Abstract
High-frequency oscillatory ventilation (HFOV) is an alternative to conventional mechanical ventilation (CMV). Recently, the use of volume guarantee (VG) combined with HFOV has been suggested as a safe strategy capable of reducing the damage induced by ventilation in immature lungs. However, the possible impact of this new ventilation technique on cerebral hemodynamics is unknown. To evaluate the cerebral hemodynamics effect of HFOV combined with VG in an experimental animal model of neonatal respiratory distress syndrome (RDS) due to surfactant deficiency compared with HFOV and CMV+VG (control group). Eighteen newborn piglets were randomized, before and after the induction of RDS by bronchoalveolar lavage, into 3 mechanical ventilation groups: CMV, HFOV and HFOV with VG. Changes in cerebral oxygen transport and consumption and cerebral blood flow were analyzed by non-invasive regional cerebral oxygen saturation (CrSO2), jugular venous saturation (SjO2), the calculated cerebral oxygen extraction fraction (COEF), the calculated cerebral fractional tissue oxygen extraction (cFTOE) and direct measurement of carotid artery flow. To analyze the temporal evolution of these variables, a mixed-effects linear regression model was constructed. After randomization, the following statistically significant results were found in every group: a drop in carotid artery flow: at a rate of -1.7 mL/kg/min (95% CI: -2.5 to -0.81; p < 0.001), CrSO2: at a rate of -6.2% (95% CI: -7.9 to -4.4; p < 0.001) and SjO2: at a rate of -20% (95% CI: -26 to -15; p < 0.001), accompanied by an increase in COEF: at a rate of 20% (95% CI: 15 to 26; p < 0.001) and cFTOE: at a rate of 0.07 (95% CI: 0.05 to 0.08; p < 0.001) in all groups. No statistically significant differences were found between the HFOV groups.
Conclusion: No differences were observed at cerebral hemodynamic between respiratory assistance in HFOV with and without VG, being the latter ventilatory strategy equally safe.
What is Known: • Preterm have a situation of fragility of cerebral perfusion wich means that any mechanical ventilation strategy can have a significant influence. High-frequency oscillatory ventilation (HFOV) is an alternative to conventional mechanical ventilation (CMV). Recently, the use of volume guarantee (VG) combined with HFOV has been suggested as a safe strategy capable of reducing the damage induced by ventilation in immature lungs. Several studies have compared CMV and HFOV and their effects at hemodynamic level. It is known that the use of high mean airway pressure in HFOV can cause an increase in pulmonary vascular resistance with a decrease in thoracic venous return. | |
What is New: • The possible impact of VAFO + VG on cerebral hemodynamics is unknown. Due the lack of studies and the existing controversy, we have carried out this research project in an experimental animal model with the aim of evaluating the cerebral hemodynamic repercussion of the use of VG in HFOV compared to the classic strategy without VG. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Respiratory distress syndrome (RDS) continues to be the main causes of morbidity and mortality in prematurity, with the risk of bronchopulmonary dysplasia (BPD) being high [1]. Although the pathogenesis of BPD is multifactorial, invasive mechanical ventilation is one of the most relevant etiological factors [2,3,4].
An alternative to conventional mechanical ventilation (CMV) is high-frequency oscillatory ventilation (HFOV). In recent years, the possibility of directly controlling high-frequency tidal volume (VThf) by incorporating volume guarantee (VG) represents a new alternative with the theoretical advantage of keeping the volumes generated at high frequency constant and reducing them to the minimum, thus avoiding exposure of the most immature lungs to large volumes [5,6,7].
Several studies have compared CMV and HFOV and their effects at hemodynamic level. It is known that the use of high mean airway pressure (mPaw) in HFOV can cause an increase in pulmonary vascular resistance with a decrease in thoracic venous return [8,9,10]. However, some studies that used optimal mPaw reported no decrease in ventricular function or cardiac output [11,12,13].
Premature infants have a high risk of long-term neurological sequelae; the etiology being multifactorial [14]. One important point is the impaired cerebral blood flow (CBF) autoregulation, making them especially vulnerable to changes in blood pressure [15, 16].
This situation of this fragility of cerebral perfusion means that any mechanical ventilation strategy can have a significant influence, so this new HFOV with VG could have a potential effect on cerebral perfusion. However, no studies have evaluated this effect. To better analyzed this new ventilatory strategy on cerebral hemodynamics, HFOV with or without VG were compared in an experimental model of neonatal RDS due to surfactant deficiency.
Materials and methods
Study subjects
Eighteen healthy at term Landrace-Large White piglets of 24–96 h of life with an average body weight of 1500–2500 g. The minimum sample size was used to find significant differences. In the preparation of the experimental model, 6 animals which were not included in the study were used. This study was approved by the Institutional Animal Care and Use Committee (Ethical Committee CEEA/OH 002-002/2019 Ref. PROEX 169/19) and carried out in accordance with the terms authorized by the European and National Regulations on the Handling of Experimental Animals (2010/63/UE and Royal Decree 53/2013). ARRIVE guidelines have been followed.
