Abstract
The aim of the study was to assess the prevalence of sleep disturbance in pediatric IBD patients and evaluate the relationship between clinical features of IBD, disease activity, inflammatory markers and quality of sleep. A total of 99 patients who were followed-up with the diagnosis of IBD (44 Crohn’s disease (CD), 55 Ulcerative colitis (UC)) between 2015–2020 and 80 healthy controls were enrolled in the study. The clinical and demographic characteristics, laboratory parameters and disease activities were obtained from medical reports retrospectively. Pittsburgh sleep quality index (PSQI) was administered to all participants. PSQI score was significantly higher in patient group than the control group (P < 0.001). The sleep time of patient group, especially patients with UC was later than the control group (P = 0.008). Sleep duration was longer in control group than the patient group (P < 0.001). A positive strong correlation was obtained in disease activity index (r = 0.886; P < 0.001) and abdominal pain (r = 0.781; P < 0.001) with PSQI scores of CD patients. Disease activity index, rectal bleeding, diarrhea and number of stool had statistically significant positive strong correlation with PSQI scores of UC patients (P < 0.001). Pediatric Crohn’s disease activity index and Pediatric ulcerative colitis activity index were the only independent risk factors affecting sleep disturbances (80% sensitivity and 91.67% specificity, 93.1% sensitivity and %96.15 specificity, respectively).
Conclusion: Increased disease activity has adverse effects on sleep quality. PSQI and PCDAI were strong tests for predicting sleep disorders in pediatric patients with IBD.
What is Known: • Sleep disturbances are common complaint in inflammatory bowel disease (IBD), even in clinical remission. • Pittsburgh sleep quality index (PSQI) was used to evaluate the subjective sleep quality of patients. | |
What is New: • PSQI and Pediatric Crohn Disease Activity index (PCDAI) were strong tests for predicting sleep disorders in pediatric patients with IBD. • PSQI and PCDAI scores correlated significantly with the severity of the sleep disturbances. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel diseases (IBDs), are chronic inflammatory diseases of the gastrointestinal tract, caused by abnormal immune response to antigens and environmental factors in genetically susceptible individuals and characterized by repetitive attacks and remissions [1].
Sleep is one of the basic needs for a healthy and quality life. Quality and adequate sleep plays a role in the regulation of the immune and neuroendocrine system [2, 3]. Recent studies showed that there is a strong relationship between sleep disorders and gastrointestinal diseases [4,5,6,7,8,9,10,11,12]. Increase in proinflammatory cytokines such as tumor necrosis factor alpha (TNF), interleukin (IL)-1 and IL-6 is related to sleep disturbances [4]. These cytokines, implicated in the regulation of the sleep-wake cycle, are also involved in the pathogenesis of chronic inflammatory conditions such as IBD, gastroesophageal reflux disease (GERD), liver diseases and colorectal cancer [4, 13]. Additionally, it has been reported that patients with active IBD had worse sleep quality than the patients with inactive IBD [5]. Poor quality sleep may cause exacerbation of symptoms in gastrointestinal diseases or conversely many gastrointestinal diseases negatively affects the sleep-wake cycle related with sleep quality [4, 5]. The possible mechanisms of sleep disturbances in IBD are nocturnal symptoms such as abdominal pain, urgency and diarrhea, disease activity and the treatments (corticosteroids, biological agents) [14].
Pittsburgh sleep quality index (PSQI) was used to evaluate the subjective sleep quality of patients [15]. Studies examining the relationship between the disease activity and sleep disturbences reported that the patients with active disease had higher PSQI scores [4, 6, 7].
The aim of this study was to assess the prevalence of sleep disturbances in pediatric IBD patients and evaluate the relationship between clinical features of IBD, disease activity, inflammatory markers and quality of sleep.
