Abstract
Lung ultrasound (LU) has emerged as a valuable tool for assessing pulmonary aeration noninvasively, rapidly, and reliably in different neonatal conditions. However, its role in the preoperative and postoperative evaluation in congenital diaphragmatic hernia (CDH) is still poorly analyzed. We present a cohort of 8 patients diagnosed with CDH who underwent lung ultrasound examinations at various time points before and after surgical correction. The lung ultrasound patterns were compared between two groups: mechanical ventilation ≤ 7 days (MV ≤ 7) and mechanical ventilation > 7 days (MV > 7). The ultrasound findings were also compared to CT scans and chest X-ray images to assess its diagnostic capacity for identifying postoperative complications: pneumothorax, pleural effusion, and pneumonia. Group MV ≤ 7 exhibited a normal pattern even at 48 h postsurgery, while group MV > 7 presented interstitial or alveolointerstitial pattern in both lungs for prolonged periods (2–3 weeks). Furthermore, contralateral LU pattern may be predictive of respiratory evolution.
Conclusion: Lung ultrasound is a valuable tool for evaluating the progressive reaeration of the lung following surgical correction in CDH patients. It demonstrates the ability to diagnose common postoperative complications without the need for radiation exposure while offering the advantages of quick and serial assessments. These findings highlight the potential of lung ultrasound as an effective alternative to conventional imaging methods in the management of CDH.
What is Known: |
• Lung ultrasound evaluates lung aeration and predicts respiratory outcomes in neonatal patients. |
What is New: |
• Lung ultrasound is useful in the postsurgical management of congenital diaphragmatic hernia patients, detecting reaeration and respiratory complications. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Congenital diaphragmatic hernia (CDH) is a rare and serious condition, with an estimated incidence of 1 in 2500–3500 live newborns [1, 2]. It is a consequence of a congenital defect in the diaphragm, so the abdominal organs move to the thorax during embryonic development, generating variable degrees of pulmonary hypoplasia, both parenchymal and vascular, with the consequent development of pulmonary hypertension. It causes significant short- and long-term morbidity and mortality due to severe respiratory failure and associated complications, both cardiovascular and neurological [1].
Prenatal diagnosis and prognostic assessment are established through fetal ultrasound and magnetic resonance imaging. After birth, simple chest X-rays, echocardiography, and CT-scan are the most commonly used techniques for diagnosis and evaluation [3,4,5].
Lung ultrasound (LU) has proven its value in diagnosing and semi-quantifying neonatal respiratory distress syndrome, pleural effusion, and pneumothorax and predicting admission for respiratory distress, the need for surfactant, and the development of bronchopulmonary dysplasia [6,7,8].
Regarding its use in patients with CDH, only two articles have been published to date: one describes its ultrasound pattern at diagnosis in a group of 7 newborns [9], and another one focuses on postoperative follow-up [10].
We present a cohort of neonates diagnosed with CDH evaluated by LU before and after surgery.
The primary objective was to describe the evolution of ultrasound findings in the lung of a cohort of neonates diagnosed with CDH, either prenatally or postnatally, before and after surgical correction.
The secondary objectives were to analyze the ultrasound findings in the group of patients who had a favorable postoperative course, requiring 7 days or less of invasive mechanical ventilation (MV) (group MV ≤ 7) versus a group of patients with a more complicated course, requiring more than 7 days of invasive ventilation (group MV > 7). We describe the complications that occurred, the role of LU in their diagnosis, and their comparison with other imaging tests, both conventional portable radiographs and computed tomography (CT scan).
Our hypothesis is that LU may be a feasible and useful tool for the follow-up of these patients, allowing for the detection of postoperative reaeration and the most commonly associated respiratory complications.
Material and methods
Study design
Observational, descriptive, and prospective study was conducted in the Neonatology Department of the Gregorio Marañón University Hospital (Madrid, Spain) from November 2020 to December 2022. This is a neonatal intensive care unit (NICU) of the maximum level of complexity that attends to all types of neonatal pathologies, including cardiac surgery and extracorporeal oxygenation support (ECMO).
Inclusion and exclusion criteria
All neonates admitted with a diagnosis of CDH were included. Exclusion criteria were as follows: clinical instability that made LU impossible, death before performing LU, and unavailability of the investigator.
Demographic and clinical data were obtained from medical records, including pre- and postnatal follow-ups.
Resuscitation followed the national guidelines of the Spanish Society of Neonatology (SENeo), and the management of patients during admission followed the European recommendations (CDH EURO Consortium Consensus) [11, 12]. Surgical correction was performed by thoracotomy or thoracoscopy, according to the criteria of the surgical team.
Lung ultrasound protocol
LU has been a routine part of the daily care of neonates admitted to our unit for the past 6 years. The scans were performed by one of the neonatologists (RGH), specifically trained in LU and with more than 5 years of experience. The scans were always performed immediately after the standard patient care using non-pharmacological measures.
A standardized ultrasound evaluation was performed using a Sonosite iViZ ultrasound machine with a high-frequency linear probe.