Anesthesia
Induction of general anesthesia was performed with 8% sevoflurane vaporized in oxygen, morphine (1 mg/kg) and muscle relaxant (rocuronium 0.6 mg/kg) as a bolus; general anesthesia was subsequently maintained by continuous intravenous infusion of propofol (5–30 mg/kg/hour).
Animal preparation
Once an adequate level of anesthesia was achieved, endotracheal intubation by tracheostomy and tracheal ligation was done, using a 3 mm diameter endotracheal tube, to prevent any air leaks. A peripheral intravenous line was placed (24G polyethylene catheter) for continuous perfusion of isotonic saline solution (10 ml/kg/hour) and propofol.
Piglets were connected to the respirator (Babylog VN500, Dräger ®, Lübeck, Germany), set to CMV using pressure support ventilation (PSV), in VG mode, with the following initial settings: a positive end-expiratory pressure (PEEP) of 6 cmH20, inspired oxygen fraction (FiO2) of 40% to maintain a transcutaneous oxygen saturation (SatO2) of 90–95%, a respiratory rate of 60 rpm and a tidal volume (Vt) 7 ml/kg to achieve an arterial partial pressure of carbon dioxide (PaCO2) of 40–55 mmHg. Peak inspiratory pressure (PIP) was defined to enable automatic adjustment by the respirator to achieve the Vt set.
Monitoring
To monitor cerebral hemodynamics, the following were used:
-
Ultrasonic flow probe in the left carotid artery (Transonic® Flowprobe, Transonic Systems INC, NY, USA) to measure cerebral blood flow using a flow meter (T206 blood flow meter, Transonic Systems INC, NY, USA).
-
INVOS 5100 Monitor® (Covidien, Dublin, Ireland) with two neonatal near-infrared spectroscopy sensors placed in the frontomedial region to monitor cerebral regional oxygen saturation (CrSO2) and another in the thoracolumbar region to monitor somatic regional oxygen saturation (RrSO2).
-
Right femoral artery (20G polyethylene catheter) and right jugular vein (22G polyethylene catheter) with retrograde cannulation to the jugular bulb: for measurement of cerebral oxygen consumption by difference between arterial and venous blood gases (IL 1306 ph/Blood Gas Analyzer, Allied Instrumentation) and continuous monitoring of blood pressure through a calibrated pressure transducer
-
Continuous monitoring of the electrocardiogram and heart rate, as well as SatO2 (using a pulse oximeter placed on the leg) and rectal temperature (maintained between 37 and 39 °C).
Experimental protocol
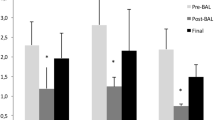
After an initial 60-min stabilization period and once adequate CMV ventilation was achieved, bronchoalveolar lavage (BAL) was performed with 5 aliquots of 10 ml/kg of isotonic saline solution warmed to body temperature (38 °C), to decrease lung compliance due to surfactant depletion. Before and after BAL, pulmonary dynamic compliance (Cdyn) was noted, and the alveolar-arterial difference of oxygen (A-aDO2) was calculated (moderate disease 200–500 mmHg) (Fig. 1) [17].
After BAL, FiO2 increased to 100% and HFOV (mPaw 10 cm H2O, at a frequency of 10 Hz and VThf 2.5 ml/kg) was initiated to perform a lung recruitment maneuver to ensure alveolar re-expansion and avoid the appearance of atelectasis [5].
After lung recruitment, animals were randomized, and the ventilation strategy was initiated in accordance with the protocol assigned to each group (Fig. 2), with data collection for a period of 2 h from stabilization after lung recruitment. Random numbers were generated using the standard = RAND() function in Microsoft Excel’. At the end of the study, animals were sacrificed with an overdose of propofol, followed by potassium chloride bolus (10 mEq/kg).
Randomization groups of the experimental model. FiO2: inspired oxygen fraction; CMV: conventional mechanical ventilation; PSV+VG: pressure support ventilation with volume guarantee; HFOV: high-frequency oscillatory ventilation; HFOV + VG: high-frequency oscillatory ventilation with volume guarantee; PaCO2: arterial partial pressure of carbon dioxide; I:E ratio: inspiration: expiration ratio; ΔPhf: oscillation pressure amplitude; Hz: frequency; VThf: high-frequency tidal volume; mPaw: mean airway pressure; SatO2: oxygen transcutaneous saturation. PEEP: positive endexpiratory pressure; VG: volume guarantee
Since this was a strict experimental model, the confusion variables were reduced to a minimum.