Material and methods
A total of 99 patients who were followed-up in the pediatric gastroenterology department between 2015 and 2020 with the diagnosis of IBD and 80 healthy controls were enrolled in the study. The medical records of the patients were analyzed retrospectively. The clinical and demographic characteristics, laboratory parameters, disease activities, and treatment modalities were obtained. PSQI was administered to all participants. Patients younger than 6 years of age and older than 18 years of age were excluded from the study.
Pediatric ulcerative colitis index (PUCAI) and Crohn’s disease activity index (PCDAI) was used for determining disease activity [16, 17]. PCDAI questionaire was consisted of abdominal pain (mild, moderate, severe), the number of stool (0–1, 2–5, > 6), general well-being, presence of extraintestinal manifestations, such as fever, arthritis, rash, and uveitis; physical examination findings (weight and height); and laboratory parameters as hematocrit, erythrocyte sedimentation rate, and serum albumin. PUCAI was determined by items including general well-being, abdominal pain (none, mild, moderate, severe), rectal bleeding (none, mild, moderate, severe), diarrhea (none, mild, severe), number of stools per day, nocturnal stools, and activity level.
PSQI was used to evaluate the subjective sleep quality of patients in the past month. PSQI was administered to all study parcipitants by the same trained person. The parcipitants were questionned in terms of sleep time, sleep latency (time to fall asleep), wake up time in the morning, and sleep duration in the past month [15, 18]. Items such as get up to use bathroom, can not breath comfortably, cough or snore loudly, feel too cold or hot, had bad dreams and had pain were also determined. Finally, the participants were asked how they rated their overall sleep quality in the past month. PSQI score > 5 was considered as a significant sleep disturbance.
The study was approved by the Ethics Committee of SBU Sisli Hamidiye Etfal Training and Research Hospital (No:1267/28.05.2019). Written informed consents were obtained from all of the parents.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) 23.0 package program (SPSS Inc, Chicago, Illinois, U.S.A.). Pearson chi-square test and Fisher's Exact test were used in the analysis of categorical data. In the analysis of the assumption of normality, when the number of samples in the group is less than 50 Shapiro Wilks test, and if the number of samples in the group is larger than 50 Kolmogorov–Smirnov test were used. In the analysis of the difference between the measurement values of the two groups, Student’s t test conforming to normal distribution and Mann–Whitney U test for variables with abnormal distribution were used. Kruskal Wallis test was used to analyze non-parametric measurements of three or more groups, and Bonferroni-Dunn test was used as post-hoc test for significant cases. When normal distribution assumption is provided, ANOVA test and Tukey HSD test was used for comparison of three or more groups, Spearman correlation test was used to determine the relationship between PSQI and other variables. Cut off analyzes were made with receiver operating characteristics (ROC) curve analysis. To determine the risk factors affecting sleep disturbances in patients, multivariate analysis was made in only with factors of P < 0.05 and the results were presented with Odds Ratio and 95% confidence intervals. P < 0.05 was considered significant for all results.
Results
The mean age of the patients with IBD was 11.8 ± 2.69 (range 7–18 years) and M/F: 1.3 and the mean age of the controls (n = 80) was 11.66 ± 2.69, and M/F: 0.9. 55.5% of the patients had ulcerative colitis (UC) (mean age: 12.82 ± 2.14, M/F: 1.75) and 44.4% had Crohn’s disease (CD) (mean age:12.16 ± 1.6, M/F:0.91). PUCAI score was 45 (0–85) and PCDAI score was 30 (5–65).
When the patients with IBD and the controls compared in terms of sleep characteristics, significant differences were observed in sleep time and sleep duration (Tables 1 and 2). The sleep time of patient group, especially patients with UC was later than the control group (P = 0.008) (Table 2). Sleep duration was longer in control group than the patient group (P < 0.001). No significant difference was obtained in sleep latency and wake up time between each groups. PSQI score was significantly lower in control group than the patient group (P < 0.001), thus the sleep disorders were more frequent in patients with IBD than the healthy controls. Comparison of patients with IBD and controls in terms of sleep characteristics and sleep disorders are shown in Tables 1 and 2.
