka T, Nigami H, Yura K, Fukaya T (2008) Methylprednisolone pulse therapy for refractory Mycoplasma pneumoniae pneumonia in children. J Infect 57(3):223–228
You SY, Jwa HJ, Yang EA, Kil HR, Lee JH (2014) Effects of Methylprednisolone Pulse Therapy on Refractory Mycoplasma pneumoniae Pneumonia in Children. Allergy Asthma Immunol Res 6(1):22–26
Shan LS, Liu X, Kang XY, Wang F, Han XH, Shang YX (2017) Effects of methylprednisolone or immunoglobulin when added to standard treatment with intravenous azithromycin for refractory Mycoplasma pneumoniae pneumonia in children. World J Pediatr 13(4):321–327
Zhu Z, Zhang T, Guo W, Ling Y, Tian J, Xu Y (2021) Clinical characteristics of refractory mycoplasma pneumoniae pneumonia in children treated with glucocorticoid pulse therapy. BMC Infect Dis 21(1):126
Zhang Y, Mei S, Zhou Y, Huang M, Dong G, Chen Z (2016) Cytokines as the good predictors of refractory Mycoplasma pneumoniae pneumonia in school-aged children. Sci Rep 6:37037
Inamura N, Miyashita N, Hasegawa S, Kato A, Fukuda Y, Saitoh A, Kondo E, Teranishi H, Wakabayashi T, Akaike H, Tanaka T, Ogita S, Nakano T, Terada K, Ouchi K (2014) Management of refractory Mycoplasma pneumoniae pneumonia: utility of measuring serum lactate dehydrogenase level. J Infect Chemother 20(4):270–273
Miyashita N, Kawai Y, Inamura N, Tanaka T, Akaike H, Teranishi H, Wakabayashi T, Nakano T, Ouchi K, Okimoto N (2015) Setting a standard for the initiation of steroid therapy in refractory or severe Mycoplasma pneumoniae pneumonia in adolescents and adults. J Infect Chemother 21(3):153–160
Chaudhry R, Ghosh A, Chandolia A (2016) Pathogenesis of Mycoplasma pneumoniae: an update. Indian J Med Microbiol 34(1):7–16
Lai JF, Zindl CL, Duffy LB, Atkinson TP, Jung YW, van Rooijen N, Waites KB, Krause DC, Chaplin DD (2010) Critical role of macrophages and their activation via MyD88-NFκB signaling in lung innate immunity to Mycoplasma pneumoniae. PLoS ONE 5(12):e14417
Dukhinova M, Kokinos E, Kuchur P, Komissarov A, Shtro A (2020) Macrophage-derived cytokines in pneumonia: linking cellular immunology and genetics. Cytokine Growth Factor Rev. : S1359-6101(20)30229-X.
Narita M, Tanaka H, Abe S, Yamada S, Kubota M, Togashi T (2000) Close association between pulmonary disease manifestation in Mycoplasma pneumoniae infection and enhanced local production of interleukin-18 in the lung, independent of gamma interferon. Clin Diagn Lab Immunol 7(6):909–914
Socan M, Ravnik I, Bencina D, Dovc P, Zakotnik B, Jazbec J (2001) Neurological symptoms in patients whose cerebrospinal fluid is culture- and/or polymerase chain reaction-positive for Mycoplasma pneumoniae. Clin Infect Dis 32(2):E31–E35
Szymanski M, Petric M, Saunders FE, Tellier R (2002) Mycoplasma pneumoniae pericarditis demonstrated by polymerase chain reaction and electron microscopy. Clin Infect Dis 34(1):E16–E17
Lu A, Wang C, Zhang X, Wang L, Qian L (2015) Lactate Dehydrogenase as a Biomarker for Prediction of Refractory Mycoplasma pneumoniae Pneumonia in Children. Respir Care 60(10):1469–1475
Oishi T, Narita M, Matsui K, Shirai T, Matsuo M, Negishi J, Kaneko T, Tsukano S, Taguchi T, Uchiyama M (2011) Clinical implications of interleukin-18 levels in pediatric patients with Mycoplasma pneumoniae pneumonia. J Infect Chemother 17(6):803–806
Luo Z, Luo J, Liu E, Xu X, Liu Y, Zeng F, Li S, Fu Z (2014) Effects of prednisolone on refractory mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol 49(4):377–380
Lu A, Wang L, Zhang X, Zhang M (2011) Combined treatment for child refractory Mycoplasma pneumoniae pneumonia with ciprofloxacin and glucocorticoid. Pediatr Pulmonol 46(11):1093–1097
Chen L, Liu J, Zhao S, Yang Y, Wu J (2014) Clinical features and treatment of refractory Mycoplasma pneumoniae pneumonia unresponded to conventional dose methylprednisolone in children [in Chinese]. Zhonghua Er Ke Za Zhi 52(3):172–176
Yan Y, Wei Y, Jiang W, Hao C (2016) The clinical characteristics of corticosteroid-resistant refractory Mycoplasma pneumoniae pneumonia in children. Sci Rep 6:39929
Shen Y, Zhang J, Hu Y, Shen K (2013) Combination therapy with immune-modulators and moxifloxacin on fulminant macrolide-resistant Mycoplasma pneumoniae infection: a case report. Pediatr Pulmonol 48(5):519–522
Bressan S, Mion T, Andreola B, Bisogno G, Da Dalt L (2011) Severe Mycoplasma pneumoniae-associated mucositis treated with immunoglobulins. Acta Paediatr 100(11):e238–e240
Attilakos A, Palaiologou P, Lagona E, Voutsioti A, Dinopoulos A (2008) Mycoplasma pneumoniae encephalopathy: recovery after intravenous immunoglobulin. Pediatr Neurol 38(5):357–359
Krause I, Wu R, Sherer Y, Patanik M, Peter JB, Shoenfeld Y (2002) In vitro antiviral and antibacterial activity of commercial intravenous immunoglobulin preparations–a potential role for adjuvant intravenous immunoglobulin therapy in infectious diseases. Transfus Med 12(2):133–139
Wang K, Gill P, Perera R, Thomson A, Mant D, Harnden A (2012) Clinical symptoms and signs for the diagnosis of Mycoplasma pneumoniae in children and adolescents with community-acquired pneumonia. Cochrane Database Syst Rev 10(10):CD009175
Fischer JE, Steiner F, Zucol F, Berger C, Martignon L, Bossart W, Altwegg M, Nadal D (2002) Use of simple heuristics to target macrolide prescription in children with community-acquired pneumonia. Arch Pediatr Adolesc Med 156(10):1005–1008
Lee H, Yun KW, Lee HJ, Choi EH (2018) Antimicrobial therapy of macrolide-resistant Mycoplasma pneumoniae pneumonia in children. Expert Rev Anti Infect Ther 16(1):23–34
Acknowledgements
This study was partly supported by the National Natural Science Foundation of China (grant 81972991).
Funding
This study was partly supported by the National Natural Science Foundation of China (grant 81972991).
Author information
Authors and Affiliations
Contributions
G.D., X.Z., Y.Y., and Y.Z. conceived and designed the study. G.D. and X.Z. wrote the first draft that was revised and formatted for publication by Y.Y. and Y.Z. A.V. and A.R. provided critical comments and substantially revised the manuscript. All authors reviewed and approved this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Tobias Tenenbaum
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, G., Zhang, X., Vinturache, A. et al. Challenges in the treatment of pediatric Mycoplasma pneumoniae pneumonia. Eur J Pediatr 183, 3001–3011 (2024). https://doi.org/10.1007/s00431-024-05519-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05519-1