Data collection
Arterial and venous blood samples were taken blood gas analysis, initially every 20 min until the target PaCO2 was reached and, subsequently, at one hour and at 2 h after the start of the assigned strategy. Ventilator and regional oxygen saturation data were obtained from the ventilator and the INVOS devices through an USB port. Data on carotid artery flow and other hemodynamic and respiratory variables, temperature, and sedation were collected. Data were collected every 10 min. Calculations were performed for cerebral fractional tissue oxygen extraction (cFTOE): (satO2 pulse oximetry- CrSO2) / satO2 pulse oximetry; somatic fractional tissue oxygen extraction (sFTOE): (satO2 pulse oximetry- RrSO2) / satO2 pulse oximetry; cerebral oxygen extraction fraction (COEF): (arterial SO2– SjO2) / arterial SO2 × 100; cerebral-somatic ratio (CSOR): RrSO2 / CrSO2.
The evolution of arterial blood gases was analyzed at 4 moments: in the 1st hour of stabilization and after randomization (at 20, 60 and 120 min, prior to animal sacrifice). The data on the COEF and jugular venous saturation (SjO2) were analyzed at 3 moments: in the 1st hour of stabilization and after randomization (at 60 and 120 min). Other hemodynamics were evaluated at 5 moments: pre-randomization and post-randomization (at 20, 60, 90 and 120 min).
Statistics
Descriptive statistics were calculated according to their nature. The median and interquartile range for the quantitative variables were reported. The possible association between the non-longitudinal quantitative variables of the study and the experimental groups was studied using the Wilcoxon signed-rank test. To analyze the temporal evolution of the quantitative variables, a mixed-effects linear regression model was constructed, in which time, the experimental group, and the interaction between the two were considered as fixed effects. A random effect was included to account for the variability of repeated measures over time for each animal. A p-value threshold of 0.05 was used to determine statistical significance. All analyses were performed with R Statistical Software (version 4.2.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
The median body weight of the piglets was 2.05 kg (IQR 1.80-2.20) with a median age of 3 days of life (IQR 2-4). 16 of the 18 animals were male (88%).
Initial stabilization and saline lung lavage
All animals were adequately ventilated and oxygenated. No differences were observed in hemodynamic parameters during the first 60 min. Similar respiratory distress occurs after BAL in all the animals without differences between groups (p = 0.2). The median of the A-a DO2 after BAL was: PSV+VG group, 382 mmHg (IQR 330-413); HOFV group, 358 mmHg (IQR 282-434); HOFV + VG group, 308 mmHg (IQR 240-378).
Pre-randomization situation
The baseline situation prior to randomization after BAL and lung recruitment was similar in all piglets, with no significant differences found between groups, except for carotid artery flow (Table 1). All animals at that moment were ventilated on HFOV+VG.
Ventilator settings and parameters
After randomization, the ΔPhf, VThf/kg and DCO2 values were modeled using linear mixed-effects regression without detecting differences between the 2 groups ventilated with HFOV. When comparing the medians, no significant differences were found either: ΔPhf 44 (IQR 41-78) vs 39 (IQR 34-55), p > 0.9; VThf/kg 3.2 (IQR 3-3.33) vs 3.19 (IQR 3.04-3.2), p > 0,9; DCO2 120 (IQR 90-113) vs 120 (IQR 93-102), p > 0.9, for HFOV with VG and HFOV without VG, respectively. A mPaw of 10 cmH20 with 10 Hz remained constant in both groups after randomization.
Hemodynamic and cerebral blood flow changes:
-
1.
Comparison between PSV+VG vs HFOV and HFOV+VG:
-
a.
Changes in arterial blood gas analyses:
No significant differences were found between groups in pH, pO2, pCO2, oxyhemoglobin saturation (SO2) and glucose at the 4 moments studied. However, significant differences were detected over time in the 18 animals, with no differences between the study groups in 3 variables. Hemoglobin (Hb): Constant decrease at a rate of -0.27 g/dl (95% CI: -0.5 to -0.03; p = 0.028); Lactic acid: constant increase at a rate of 0.52 mmol/L (95% CI: 0.29 to 0.75; p < 0.001); Base excess (BE): constant decrease at a rate of -1.9 mmol/L (95% CI: -2.7 to -1.1; p < 0.001) (Table 2).
-
b.
Changes in hemodynamic parameters:
Mean arterial pressure (MAP) steadily decreased at a rate of -2.6 mmHg (95% CI: -4.1 to -1.1; p < 0.001) over time regardless of the treatment group. The HFOV with VG group had on average 16 mmHg higher MAP than PSV + VG group, these differences being significant (95% CI: 3.8 to 27; p = 0.011) (Table 3).
-
c.