When the patient groups were examined in terms of factors affecting sleep disturbances, significant differences were observed in sleep latency, wake up time and sleep duration in patients with CD (Table 3), and in sleep time, sleep latency and sleep duration in patients with UC (Table 4). CD patients with PSQI score > 5 had longer sleep latency (P = 0.003), later wake up time (P = 0.015), and shorter sleep duration (P = 0.013) than CD patients with PSQI score ≤ 5. PCDAI score was higher in patients with PSQI score > 5 than the patients with PSQI score ≤ 5 (P < 0.001). UC patients with PSQI score > 5 had later sleep time (P = 0.025), longer sleep latency (P = 0.041), and shorter sleep duration (P < 0.001) than UC patients with PSQI score ≤ 5 (Table 4). PUCAI score was higher in patients with PSQI score > 5 than UC patients with PSQI score ≤ 5.
The number of attacks was significantly differ with sleep disorders in CD patients, but not in UC patients (Tables 3 and 4). Treatment with biological agents did not affect the sleep characteristics of the patients in both patient group.
A statistically significant weak positive correlation was observed between PSQI score and sleep time (r = 0.346; P = 0.022) and wake up time (r = 0.422; P = 0.004), a positive moderate correlation with sleep latency (r = 0.538; P < 0.001), and a negative weak correlation with sleep duration in each patient group (Table 5). When the relationship between PSQI scores and clinical and laboratory parameters of CD patients were examined, the most strong correlation was obtained in disease activity index (r = 0.886; P < 0.001) and abdominal pain (r = 0.781; P < 0.001). In addition, disease activity index, rectal bleeding, diarrhea and number of stool had statistically significant positive strong correlation with PSQI scores of UC patients (r = 0.924; P < 0.001, r = 0.920; P < 0,001, r = 0.839; P < 0.001, r = 0.870; P < 0.001, respectively).
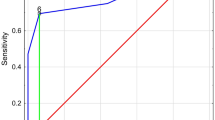
According to Binary logistic regression analysis sleep latency, sleep duration, and the number of attacks were not an independent risk factor affecting sleep disturbances for CD patients (P = 0.222, P = 0.804, P = 0.142, respectively), whereas PCDAI was the only independent risk factor affecting sleep disturbances (80% sensitivity and 91.67% specificity). It was observed that increase in each unit of PCDAI score, the risk of sleep disturbances increased 1.16 times (OR:1.165; %95 CI: 1.026–1.322; P = 0.018). Although age, gender, sleep latency and sleep duration were not an independent risk factor affecting sleep disturbances for UC patients, PUCAI was the only independent risk factor affecting sleep disturbances (93.1% sensitivity and %96.15 specificity). Increase in each unit of PUCAI score, the risk of sleep disturbances increased 1.24 times (OR:1.247; %95 CI: 1.047–1.486; P = 0.013).
Discussion
In recent studies, it has been stated that there was a strong relationship between sleep disorders and chronic diseases, increasing the severity of chronic diseases and making it difficult to control them [6, 19, 20]. Poor sleep quality leads to negative consequences such as poor quality of life, limitation in physical activity, and extraintestinal symptoms unrelated to the disease, independent of IBD itself, its activity and treatment [20]. Poor quality sleep may cause exacerbation of gastrointestinal symptoms, and conversely gastrointestinal diseases may impair individual's sleep quality by affecting the sleep-wake cycle [6].
Some changes such as decreased swallowing, decreased salivary secretion, decreased esophageal peristalsis, and increased gastric acid reflux occur in the gastrointestinal system during sleep. The migrating motor complexes (MMCs) are the waves of electrical activity that move through the gastrointestinal tract in a regular cycle [9, 21]. Kumar et al. [22] found that during sleep, there is a significant reduction in the MMC cycle length. It has been demonstrated that the amplitude of the gastric cycle declines in NREM sleep and returns in REM sleep [9, 23]. In addition, small intestine motility and colonic peristalsis also decrease during sleep.
