Abstract
Hearing loss is a common disability in infants that significantly impacts their cognitive, language, and literacy development. This study aimed to systematically assess the risk factors for the early identification and intervention in infant hearing loss. Databases were searched for meta-analyses of observational studies until November 2023. The quality assessment was performed using the Cochrane risk of bias tool, and the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the certainty of the evidence. A meta-analysis identified 14 risk factors significantly associated with infant hearing loss. According to the GRADE approach, there were four factors with moderate-certainty evidence (low birth weight(LBW), congenital anomalies, craniofacial anomalies, intracranial hemorrhages), seven factors with low-certainty evidence (ototoxic medications, family history of hearing loss, mechanical ventilation > 5 days, intrauterine infection, admission to neonatal intensive care unit (NICU) > 5 days, mechanical ventilation and asphyxia) and six with extremely-low-certainty evidence (very low birth weight < 1500 g (VLBW), hyperbilirubinemia, sepsis or meningitis, male sex, premature birth, small for gestational age (SGA)). Nevertheless, no significant association was found between infant hearing loss and factors such as small for gestational age (SGA), male sex, and premature birth (P > 0.05).
Conclusion: The identification of these 14 interrelated risk factors can prove advantageous in clinical practice, as these findings could guide hearing screening and parental counseling. Furthermore, prospective research could be conducted to develop risk-based scoring systems based on these factors.
What is Known: • Infant hearing loss is a worldwide issue. • Risk factors for this condition are debated. | |
What is New: • This is the first meta-analysis to comprehensively evaluate perinatal and postnatal risk factors for hearing loss in infants. • Intracranial hemorrhage, mechanical ventilation, and low birth weight are associated with infant hearing loss. However, no evidence of an association was found between premature birth, being small for gestational age, or male sex and hearing loss. |
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Hearing loss has become the fourth leading cause of disability worldwide [1], affecting approximately one to two out of every 1000 children and significantly impacting their normal development [2]. The commonly used hearing screening methods in clinical practice are the Otoacoustic Emission (OAE) and Auditory Brainstem Response (ABR) tests. Guidelines recommend a two-step screening program for healthy and low-risk newborns, with the ABR test performed if the OAE test is not passed. However, for individuals with auditory neuropathy spectrum disorders (ANSDs), both the OAE and ABR should be used to avoid missed diagnoses [3]. The updated 2019 JCIH guidelines recommend conducting a comprehensive audiological evaluation between hospital discharge and 9 months of age when risk factors for delayed-onset or progressive hearing loss are present [4]. The recommended hearing screening plan for neonatal intensive care unit (NICU) infants and infants in the well-baby nursery (WBN) also differs. Infants admitted to the NICU face a 10–20 times greater risk of permanent hearing loss due to underlying health conditions. Furthermore, 50% of cases involve genetic factors and are not related to other risk factors [5]. Therefore, hearing screening is necessary for both infants in the NICU and healthy infants without related risk factors.
A timely diagnosis of hearing loss in children is crucial, as studies have confirmed that hearing loss is typically diagnosed between 24 and 30 months of age. A delayed diagnosis significantly impacts normal growth and brain development in infants [3]. The severity of hearing loss in children is directly proportional to its negative effects on cognitive, language, and literacy skills [6, 7]. Additionally, communication difficulties in childhood can lead to psychological symptoms such as anger, loneliness, and burnout [8].
Studies have demonstrated that appropriate intervention measures during the first 6 months of life are essential for mitigating the adverse effects of hearing loss [9]. Numerous studies have consistently shown that infants with risk factors for hearing loss are more likely to experience impairment, highlighting the importance of identifying these risk factors and implementing standardized hearing screening programs for early detection and intervention.
This study aimed to systematically review the recent literature on the risk factors for infant hearing loss, conduct a meta-analysis to identify the main risk factors, and provide reliable, evidence-based medical evidence for the prevention and treatment of infant hearing loss.
Methods
The study was conducted according to the 2020 Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines.
Inclusion and exclusion criteria
Inclusion criteria: (i) case‒control studies, cross-sectional studies, or cohort studies; (ii) studies reporting odds ratios (ORs) or relative risks (RRs) with 95% confidence intervals (CIs); and (iii) studies in which the research subjects were infants diagnosed with hearing loss by the ABR, OAE, automatic auditory brainstem response (A-ABR), brainstem auditory evoked response (BAER) and auditory event-related potential (AERP) hearing tests.