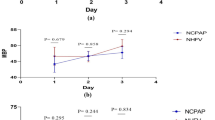
Changes in cerebral oxygenation: (Fig. 3)
CrSO2 (%) decreased steadily at a rate of -6.2% (95% CI: -7.9 to -4.4; p < 0.001) over time regardless of the treatment group. At 60 minutes after randomization, a drop in CrSO2 > 20% in 5 animals per group was observed (5/6; 83%); at 120 minutes after randomization, in 5 animals in the groups ventilated with HFOV (5/6; 83%) and in all animals in the PSV+VG group (6/6; 100%) with no significant differences between groups. cFTOE increased steadily at a rate of 0.07 (95% CI: 0.05 to 0.08; p < 0.001) over time regardless of the treatment group. Carotid artery flow fell steadily at a rate of -1.7 mL/kg/min (95% CI: -2.5 to -0.81; p < 0.001) over time regardless of the treatment group. COEF increased steadily at a rate of 20% (95% CI: 15 to 26; p < 0.001) over time regardless of the treatment group. SjO2 decreased steadily at a rate of -20% (95% CI: -26 to -15; p < 0.001) over time regardless of the treatment group.
-
d.
Changes in systemic tissue oxygenation:
RrSO2 (%) decreased steadily at a rate of -2.5% (95% CI: -3.7 to -1.3; p < 0.001) over time regardless of the treatment group. At 60 minutes after randomization, a drop in RrSO2 > 20% in one animal per group was observed (1/6; 17%); at 120 minutes after randomization, in 2 animals per group (2/6; 33%), without finding significant differences between groups. cFTOE increased steadily at a rate of 0.03 (95% CI: 0.02, to 0.04; p < 0.001) over time regardless of the treatment group. CSOR increased consistently at a rate of 0.06 (95% CI: 0.03 to 0.10; p < 0.001) over time regardless of the treatment group.
-
e.
Changes in other factors:
No statistically significant variations were observed in temperature throughout the study. As regards propofol infusion (mg/kg/hour), no significant differences were found either: PSV + VG group median 19 (IQR 14, 24); HFOV without VG group 18 (IQR 13, 22); HFOV without VG group 22 (IQR 18, 27).
-
a.
-
2.
Comparison between HFOV vs HFOV + VG:
-
a.
Changes in arterial blood gas analyses:
No significant differences were found in pH, pO2, pCO2, SO2, glucose and hemoglobin in terms of global values at the 4 moments studied. However, we observed significant differences in 2 variables over time in the 12 animals included, with no differences between the two groups. Lactic: steady increase at a rate of 0.39 mmol/L (95% CI: 0.17 to 0.62; p = 0.001); BE: this decreased constantly at a rate of -2 mmol/L (95% CI: -2.8 to -1.1; p < 0.001). (Table 2).
-
b.
Changes in hemodynamic parameters:
MAP steadily decreased at a rate of -3.2 mmHg (95% CI: -4.9 to -1.6; p < 0.001) over time regardless of the treatment group. (Table 3)
-
c.
Changes in cerebral oxygenation: (Fig. 3)
CrSO2 (%) decreased steadily at a rate of -5.3% (95% CI: -7.1 to -3.6; p < 0.001) over time regardless of the treatment group. cFTOE increased steadily at a rate of 0.05 (95% CI: 0.04 to 0.07; p < 0.001) over time regardless of the treatment group. Carotid artery flow fell steadily at a rate of -3.1 mL/kg/min (95% CI: -4.2 to -2.1; p < 0.001) over time regardless of the treatment group. COEF increased steadily at a rate of 8.7% (95% CI: 2.6 to 15; p < 0.007) over time regardless of the treatment group. SjO2 decreased steadily at a rate of -8.6% (95% CI: -15 to -2.7; p < 0.001) over time regardless of the treatment group.
-
d.
Changes in systemic tissue oxygenation:
RrSO2 (%) decreased constantly at a rate of -1.4% (95% CI: -2.6 to -0.22; p < 0.021) over time regardless of the treatment group. sFTOE increased steadily at the rate of 0.01 (95% CI: 0.00 to 0.03; p < 0.022) over time regardless of the treatment group.
-
a.
A Linear mixed-effects model. Changes in cerebral regional oxygen saturation, cerebral fractional tissue oxygen extraction and carotid artery flow. The HFOV groups exhibited a sharper reduction in carotid flow. In the HFOV with VG group, this fell at a rate of -1.6 ml/kg/min compared to the PSV+VG group (95%CI: -2.8, -0.38; p = 0.011) and in the HFOV without VG group, the decrease was at a rate of -1.5 ml/kg/min compared to the PSV+VG group (95%CI: -2.7, -0.26; p = 0.011). Times (1: pre-randomization; 2: 20 min post-randomization; 3: 60 min post-randomization; 4: 90 min post-randomization; 5: 120 min post-randomization, prior to animal sacrifice). B Linear mixed-effects model. Changes in the cerebral oxygen extraction fraction and jugular venous oxygen saturation. The HFOV without VG group presented a less pronounced increase in COEF, being -12% compared to the PSV+VG group (95% CI: -19, -3.9; p = 0.004). The HFOV without VG group exhibited a less pronounced decrease in SjO2, being 12% higher compared to the PSV+VG group. (95% CI: 4.1,20; p = 0.004). Times (1: in the 1st hour of stabilization; 2: 60 min post-randomization; 3: 120 min post-randomization, prior to animal sacrifice). CrSO2: Cerebral regional oxygen saturation; cFTOE: cerebral fractional tissue oxygen extraction; COEF: cerebral oxygen extraction fraction; SjO2: jugular venous saturation PSV+VG: pressure support ventilation with volume guarantee; VAFO: high-frequency oscillatory ventilation; VAFO+VG: high-frequency oscillatory ventilation with volume guarantee
Discussion
This experimental study in a neonatal animal respiratory distress model suggests to us that the use of VG in HFOV does not have any impact on cerebral hemodynamics compared to the classic strategy of HFOV without VG.