Marinelli et al. [14] has reported that two-thirds of their patients with IBD had sleep disorders. Jarasvaraparn et al. [24] has reported that pediatric patients with UC had significantly more sleep disturbance than patients with CD. 59.2% of our patients with UC, 40.8% of our patients with CD, and overall 49% of all our patients had sleep disorders. Kugathasan et al. [25] has stated that PSQI score was abnormal all of the patients with active IBD. Although Gingold et al. [26] has obtained higher PSQI scores in patients with CD, they observed no difference in sleep duration between patients and controls. We observed that the most important difference between IBD patients and the control group is that the control group had longer sleep duration (mean 8 h in patients vs 9 h in controls).
Iskandar et al. [20] also reported that the sleep quality of patients with CD was worse than the controls, and those with active disease were worse than those in remission similar with the studies conducted by Sobolewska et al. [6], Ali et al. [7] and our study. Nachmias et al. [27] found that 54% of pediatric patients with IBD had moderate to severe sleep disturbances, but did not find any association between the presence of sleep disturbance and disease activity or treatment regimen. It has been reported that abdominal pain is the most common symptom causing sleep disturbances in CD patients and those with sleep disturbances described more frequent and severe diarrhea than those without [5, 26]. Similar results were obtained in our study as our CD patients with sleep disorder had more severe abdominal pain and diarrhea than those without sleep disorder.
Benhayon et al. [8] reported that sedimentation rate is the most important marker among laboratory parameters associated with sleep disturbances due to the fact that their study consisted of only patients in acute flare. In another study, it was stated that the only significant laboratory marker was albumin level related with sleep disturbances in IBD patients [5], due to longer duration of disease, the follow-up period was 14 years, and chronic complications. We observed higher sedimentation rate and lower albumin level in IBD patients with sleep disorder. Although our follow-up period was 5 years, low albumin levels can be explained by inadequate intake or lack of absorption.
Study conducted by Hood et al. [28], including only UC patients with sleep disorder, has reported that the most frequent symptom was diarrhea, followed by bleeding and abdominal pain, whereas the most frequent symptom was defecation at night, followed by abdominal pain and diarrhea in acute flare. They observed that the most common laboratory parameter was low albumin level in UC patients, and high sedimentation rate in acute flare. In our study, the most frequent symptom was severe abdominal pain, followed by bleeding and diarrhea in UC patients with sleep disorder. We also observed that those patients had frequent defecation at night, higher sedimentation rate and lower albumin level. As the disease severity increases, PSQI score and incidence of sleep disorders were found to be increased in our study. This was the first study demonstrated a positive strong correlation between abdominal pain and disease severity (PCDAI) by correlation analsis in CD patients with sleep disorder. A positive moderate correlation with sedimentation rate, negative moderate correlation with albumin, and negative weak correlation with hematocrit can be used as a guide in terms of indicating disease activity. We found a strong positive correlation between PUCAI, PCDAI and PSQI score in this study, which may help to obtaine information about the activity of the disease by questioning sleep patterns in these patients. If PCDAI score is above 30, and PUCAI score above 40 (according to cutt off value in ROC analysis), sleep disturbances should be seriously suspected and further advanced analysis to detect sleep disturbances should be made by the clinicians.
In this study, diarrhea and PUCAI score in UC patients, and abdominal pain and PCDAI score in patients with CD were found to be important variables that disrupt sleep. This is the first study in the literature in which abdominal pain and PCDAI score for CD patients, and PUCAI score, bleeding level, diarrhea, number of stool for UC patients were reported to be correlated with the PSQI score according to correlation analysis. Additionally, PCDAI was determined as the only independent variable for CD patients and PUCAI score for UC patients in multivariate analyzes, and each unit increase in the scores increased the risk of sleep disorder 1.16 and 1.24 times, respectively. ROC analysis revealed that PSQI had 93.1% sensitivity and 96.15% specificity, and PCDAI had 80% sensitivity and 91.67% specificity, demonstrating how strong tests they are.