Exclusion criteria: (i) repeated publications; (ii) reviews, case reports, lectures and conference abstracts; (iii) studies of nonhuman subjects; and (iv) studies with incomplete information on ORs or lacking sufficient information to calculate OR values.
Search strategy and selection criteria
Searches were conducted in various databases, including the China National Knowledge Infrastructure, China Science and Technology Journal (VIP), Wanfang, Chinese Biology Medicine Disc, PubMed, Web of Science, Scopus, Cochrane Library, SinoMed, Embase, and Clinical Trial Registry databases in China and the USA. The search spanned from inception to November 2023 and involved the use of a combination of subject and free word retrieval methods. The English subject terms used were determined based on PubMed’s MeSH thesaurus. The search terms used included ‘Infant’, ‘Infants’, ‘Infant, Newborn”, ‘Newborn Infant’, ‘Hearing Loss’, ‘Hypoacusis’, ‘Hypoacuses’, ‘Hearing Impairment’, ‘Transitory Deafness’, ‘cohort studies’ and ‘relative risk’. Additionally, a manual search of the reference lists of the included studies was performed. The detailed search strategy for PubMed is shown as an example; details are provided in the supplementary online material (Box 1).
Study selection and data extraction
Two researchers independently conducted the literature screening, and any discrepancies were resolved through discussion or consultation with a third party. The following data were extracted from the eligible studies: (i) basic information about the study (e.g. first author, publication date, research country, and research type), (ii) baseline characteristics such as sample size and age, and (iii) risk factors and specific data on infant hearing loss. Endnote X9 was used for managing and screening the literature. The abstracts and full texts were further reviewed to determine eligibility.
Quality assessment
Two investigators independently assessed bias in the included studies and cross-verified the results. Any disagreements were resolved through discussion with a third party until consensus was reached. The quality of the case‒control and cohort studies was evaluated using the Newcastle–Ottawa Scale (NOS), while cross-sectional studies were assessed for bias based on criteria recommended by the Agency for Healthcare Research and Quality (AHRQ).
Data analysis
RevMan 5.4 software was used for meta-analysis, with ORs/RRs and 95% CIs as the effect indices. Heterogeneity among the included studies was assessed using the χ2 test (α = 0.1) and I2 statistic. A fixed-effects model was employed if heterogeneity was acceptable (P > 0.10 and I2 ≤ 50%); otherwise, a random-effects model was used. The significance level for the meta-analysis was set at α = 0.05. Furthermore, the influence of individual studies on the overall results was evaluated by conducting a sensitivity analysis, whereby studies were eliminated one by one. In addition, funnel plots were drawn for outcome indicators with data from ≥ 6 studies to observe whether publication bias existed. Additionally, the quality of evidence for each risk factor was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to evaluate the quality of evidence for each risk factor [10].
Results
Study identification
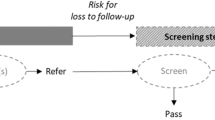
A total of 6008 relevant studies were obtained during the preliminary examination, and 18 studies were ultimately identified after screening, including 1,110,943 participants. The literature screening process and results are shown in Fig. 1.
Characteristics of the included studies
All the basic characteristics of the included studies are shown in Table 1. The risk of bias assessment results of the included case‒control studies, cohort studies, and cross-sectional studies is shown in Supplementary document 1.