In the control CMV group after BAL there was a gradual drop in CBF: a decrease in carotid artery flow, CrSO2 and SjO2 accompanied by an increase in COEF and cFTOE. These same changes were observed in the two groups of HFOV with no significant differences between them (Fig. 3).
Brain oxygenation depends on oxygen delivery (DO2), that is arterial oxygen content (which, in turn, depends on Hb concentration, its saturation status, and its affinity for O2) and blood flow (which is influenced by cardiac output and vascular resistance). O2 extraction and consumption (VO2) by tissues is mostly related to oxygen transport and the metabolic status [18]. Under normal conditions, VO2 does not depend on DO2 since O2 transported is greater than VO2. When an imbalance occurs, normally due to a decrease in DO2, a number of compensation mechanisms are activated, notably the increase in COEF to keep VO2 constant [19]. If this situation is maintained over time, a maximum point of extraction (critical oxygen transport point) will be reached, where VO2 will depend on DO2 and new decrease in DO2 will reduce VO2, resulting in anaerobiosis and acidosis [20].
There are different techniques to monitor cerebral hemodynamics. One is jugular oximetry [21], with blood gas extraction through a catheter positioned retrograde through the internal jugular vein into the jugular bulb to calculate COEF, which provides an indirect estimate of CBF [22]. Another method is by means of cerebral oximetry with near-infrared spectroscopy (NIRS) [23], which measures CrSO2, analyzing the balance between O2 supply and demand. The degree of saturation is measured at the level of the exchange vessels where 75–80% correspond to the venous and tissular compartment. It therefore reflects the amount of O2 not extracted by tissues. The changes will be directly proportional to the supply and metabolic demand of cerebral [16, 24, 25].
During the 120 min of the study protocol, a decrease was observed in CrSO2 and SjO2 with an increase in the cerebral COEF and cFTOE. Why does this decrease in CBF occur in the 3 modalities of ventilatory support? The three main metabolic factors that can affect CBF are pCO2, pO2 and hydrogen ions. Hypercapnia, hypoxemia, and acidosis cause cerebral vasodilatation with increased in CBF. Initially, VO2 should remain constant. DO2 depends on cardiac output and arterial O2 content, which in turn depends on Hb content and its O2 saturation. No significant differences were observed for these previously described values in the 3 groups. Although there seemed to be a statistically significant decrease in Hb, we did not consider this to be clinically relevant. Nor did we find factors that might have shifted the oxyhemoglobin dissociation curve, resulting in a lower affinity of Hb for O2 (increases in pCO2, hydrogen ion or temperature) [26]. Despite the statistically significant increase in lactic acid and BE, we consider that this was not a clinically significant change, with arterial blood gases being maintained at pH > 7.20 (Table 2).
Sedation is also a conditioning factor in CBF. In our study, a continuous infusion of propofol was maintained. This drug produces a decrease in CBF and in cerebral metabolic oxygen demand, preserving autoregulation and performing a neuroprotective role [27, 28]. No significant differences were found in the doses administered between groups after randomization.
Another important point to evaluate is the autoregulation of CBF. In the immature brain of a newborn, especially of a premature one, CBF regulation capacity is altered, resulting in fluctuations in perfusion and cerebral oxygenation [16, 29]. This loss of autoregulation in the first days of life has been associated with brain damage, namely decreased CBF with silent ischemic lesions such as periventricular leukomalacia [29, 30] and the increase in CBF with cerebral hyper perfusion with risk of germinal matrix hemorrhage or intraventricular hemorrhage [29, 31, 32]. It is therefore important to determine the degree of involvement of the different MV strategies in variations in CBF. Little has been described so far regarding HFOV with VG.
In 2021 Bhogal et al. [33] published an experimental study in piglets with RDS in which they concluded that animals ventilated with HFOV+VG presented a significant drop in CBF with a decrease in CrSO2, compared to those ventilated with HFOV without VG and CMV. The explanation given for this finding was that the piglets assisted with HFOV + VG reached the end of the study with statistically lower blood pressure figures (MAP 35 mmHg), and they considered that, based on these figures, there was a loss of CBF autoregulation [34]. Other studies have demonstrated the persistence of CBF autoregulation in piglets with MAP values of around 30 and 100 mmHg [35]. In preterm newborns, Munro et al. [36] also reported persistent cerebral autoregulation with MAP values higher than this figure (30 mmHg). However, other authors been unable to define the optimal blood pressure for maintaining adequate cerebral perfusion in this population [15, 37].