Three longitudinal planes were intended to be obtained per hemithorax: midclavicular, anterior, and posterior axillary lines. In case of dressings, some of them could be impossible. Qualitative descriptions of the findings followed the classical description according to Raimondi et al. [13].
LU was performed at the following times: preoperatively, in the first 24 h after surgery, 48–72 h after surgery, 7 days later, and then weekly while the patient was admitted to the NICU.
The ultrasound images were recorded on the portable ultrasound machine and subsequently stored in a dedicated memory controlled by the principal investigator.
The team responsible for the patient was informed about the ultrasound results, but patients were managed based on the usual protocols of the unit, relying on clinical, analytical, and portable X-ray information.
Statistical analysis
Qualitative variables of interest are expressed as percentages and frequencies. Continuous variables are evaluated using median and interquartile range (IQR). Comparisons were made between the group that required MV 7 days or less (group MV ≤ 7) and the group that required more than 7 days (group MV > 7) using the Mann–Whitney U test, with p < 0.05 being considered statistically significant. Data were analyzed using StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.
Results
During the study period, a total of 8 patients were admitted with a diagnosis of CDH. All of them were included. Table 1 shows their clinical characteristics. One of them died. Between 1 and 11 LUs were obtained during the postoperative period.
Group MV ≤ 7 consisted of 4 patients (cases 1, 2, 3, 4), while the other 4 patients (cases 5, 6, 7, 8) were included in group MV > 7. No significant differences were found in gestational age (38.9 vs. 37.8, p 0.69 weeks), birth weight (2715 vs. 3035 g, p 0.06), Apgar at 5 min (8 vs. 7, p 0.74), non-invasive ventilation (NIV) duration (5 vs. 19.5 days, p 0.69), global admission duration (23 vs. 101, p 0.11), age at surgical correction (3.5 vs. 6.5 days, p 0.49), or at first LU between the groups (0 vs. 1.5 days, p 0.17). However, there were significant differences in MV (3.5 vs. 33.5 days, p 0.03) and total NICU admission (10 vs. 66 days, p 0.03).
Preoperative evolution
All patients received conventional MV from admission, except for the two cases postnatally diagnosed who were intubated after diagnosis (1 day of life, DOL). All but two required high-frequency oscillator ventilation (HFOV) before surgery. Two of them required ECMO support due to refractory respiratory failure.
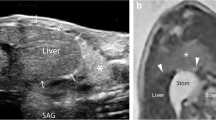
Preoperative ultrasound was performed at a median of 1 DOL (IQR 0–2). In the CDH-affected hemithorax, images consistent with intrathoracic intestinal loops, spleen, stomach, or liver were observed, with no visible pleural line, lung sliding, or A lines (Fig. 1 and supplementary material 1). In this hemithorax, a pattern of lung parenchyma with thickened pleural line and interstitial or alveolointerstitial pattern was only observed in the anterior field, while the other areas were occupied by intra-abdominal content, except in case 6, where an interstitial pattern was also seen in the lateral and posterior upper fields.
The contralateral lung exhibited a normal pattern (A lines with pleural sliding) in group MV ≤ 7, while an interstitial or alveolointerstitial pattern was observed in group MV > 7. Additionally, in the right anterior field, a normal pattern was observed in group MV ≤ 7 patients, whereas in group MV > 7, the mediastinum and great vessels were observed (mediastinal shift). Figure 2 illustrates the comparison of classical radiological images of cases number 3 (group MV ≤ 7) and case number 8 (group MV > 7).
Surgical correction
The median age of patients at the time of surgical correction was 4.5 days (IQR 2–8.5). Five cases were corrected by thoracoscopy and 3 by thoracotomy. After thoracoscopy, 5 cases had ipsilateral pleural drainage in place. The main postoperative complications are shown in Table 1.
Postoperative evolution
All patients were readmitted intubated, and on conventional support. In the whole sample, the median MV duration was 18.5 days (IQR 3.5–33.5). The median NIV duration was 10.5 days (IQR 2–35.5). Five of the patients received inhaled or oral treatment for pulmonary hypertension with nitric oxide and/or oral sildenafil. The patients were admitted for a median of 54.5 days (IQR 22.5–101), of which 26.5 (IQR 10–66) were in the NICU. The most common postoperative complications were pulmonary hypertension (5 patients), pleural effusion (2 patients), and pneumothorax (3 patients).
Group MV ≤ 7 days
These four cases required 7 days or less of postoperative MV (median 3.5 days, IQR 2–5.5) and a maximum of 47 days of NIV (median 5 days, IQR 2–26.5). Only one of them required HFO ventilation (case 4). Median NICU admission was 10 days (IQR 6.5–18), and median global admission was 23 days (IQR 13–54.5). Cases 1 and 4 were prenatal diagnosis, while the other two were diagnosed postnatally. Cases 1–3 had a normal pattern (less than 3 B lines per scanned area) even at 48 postoperative hours in the lateral and posterior fields in the affected lung, without pleural effusion or pneumothorax, although they had apical consolidations smaller than 1 cm. In all these cases, the contralateral lung maintained a pattern of A lines with some isolated B lines and a thin pleural line with lung sliding.