The limitations of the study were retrospective nature of the study, datas based on the declaration of the patient and/or parents, the duration of the disease is not known due to centers where the patients are diagnosed are different, thus lack of information clearly identifying accompanying chronic complications or psychological states which affects sleep quality.
In conclusion, assessment of sleep disturbances in pediatric patients with IBD can play an important role in optimizing treatment and keeping disease in remission. In this study, abdominal pain in patients with CD and diarrhea in patients with UC were identified as the most important complaints disrupting sleep. Further, larger studies are needed to evaluate the impact of sleep assessment on the disease outcomes of patients with IBD and clinicians should keep in mind that PSQI and PCDAI scores correlated significantly with the severity of the sleep disturbances, and were strong tests for predicting sleep disorders.
Data availability
N/a.
Abbreviations
- CD:
-
Crohn’s disease
- IBD:
-
Inflammatory bowel disease
- PCDAI:
-
Pediatric Crohn’s disease activity index
- PSQI:
-
Pittsburgh sleep quality index
- PUCAI:
-
Pediatric ulcerative colitis activity index
- UC:
-
Ulcerative colitis
References
Ananthakrishnan AN, Xavier RJ, Podolsky DK (2017) Epidemiology and Pathogenesis. In Inflammatory Bowel Diseases (eds A.N.Ananthakrishnan, R.J. Xavier and D.K. Podolsky) 1–15. https://doi.org/10.1002/9781119077633.ch1
Besedovsky L, Lange T, Born J (2012) Sleep and immune function. Pflugers Arch 463(1):121–137. https://doi.org/10.1007/s00424-011-1044-0
Lange T, Luebber F, Grasshoff H, Besedovsky L (2022) The contribution of sleep to the neuroendocrine regulation of rhythms in human leukocyte traffic. Semin Immunopathol 44(2):239–254. https://doi.org/10.1007/s00281-021-00904-6
Ali T, Choe J, Awab A, Wagener TL, Orr WC (2013) Sleep, immunity and inflammation in gastrointestinal disorders. World J Gastroenterol 19(48):9231–9239
Ananthakrishnan AN, Long MD, Mar n CF, Sandler RS, Kappel- man MD, (2013) Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin Gastroenterol Hepatol 11(8):965–971
Sobolewska-Wlodarczyk A, Wlodarczyk M, Banasik J, Gasiorowska A, Wisniewska-Jarosinska M, Fichna J (2018) Sleep disturbance and disease activity in adult patients with inflammatory bowel diseases. J Physiol Pharmacol 69:423–428. https://doi.org/10.26402/jpp.2018.3.09
Ali T, Madhoun MF, Orr WC, Rubin DT (2013) Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis 19:2440–2443
Benhayon D, Youk A, McCarthy FN et al (2013) Characterization of relations among sleep, inflammation, and psychiatric dysfunction in depressed youth with Crohn disease. J Pediatr Gastroenterol Nutr 57(3):335–342. https://doi.org/10.1097/MPG.0b013e31829641df
Khanijow V, Prakash P, Emsellem HA, Borum ML, Doman DB (2015) Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol Hepatol (N Y) 11(12):817–825
Orr WC, Fass R, Sundaram SS, Scheimann AO (2020) The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol 5(6):616–624. https://doi.org/10.1016/S2468-1253(19)30412-1
Vernia F, Di Ruscio M, Ciccone A et al (2021) Sleep disorders related to nutrition and digestive diseases: a neglected clinical condition. Int J Med Sci 18(3):593–603. https://doi.org/10.7150/ijms.45512
Hyun MK, Baek Y, Lee S (2019) Association between digestive symptoms and sleep disturbance: a cross-sectional community-based study. BMC Gastroenterol 19(1):34. https://doi.org/10.1186/s12876-019-0945-9
Krueger JM (2008) The role of cytokines in sleep regulation. Curr Pharm Des 14(32):3408–3416. https://doi.org/10.2174/138161208786549281