We observed a decrease in cardiac output (gradual fall in MAP) with a decrease in CBF together with a progressive increase in COEF to maintain VO2 without reaching the maximum extraction point. Additionally, we noted a greater impact on CBF than on somatic flow. There was a decrease of > 20% in CrSO2 at 120 min in almost 100% of the animals. However, the decrease of > 20% in RrSO2 was only observed in 33% of the animals. As regards the differences found between both groups, the 3 modes of respiratory assistance presented similar behavior, although a sharper drop in carotid artery flow was observed in piglets ventilated with HFOV (with and without VG) compared to the CMV group but maintaining adequate metabolic compensation with increased COEF. This was possibly due to the absence of expiration and maintained pressure, which reduced venous return in the thorax and most likely interfered with carotid flow. The piglets in the CMV group started from a more compromised pre-randomization situation, which perhaps explains the greater increase in COEF with a sharper decrease in SjO2.
No significant differences were found in any of the parameters studied between HFOV with and without VG. Both groups remained normally ventilated with a constant mPaw (10 cm H20) and 10 Hz. This differs from the results reported by Bhogal et al., who found that piglets in the HFOV + VG group needed a significantly higher ΔPhf to achieve the same Vt/kg as in the group without VG. They theorized that this increase in ΔPhf would have caused a fluctuation in intrathoracic pressure, causing CBF to decrease. This result is also not consistent with other published studies which have shown that amplitude variations are not transmitted at the level of the distal airway [35, 38]. Furthermore, in Bhogal’s study there were significant differences in pCO2 values at 60 min among the three groups. However, in our study the most stable pCO2 values were maintained.
Since this was an experimental model with term newborn piglets, the main limitation of our study is that it may not be possible to extrapolate the results to the extremely preterm population. In addition, the study period was limited to 120 min. Nor was surfactant administered, which is the current treatment indicated for RDS. Therefore, we do not know how this treatment could have influenced lung mechanics and, consequently, the repercussions at cerebral hemodynamic level.
Conclusions
In our experimental model of neonatal respiratory distress with invasive ventilation, a greater decrease was observed in CBF than in systemic flow, probably because of changes in thoracic pressure and lung compliance. No differences were observed at cerebral hemodynamic between respiratory assistance in HFOV without and with VG, being the latter ventilatory strategy equally safe. It is worth highlighting the importance that the modifications in CBF may have in RDS in this highly susceptible population.
Abbreviations
- A-aDO2 :
-
Alveolar-arterial difference of oxygen
- BAL:
-
Bronchoalveolar lavage
- BE:
-
Base excess
- BPD:
-
Bronchopulmonary dysplasia
- CBF:
-
Cerebral blood flow
- Cdyn:
-
Dynamic compliance
- cFTOE:
-
Cerebral fractional tissue oxygen extraction
- CMV:
-
Conventional mechanical ventilation
- COEF:
-
Cerebral oxygen extraction fraction
- CrSO2 :
-
Non-invasive regional cerebral oxygen saturation
- CSOR:
-
Cerebral-somatic ratio
- DO2:
-
Oxygen delivery
- FiO2 :
-
Inspired oxygen fraction
- Hb:
-
Hemoglobin
- HFOV:
-
High-frequency oscillatory ventilation
- HFOV + VG:
-
High-frequency oscillatory ventilation with volume guarantee
- HR:
-
Heart rate.