Meta-analysis results
The findings of the comprehensive meta-analysis are presented in Table 2. The studies were categorized into perinatal factors, perinatal or postnatal factors, and other factors. Among all factors, eight were perinatal factors; all of them showed statistical significance. A family history of hearing loss [11,12,13,14,15,16,17,18,19] (OR = 2.20, P < 0.001) and the use of ototoxic medications [11,12,13,14,15,16, 20,21,22,23] (OR = 2.75, P < 0.001) were risk factors for hearing loss. Admission to neonatal intensive care unit (NICU) for > 5 days [14, 16, 23] (OR = 2.08, P < 0.001) and hyperbilirubinemia [11,12,13,14,15, 20, 21, 24,25,26] (OR = 2.17, P = 0.009) were strongly associated with hearing loss. Factors such as intrauterine infection [11, 13, 18, 22, 26] (OR = 6.07, P < 0.001), asphyxia [12, 13, 27] (OR = 1.76, P = 0.009), craniofacial anomalies [11, 15, 16, 21, 24, 27] (OR = 6.43, P < 0.001), and congenital malformations and syndromes [14, 19, 28] (OR = 5.01, P < 0.001) strongly increased the risk of hearing loss. Because of the high heterogeneity, this study divided participants with ototoxic medication use into two subgroups and investigated the risk factors for hearing loss in infants in the Asian group and the non-Asian group; the results were statistically significant. Two perinatal or postnatal factors, sepsis or meningitis [12,13,14, 18,19,20, 25](OR = 2.99, P = 0.005) and intracranial hemorrhages [11, 13, 25] (OR = 2.67, P < 0.001), which were closely related to the occurrence of infant hearing loss, were included in this meta-analysis. Among the other factors, SGA [12, 18, 19, 25] (OR = 1.71, P = 0.05), premature birth [11, 13, 26, 28] (OR = 1.95, P = 0.20 > 0.05) and male sex [15, 20, 21] (OR=1.04, P = 0.77 > 0.05) had no statistical significance. Mechanical ventilation [11, 14, 21, 25] (OR = 1.71, P < 0.001) and mechanical ventilation duration > 5 days [12, 13, 18, 22, 27] (OR = 2.10, P = 0.03) were significant risk factors for infant hearing loss. LBW [11, 19, 23, 28] (OR = 1.78, P = 0.001) and VLBW [12, 13, 15, 26] (OR = 3.47, P = 0.003) were also associated with hearing loss.
GRADE assessment
Table 3 shows all the results found in the GRADE evaluation. Four factors with moderate-certainty evidence were identified, while the seven factors had low-certainty evidence. In addition, the certainty of evidence for six factors was extremely low.
Publication bias
The results revealed a symmetrical distribution of research sites, indicating the absence of publication bias (Fig. 2).
Discussion
This study is the first to identify 14 risk factors for infant hearing loss based on meta-analysis and hierarchical evidence assessment.
The findings of this study provide moderate evidence that low birth weight, craniofacial anomalies, congenital malformations, and intracranial hemorrhages are significant risk factors for hearing loss in infants. Craniofacial anomalies may increase infants’ risk of developing hearing loss, consistent with the findings of previous research [29]. According to the JCIH statement, craniofacial anomalies and more than 400 syndromes and genetic disorders associated with atypical hearing thresholds are classified as risk factors for perinatal hearing loss [4]. Therefore, early hearing screening for infants with congenital malformations, especially those involving craniofacial anomalies, may be needed. The present study showed a significantly increased risk of hearing loss in infants who were admitted to the NICU for more than 5 days. With respect to LBW, the lower an infant’s weight is, the greater their risk of hearing loss. Newborns residing in the NICU often have complex congenital diseases and poor physical conditions, resulting in an incidence of hearing loss ranging from 2 to 5% [30]. The immature development of various organs in LBW infants, especially in VLBW infants in the NICU, coupled with potential malnutrition, increases susceptibility to auditory nerve cell damage and subsequent hearing impairment due to prolonged exposure to sound sources such as ventilator alarms and vital sign monitors [31]. It can be inferred from these findings that a history of NICU hospitalization, LBW, and mechanical ventilation factors have synergistic effects on infant hearing loss and lead to a greater incidence of hearing loss in the NICU than in the WBN. Therefore, it is necessary to strengthen hearing screening for infants with a history of NICU hospitalization and implement appropriate measures to prevent and control this risk factor. In addition, some VLBW infants may not pass their first OAE test due to the problem of middle ear effusion; as the effusion subsides a few weeks after birth, a large proportion of these infants will pass the subsequent ABR test [32]. Therefore, for hearing assessment in VLBW infants, careful examination and the performance of the ABR by a professional audiologist is needed. There is a strong correlation between intracranial hemorrhage and hearing loss; a large amount of intracranial hemorrhage will lead to serious brain damage, resulting in auditory and various system dysfunction. This study also confirmed that the following risk factors had low or extremely low evidence levels. Our study showed that asphyxia is a risk factor for hearing loss in infants, which is