Marinelli C, Savarino EV, Marsilio I et al (2020) Sleep disturbance in Inflammatory Bowel Disease: prevalence and risk factors - A cross-sectional study. Sci Rep 10(1):507. https://doi.org/10.1038/s41598-020-57460-6
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Harvey RF, Bradshaw JM (1980) A simple index of Crohn’sdisease activity. Lancet 1:514
Sutherland LR, Martin F, Greer S et al (1987) 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology 92:1894–1898
Agargun MY, Kara H, Anlar O (1996) Validity and reliability of the Pittsburgh Sleep Quality Index. Turk J Psychiatry 7(2):107–115
Dai H, Mei Z, An A, Wu J (2019) Association between sleep problems and health-related quality of life in Canadian adults with chronic diseases. Sleep Med 61:26–30. https://doi.org/10.1016/j.sleep.2019.04.015
Iskandar HN, Linan EE, Patel A et al (2020) Self-reported sleep disturbance in Crohn’s disease is not confirmed by objective sleep measures. Sci Rep 10(1):1980. https://doi.org/10.1038/s41598-020-58807-9
Paruthi S, Brooks LJ, D’Ambrosio C et al (2016) Consensus Statement of the American Academy of Sleep Medicine on the Recommended Amount of Sleep for Healthy Children: Methodology and Discussion. J Clin Sleep Med 12(11):1549–1561. https://doi.org/10.5664/jcsm.6288
Kumar D, Idzikowski C, Wingate DL, Soffer EE, Thompson P, Siderfin C (1990) Relationship between enteric migrating motor complex and the sleep cycle. Am J Physiol 259(6 pt 1):G983–G990
Orr WC, Chen CL (2005) Sleep and the gastrointestinal tract. Neurol Clin 23(4):1007–1024
Kugathasan S, Judd RH, Hoffmann RG et al (2003) Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr 143(4):525–531
Jarasvaraparn C, Zlomke K, Vann NC, Wang B, Crissinger KD, Gremse DA (2019) The Relationship Between Sleep Disturbance and Disease Activity in Pediatric Patients With Inflammatory Bowel Disease. J Pediatr Gastroenterol Nutr 68(2):237–243. https://doi.org/10.1097/MPG.0000000000002156
Gingold-Belfer R, Peled N, Levy S et al (2014) Impaired sleep quality in Crohn’s disease depends on disease activity. Dig Dis Sci 59(1):146–151. https://doi.org/10.1007/s10620-013-2890-8
Nachmias V, Sheinberg A, Weiss B, Fradkin A, Bujanover Y (2006) Sleep disturbances among young patients with IBD in Israel. J Pediatr Gastroenterol Nutr 43(suppl 2):S48
Hood MM, Wilson R, Gorenz A et al (2018) Sleep Quality in Ulcerative Colitis: Associations with Inflammation, Psychological Distress, and Quality of Life. Int J Behav Med 25(5):517–525. https://doi.org/10.1007/s12529-018-9745-9
Author information
Authors and Affiliations
Contributions
Conceptualization: Dilsat Gundogdu, Nafiye Urganci; Methodology: Dilsat Gundogdu, Nafiye Urganci; Formal analysis and investigation: Dilsat Gundogdu, Nafiye Urganci, Merve USTA; Writing -original draft preparation: Dilsat Gundogdu, Nafiye Urganci,; Writing - review and editing: Dilsat Gundogdu, Nafiye Urganci, Merve USTA.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of T SBU Sisli Hamidiye Etfal Training and Research Hospital (No:1267/28.05.2019).
Consent to participate
Written informed consent was obtained from the parents.
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by Peter de Winter
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gundogdu, D., Urgancı, N. & Usta, M. Relationship between disease activity index and sleep disorders in children with inflammatory bowel diseases. Eur J Pediatr 182, 4095–4102 (2023). https://doi.org/10.1007/s00431-023-05081-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05081-2