- Hz:
-
Frequency
- MAP:
-
Mean arterial pressure
- mPaw:
-
Mean airway pressure
- NIRS:
-
Near-infrared spectroscopy
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- PaO2 :
-
Arterial partial pressure of oxygen
- PEEP:
-
Positive end-expiratory pressure
- PIP:
-
Peak inspiratory pressure
- PSV+VG:
-
Pressure support ventilation with volume guarantee
- RDS:
-
Respiratory distress syndrome
- RrSO2 :
-
Somatic regional oxygen saturation
- SatO2 :
-
Transcutaneous oxygen saturation
- sFTOE:
-
Somatic fractional tissue oxygen extraction
- SjO2 :
-
Jugular venous saturation
- SO2 :
-
Oxyhemoglobin saturation
- Tª:
-
Temperature
- VG:
-
Volume guarantee
- VO2:
-
Oxygen consumption
- VThf:
-
High-frequency tidal volume
- Vt:
-
Tidal volume
- ΔPhf:
-
Oscillation pressure amplitude
References
Gilfillan M, Bhandari A, Bhandari V (2021) Diagnosis and management of bronchopulmonary dysplasia. BMJ 375:n1974. https://doi.org/10.1136/bmj.n1974
Dolinay T, Himes BE, Shumyatcher M et al (2017) Integrated stress response mediates epithelial injury in mechanical ventilation. Am J Respir Cell Mol Biol 57:193–203. https://doi.org/10.1165/rcmb.2016-0404OC
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116:9S-15S. https://doi.org/10.1378/chest.116.suppl_1.9s-a
Gupta S, Sinha SK, Donn SM (2009) Ventilatory management and bronchopulmonary dysplasia in preterm infants. Semin Fetal Neonatal Med 14:367–373. https://doi.org/10.1016/j.siny.2009.08.011
González-Pacheco N, Sánchez-Luna M, Chimenti-Camacho P et al (2019) Use of very low tidal volumes during high-frequency ventilation reduces ventilator lung injury. J Perinatol 39:730–736. https://doi.org/10.1038/s41372-019-0338-5
González-Pacheco N, Sánchez-Luna M, Ramos-Navarro C et al (2016) Using very high frequencies with very low lung volumes during high-frequency oscillatory ventilation to protect the immature lung. A pilot study. J Perinatol 36:306–310. https://doi.org/10.1038/jp.2015.197
González-Pacheco N, Sánchez-Luna M, Arribas-Sánchez C et al (2020) DCO2/PaCO2 correlation on high-frequency oscillatory ventilation combined with volume guarantee using increasing frequencies in an animal model. Eur J Pediatr 179:499–506. https://doi.org/10.1007/s00431-019-03503-8
de Waal K, Evans N, van der Lee J, van Kaam A (2009) Effect of lung recruitment on pulmonary, systemic, and ductal blood flow in preterm infants. J Pediatr 154:651–655. https://doi.org/10.1016/j.jpeds.2009.01.012
Tana M, Polglase GR, Cota F et al (2015) Determination of lung volume and hemodynamic changes during high-frequency ventilation recruitment in preterm neonates with respiratory distress syndrome. Crit Care Med 43:1685–1691. https://doi.org/10.1097/CCM.0000000000000967
Simma B, Fritz M, Fink C, Hammerer I (2000) Conventional ventilation versus high-frequency oscillation: hemodynamic effects in newborn babies. Crit Care Med 28:227–231. https://doi.org/10.1097/00003246-200001000-00038
Cambonie G, Guillaumont S, Luc F et al (2003) Haemodynamic features during high-frequency oscillatory ventilation in preterms. Acta Paediatr 92:1068–1073. https://doi.org/10.1080/08035250310004856
Kinsella JP, Gerstmann DR, Clark RH et al (1991) High-frequency oscillatory ventilation versus intermittent mandatory ventilation: early hemodynamic effects in the premature baboon with hyaline membrane disease. Pediatr Res 29:160–166. https://doi.org/10.1203/00006450-199102000-00012
Osborn DA, Evans N (2003) Randomized trial of high-frequency oscillatory ventilation versus conventional ventilation: effect on systemic blood flow in very preterm infants. J Pediatr 143:192–198. https://doi.org/10.1067/S0022-3476(03)00359-7
Moore T, Hennessy EM, Myles J et al (2012) Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 345:e7961. https://doi.org/10.1136/bmj.e7961
Rhee CJ, da Costa CS, Austin T et al (2018) Neonatal cerebrovascular autoregulation. Pediatr Res 84:602–610. https://doi.org/10.1038/s41390-018-0141-6
Kooi EMW, Verhagen EA, Elting JWJ et al (2017) Measuring cerebrovascular autoregulation in preterm infants using near-infrared spectroscopy: an overview of the literature. Expert Rev Neurother 17:801–818. https://doi.org/10.1080/14737175.2017.1346472
Grupo Respiratorio y Surfactante de la Sociedad Española de Neonatología (2008) Recommendations for respiratory support in the newborn (I). An Pediatr (Barc) 68:516–524. https://doi.org/10.1157/13120054
Baquero Cano M, Sánchez Luna M, Elorza Fernandez MD et al (1996) Oxygen transport and consumption and oxygen saturation in the right atrium in an experimental model of neonatal septic shock. An Esp Pediatr 44:149–156
Russell JA, Phang PT (1994) The oxygen delivery/consumption controversy. Approaches to management of the critically ill. Am J Respir Crit Care Med 149:533–537. https://doi.org/10.1164/ajrccm.149.2.8306058
Fiddian-Green RG, Haglund U, Gutierrez G, Shoemaker WC (1993) Goals for the resuscitation of shock. Crit Care Med 21:S25-31. https://doi.org/10.1097/00003246-199302001-00006
Gibbs EL, Lennox WG, Nims LF et al (1942) Arterial and cerebral venous blood arterial-venous differences in man. J Biol Chem 144:325–332