similar to the results of the present study [20]. When asphyxia combined with a history of NICU residence increases the risk of hearing loss in 2-year-old infants [33].Thus, the essence of asphyxia is hypoxia, which can have some effect on inner and outer hair cells, mainly outer hair cells [34]. The damage to cochlear cells caused by severe hypoxia is irreversible, but there is currently no clear threshold of hypoxia available to define the critical point of hearing risk [32]. Therefore, it is necessary to follow up on the hearing of this high-risk population to take preventive measures as soon as possible. The administration of ototoxic drugs is associated with a substantial increase in susceptibility to hearing impairment among infants. In particular, the vestibular or cochlear toxicity of aminoglycoside drugs results in irreversible hearing loss [35]. Moreover, the A155G mutation carried on the 12s rRNA gene in mitochondria, and the simultaneous use of ethylene propionic acid can increase the ototoxicity of aminoglycosides [29]. Subgroup analysis of this factor revealed that the non-Asian group exhibited a high degree of heterogeneity, comprising only developing countries. Conversely, the Asian group encompassed not only developing nations but also three developed countries. This observed heterogeneity is tentatively associated with the limited prevalence of domestic hearing screening in developing countries, insufficient funding and a shortage of testing professionals. Septicemia or meningitis, an infectious disease in infants, is one of the risk factors. Sensorineural hearing loss is the most common serious adverse effect of bacterial meningitis [36]. Swedish guidelines recommend that all patients with meningitis undergo otoscopy and be followed up with audiometry [37]. An association between factors such as male sex and hearing loss was not found in this study. The low level of evidence might be due to the collection of data from different countries with a potential admission and detection bias. This study found no significant association between SGA and infant hearing loss, contradicting previous studies [12, 25]. The variation in the proportion of non-LBW infants classified as SGA across different studies and the heterogeneity between studies may explain this discrepancy, suggesting the need for larger sample sizes and rigorous clinical designs to clarify their relationship. Our study also did not observe a significant correlation between infant hearing loss and preterm birth, which may be caused by the improvement of perinatal care conditions and the overall decrease of complications in preterm infants [38].This study also revealed strong correlations between family history of hearing impairment and intrauterine infection and hearing loss in infants. While 60–70% of deafness cases are caused by genetic factors [39], such as the GJB2, GJB3 and SLC26A4 genes, mutations in the GJB2 gene are the most common [40]. In contrast to the results of Karaca et al. [41], we found that hyperbilirubinemia is a significant risk factor for hearing loss, possibly due to variations in hearing screening methods. Elevated bilirubin levels in the blood can damage the auditory nerve and central nervous system. At this time, auditory brainstem response (ABR) tests, which assess the complete function of the outer ear to the lower brainstem pathway, have a greater detection rate for hearing loss than otoacoustic emission (OAE) tests. There has been increasing evidence that the auditory nervous system is the most sensitive nervous system to bilirubin toxicity [42]. Infants with severe jaundice are at increased risk for auditory nerve disorders [43]. Without timely intervention, these children may face problems related to abnormal language development [44].
The limitations of this study are as follows: (1) The exclusion of grey literature in the analyzed studies may introduce publication bias. (2) Several influencing factors, such as racial differences in Africa and Latin America, were not included due to the limited sample size. It is worth noting that 80% of hearing-impaired children worldwide come from low- and middle-income countries, which further reduces confidence in assessing certain risk factors. (3) Inconsistencies between subgroup results and overall findings suggest potential instability in the results of these studies. Future research should involve larger sample sizes from multiple centers to clarify the risk of hearing loss.
Conclusion
The study revealed 14 risk factors that are strongly linked to infant hearing loss, with moderate evidence for four of these risk factors. Health care professionals need to perform premarital counseling, provide medical screening and fertility guidance and perform TORCH screening for pregnant women. Raising awareness and educating the public on the importance of new-born hearing screening are crucial for identifying infants with hearing loss and intervening as soon as possible. Future large-scale, multicenter studies are needed to investigate the combined impact of multiple risk factors on infant hearing loss and to translate these factors into risk-based scoring systems through prospective research.
Data availability
Data supporting the article may be reasonably requested from the corresponding author.
References
Organization WH (2018) Addressing the rising prevalence of hearing loss
Wood SA, Sutton GJ, Davis AC (2015) Performance and characteristics of the Newborn Hearing Screening Programme in England: the first seven years. Int J Audiol 54(6):353–358
American Academy of Pediatrics, Joint Committee on Infant Hearing (2007) Year 2007 position statement: Principles and guidelines for early hearing detection and intervention programs. Pediatrics 120(4):898–921
Hearing TJCoI (2019) Year 2019 position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detect Interv 1–44
Saluja S, Agarwal A, Kler N, Amin S (2010) Auditory neuropathy spectrum disorder in late preterm and term infants with severe jaundice. Int J Pediatr Otorhinolaryngol 74(11):1292–1297
Fulcher A, Purcell AA, Baker E, Munro N (2012) Listen up: children with early identified hearing loss achieve age-appropriate speech/language outcomes by 3 years-of-age. Int J Pediatr Otorhinolaryngol 76(12):1785–1794
Stika CJ, Eisenberg LS, Johnson KC, Henning SC, Colson BG, Ganguly DH, DesJardin JL (2015) Developmental outcomes of early-identified children who are hard of hearing at 12 to 18 months of age. Early Hum Dev 91(1):47–55
Whicker JJ, Muñoz K, Nelson LH (2019) Parent challenges, perspectives and experiences caring for children who are deaf or hard-of-hearing with other disabilities: a comprehensive review. Int J Audiol 58(1):5–11
Sininger YS, Grimes A, Christensen E (2010) Auditory development in early amplified children: factors influencing auditory-based communication outcomes in children with hearing loss. Ear Hear 31(2):166–185
Abiramalatha T, Bandyopadhyay T, Ramaswamy VV, Shaik NB, Thanigainathan S, Pullattayil AK, Amboiram P (2021) Risk factors for periventricular leukomalacia in preterm infants: a systematic review, meta-analysis, and GRADE-based assessment of certainty of evidence. Pediatr Neurol 124:51–71
Hajare P, Mudhol R (2022) A study of JCIH (Joint Commission on Infant Hearing) risk factors for hearing loss in babies of NICU and well baby nursery at a tertiary care center. Indian J Otolaryngol Head Neck Surg 74(Suppl 3):6483–6490
Mannan MA, Choudhury SM, Dey AC, Dey SK, Naher BS, Shahidullah M (2014) Newborn hearing screening: what are we missing? Bangladesh Med Res Counc Bull 40(1):1–5
Harbi NB et al (2008) Hearing screening in at risk newborn. J Med Sci 8(7):648–653
Abu-Shaheen A, Al-Masri M, El-Bakri N, Batieha A, Nofal A, Abdelmoety D (2014) Prevalence and risk factors of hearing loss among infants in Jordan: initial results from universal neonatal screening. Int J Audiol 53(12):915–920
Olusanya BO (2009) Newborns at risk of sensorineural hearing loss in low-income countries. Arch Dis Child 94(3):227–230
Anastasio ART, Yamamoto AY, Massuda ET, Manfredi AKS, Cavalcante JMS, Lopes BCP, Aragon DC, Boppana S, Fowler KB, Britt WJ, Mussi-Pinhata MM (2021) comprehensive evaluation of risk factors for neonatal hearing loss in a large Brazilian cohort. J Perinatol 41(2):315–323
Beswick R, Driscoll C, Kei J, Khan A, Glennon S (2013) Which risk factors predict postnatal hearing loss in children? J Am Acad Audiol 24(3):205–213
Meyer C, Witte J, Hildmann A, Hennecke KH, Schunck KU, Maul K, Franke U, Fahnenstich H, Rabe H, Rossi R et al (1999) Neonatal screening for hearing disorders in infants at risk: incidence, risk factors, and follow-up. Pediatrics 104(4 Pt 1):900–904
Mäki-Torkko EM, Järvelin MR, Sorri MJ, Muhli AA, Oja HF (1998) Aetiology and risk indicators of hearing impairments in a one-year birth cohort for 1985–86 in northern Finland. Scand Audiol 27(4):237–247
Maharani NLP (2015) Risk factors for hearing loss in neonates. The Indonesian Journal of Pediatrics and Perinatal Medicine 55:328–332
Umehara T, Hosokawa S, Kita JY, Takahashi G, Okamura J, Nakanishi H, Hosokawa K, Kyou K, Hayashi Y, Mineta H (2019) Risk factors and prognostic factors of hearing impairment in neonatal intensive care unit-treated infants. Audiol Neurootol 24(2):84–89
Eras Z, Konukseven O, Aksoy HT, Canpolat FE, Genç A, Sakrucu ED, Develioğlu O, Dilmen U (2014) Postnatal risk factors associated with hearing loss among high-risk preterm infants: tertiary center results from Turkey. Eur Arch Otorhinolaryngol 271(6):1485–1490