Poca MA, Sahuquillo J, Monforte R, Vilalta A (2005) Global systems for monitoring cerebral hemodynamics in the neurocritical patient: basic concepts, controversies and recent advances in measuring jugular bulb oxygenation. Neurocirugia (Astur) 16:301–322. https://doi.org/10.4321/s1130-14732005000400002
Korček P, Straňák Z, Širc J, Naulaers G (2017) The role of near-infrared spectroscopy monitoring in preterm infants. J Perinatol 37:1070–1077. https://doi.org/10.1038/jp.2017.60
Cabañas F, Pellicer A, Valverde E (2000) Non-invasive monitoring of cerebral hemodynamics in the newborn. Rev Neurol 31:775–783
Kalteren WS, Verhagen EA, Mintzer JP et al (2021) Anemia and red blood cell transfusions, cerebral oxygenation, brain injury and development, and neurodevelopmental outcome in preterm infants: a systematic review. Front Pediatr 9:644462. https://doi.org/10.3389/fped.2021.644462
Vali P, Underwood M, Lakshminrusimha S (2019) Hemoglobin oxygen saturation targets in the neonatal intensive care unit: is there a light at the end of the tunnel? Can J Physiol Pharmacol 97:174–182. https://doi.org/10.1139/cjpp-2018-0376
Kotani Y, Shimazawa M, Yoshimura S et al (2008) The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther 14:95–106. https://doi.org/10.1111/j.1527-3458.2008.00043.x
Mikkelsen MLG, Ambrus R, Miles JE et al (2016) Effect of propofol and remifentanil on cerebral perfusion and oxygenation in pigs: a systematic review. Acta Vet Scand 58:42. https://doi.org/10.1186/s13028-016-0223-6
Tsuji M, Saul JP, du Plessis A et al (2000) Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106:625–632. https://doi.org/10.1542/peds.106.4.625
Volpe JJ (2001) Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50:553–562. https://doi.org/10.1203/00006450-200111000-00003
Haruda FD (2001) The structure of blood vessels in the germinal matrix and the autoregulation of cerebral blood flow in premature infants. Pediatrics 108:1050–1051. https://doi.org/10.1542/peds.108.4.1050
Volpe JJ (2008) Neurology of the newborn. Philadelphia
Bhogal J, Solevåg AL, O’Reilly M et al (2021) Hemodynamic effects of high frequency oscillatory ventilation with volume guarantee in a piglet model of respiratory distress syndrome. PLoS ONE 16:e0246996. https://doi.org/10.1371/journal.pone.0246996
Laptook A, Stonestreet BS, Oh W (1982) Autoregulation of brain blood flow in the newborn piglet: regional differences in flow reduction during hypotension. Early Hum Dev 6:99–107. https://doi.org/10.1016/0378-3782(82)90063-9
Silvera F, Gagliardi T, Vollono P et al (2022) Study of the relationship between regional cerebral saturation and pCO2 changes during mechanical ventilation to evaluate modifications in cerebral perfusion in a newborn piglet model. Braz J Med Biol Res 55:e11543. https://doi.org/10.1590/1414-431X2022e11543
Munro MJ, Walker AM, Barfield CP (2004) Hypotensive extremely low birth weight infants have reduced cerebral blood flow. Pediatrics 114:1591–1596. https://doi.org/10.1542/peds.2004-1073
Evans N, Osborn D, Kluckow M (2005) Preterm circulatory support is more complex than just blood pressure. Pediatrics 115:1114–5; author reply 1115–6. https://doi.org/10.1542/peds.2005-0009
Mukerji A, Belik J, Sanchez-Luna M (2014) Bringing back the old: time to reevaluate the high-frequency ventilation strategy. J Perinatol 34:464–467. https://doi.org/10.1038/jp.2014.39
Acknowledgements
The authors thank SENEO for funding the study.
Funding
This study was funded by de Spanish Society of Neonatology (SENEO).
Author information
Authors and Affiliations
Contributions
Alba Pérez-Pérez, Noelia González-Pacheco and Manuel Sánchez-Luna designed the study. Martín Santos-González directed the experimental study in the neonatal animal model. Alba Pérez-Pérez, Noelia González-Pacheco, María Arriaga-Redondo, Cristina Ramos-Navarro, Elena Rodríguez-Corrales and Ana Rodríguez-Sánchez de la Blanca conducted the data collection. Pablo González-Navarro performed the statistical analysis of the data. All authors made contributions to the conception of the work, revised the work critically and approved the final version of the text.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Animal Care and Use Committee (Ethical Committee CEEA/OH 002-002/2019 Ref. PROEX 169/19) and carried out in accordance with the terms authorized by the European and National Regulations on the Handling of Experimental Animals (2010/63/UE and Royal Decree 53/2013).
Competing interests
MSL has received advisory board consulting fees from Dräger. The authors declare that they have no conflicts of interest.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pérez-Pérez, A., González-Pacheco, N., Arriaga-Redondo, M. et al. Impact on cerebral hemodynamics of the use of volume guarantee combined with high frequency oscillatory ventilation in a neonatal animal respiratory distress model. Eur J Pediatr 183, 157–167 (2024). https://doi.org/10.1007/s00431-023-05245-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05245-0