Jeong J, Youk TM, Oh J, Eo TS, Choi HS (2021) Neonatal and maternal risk factors for hearing loss in children based on population-based data of Korea. Int J Pediatr Otorhinolaryngol 147:110800
Bhat JA, Kurmi R, Kumar S, Ara R, Mittal AK (2018) Targeted screening for hearing impairment in neonates: a prospective observational study. Indian J Otol 24(1):42–46
Hirvonen M, Ojala R, Korhonen P, Haataja P, Eriksson K, Gissler M, Luukkaala T, Tammela O (2018) Visual and hearing impairments after preterm birth. Pediatrics 142(2)
Gupta AK, Anand NK, Raj H (1991) Evaluation of risk factors for hearing impairment in at risk neonates by brainstem evoked response audiometry (BERA). Indian J Pediatr 58(6):849–855
Hille ET, van Straaten HI, Verkerk PH (2007) Prevalence and independent risk factors for hearing loss in NICU infants. Acta Paediatr 96(8):1155–1158
Megantara I, T’sidkenu MI, Chaidir L, Anggraeni R, Sylviana N (2021) Relation between risk factor of hearing loss and the result of otoacoustic emission in newborns at Santosa Hospital Bandung Central. Hearing Balanc Commu 19(3):167–174
Thangavelu K, Martakis K, Fabian S, Venkateswaran M, Roth B, Beutner D, Lang-Roth R (2019) Prevalence and risk factors for hearing loss in high-risk neonates in Germany. Acta Paediatr 108(11):1972–1977
Wien MA, Whitehead MT (2017) The association among prematurity, cochlear hyperintensity, and hearing loss. Neuroradiol J 30(5):448–453
American Academy of Pediatrics and Committee on Environmental Health (1997) Noise: a hazard for the fetus and newborn. Pediatrics 100(4):724–727
Cristobal R, Oghalai JS (2008) Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed 93(6):F462-468
Hemmingsen D, Moster D, Engdahl B, Klingenberg C (2023) Hearing impairment after asphyxia and neonatal encephalopathy: a Norwegian population-based study. Eur J Pediatr
Jiang ZD, Zang Z, Wilkinson AR (2012) Cochlear function in 1-year-old term infants born with hypoxia-ischaemia or low Apgar scores. J Paediatr Child Health 48(2):160–165
Schacht J, Talaska AE, Rybak LP (2012) Cisplatin and aminoglycoside antibiotics: hearing loss and its prevention. Anat Rec (Hoboken) 295(11):1837–1850
de Jonge RC, Sanders MS, Terwee CB, Heymans MW, Gemke RJ, Koomen I, Spanjaard L, van Furth AM (2013) Independent validation of an existing model enables prediction of hearing loss after childhood bacterial meningitis. PLoS One 8(3):e58707
Infektionsläkarföreningen S, Bläckberg J, Brink M et al. Bakteriella CNS-infektioner[J]
Hack M, Friedman H, Fanaroff AA (1996) Outcomes of extremely low birth weight infants. Pediatrics 98(5):931–937
Ling Q, Li M, Xu B, Zhon J, Huang Z (2016) Clinical study on combined screening of hearing and deafness genes in 1280 newborns. Chinese J Eugenics and Heredity 24(5):82–84
Zhao HB (2017) Hypothesis of K(+)-recycling defect is not a primary deafness mechanism for Cx26 (GJB2) deficiency. Front Mol Neurosci 10:162
Karaca CT, Oysu C, Toros SZ, Naiboǧlu B, Verim A (2014) Is hearing loss in infants associated with risk factors? Evaluation of the frequency of risk factors. Clin Exp Otorhinolaryngol 7(4):260–263
Amin SB (2004) Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol 28(5):340–347
Berlin CI, Morlet T, Hood LJ (2003) Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatr Clin North Am 50(2):331–340, vii-viii
Amin SB, Prinzing D, Myers G (2009) Hyperbilirubinemia and language delay in premature infants. Pediatrics 123(1):327–331
Funding
This article was funded by the Hebei Provincial Bureau of Foreign Experts Affairs (YZ202302).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yiwei Han, Shangbin Li. Data curation: Jingfei Sun, Yankun Song. Formal analysis: Yiwei Han, Shangbin Li. Investigation: Yiwei Han, Shangbin Li, Qian Zhao. Methodology: Yiwei Han, Shangbin Li, Weichen Yan. Supervision: Xiong Gao, Qian Zhao, Changjun Ren. Validation: Jingfei Sun, Yankun Song, Xueying Li. Visualization: Jie Wang, Changjun Ren. Writing-original draft: Yiwei Han, Shangbin Li. Writing-review and editing: Yiwei Han, Shangbin Li, Changjun Ren.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Gregorio Milani
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, Y., Li, S., Song, Y. et al. Risk factors for infant hearing loss: a meta-analysis. Eur J Pediatr 183, 2401–2409 (2024). https://doi.org/10.1007/s00431-024-05498-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-024-05498-3