However, patient no. 4 presented a pleural effusion ipsilateral to the hernia which did not require drainage, considered secondary to the pulmonary hypoplasia in the lateral and posterior fields. An anterior pneumothorax was also observed only in the first postoperative ultrasound. Although this patient only received 7 days of MV, she required non-invasive respiratory support for 46 days, parallel to the ultrasound pattern observed, since the right lung persisted with an interstitial pattern with some alveolointerstitial areas until discharge from the NICU (23 DOL).
Figure 2 shows the ultrasound and radiological evolution of case 3, with ultrasound accurately detecting left pulmonary reaeration after surgery. None of the patients presented serious respiratory complications after surgery.
Group MV > 7 days
These four patients had a prenatal diagnosis (Table 1). The median NICU admission was 66 days (IQR 41–85), and the median global admission was 101 days (IQR 59–162). The duration of postoperative MV was 33.5 days (IQR 31.5–37.5) and, for non-invasive, 19.5 days (IQR 7–36). All of them experienced prolonged periods of respiratory support, including HFOV, and had extended hospital stays, along with various complications that were easily identified by ultrasound as described below.
In these four patients, the affected lung showed a generalized alveolointerstitial pattern during the first 15 postoperative days, evolving to an interstitial pattern, that persisted until discharge from the NICU. The pleural line was initially found to be thickened, becoming thinner and better defined in parallel as the pulmonary pattern improved.
The contralateral lung exhibited an interstitial pattern with a variable number of B lines from the first postoperative day in anterior and lateral areas and an alveolointerstitial pattern in posterior fields, which persisted until discharge from the NICU (Fig. 2).
Patient no. 5 presented a persistent left pneumothorax that required multiple drainages. Ultrasound follow-up in this case allowed daily evaluation of the pneumothorax, identifying re-accumulation and resolution (SM 2: lung point in HFOV) and even facilitating ultrasound-guided drainage of the associated pneumoperitoneum by performing an ultrasound-guided puncture 13 days after surgery. Finally, surgical revision was performed to seal the parenchymal leak 22 days after the first intervention resulting in a resolution in the follow-up ultrasound.
Patient no. 6 presented a left pneumothorax due to a surgical parenchymal injury, which required drainage for 14 days. On the 19th postoperative day, a right posterolateral consolidation, up to 5 cm deep, with a dynamic air bronchogram (SM3) was shown. Despite postural changes and recruitment maneuvers, the consolidation did not change. These findings, combined with the patient’s respiratory deterioration and laboratory results, confirmed the diagnosis of pneumonia associated with mechanical ventilation, with isolation of E. coli in bronchoalveolar lavage. On ultrasound follow-up, this consolidation persisted, even at the time of home discharge, progressively becoming into an anechoic cavity with thickening of the pleural line, compatible with necrotizing pneumonia, which was confirmed by a CT scan. Ultrasound, portable X-rays, and CT scan images are shown in Fig. 3.
Patient no. 7 required ECMO support for 10 days because of severe respiratory insufficiency and died at 1 month of life as a result of redirection of care related to severe hypoxic-ischemic encephalopathy and severe lung disease, not able to wean MV. Bilateral homogeneous alveolointerstitial pattern was observed during his evolution.
Patient no. 8 required ECMO support for 19 days before surgery. Afterward, alveolointerstitial pattern was seen in the anterior areas of the left lung and pleural effusion in the lateral and posterior areas. In the right lung, interstitial pattern in the anterior area and alveolointerstitial in the lateral and posterior areas were shown during the entire study period (54 DOL).
Discussion
CDH is an extremely rare condition in neonatology nowadays. In our study, we present a point-of-care lung ultrasound follow-up of a cohort of neonates before and after surgical correction.
There are some international guidelines about management [14,15,16], but they do not include LU. However, based on our experience, LU is a really interesting tool for evaluating the progressive reaeration of the affected lung and identifying postoperative complications, such as pleural effusion, pneumothorax, atelectasis, or pneumonia [17, 18].
In terms of preoperative complementary tests, portable chest X-ray is the most commonly used and available. CT scan may be useful in some selected cases, especially when there are diagnostic challenges. However, both of these tests have the disadvantage of ionizing radiation, a major problem when dealing with these vulnerable children.
LU has demonstrated its value in diagnosing and semi-quantifying neonatal respiratory distress syndrome, pleural effusion, and pneumothorax and predicting admission for respiratory distress and need for surfactant, with the advantage of being non-ionizing, fast, sensitive, easy to learn, and at the bedside [19,20,21]. However, its use in neonatal populations with severe congenital malformations is limited, which further highlights the importance of our follow-up proposal.
Regarding the preoperative ultrasound image of CDH, our cohort confirms the findings previously described by Corsini et al. [9] and offers representative video images for reference (see Fig. 1 and SM 1, 2, 3). Moreover, in patients without a prenatal diagnosis, ultrasonography allowed a quick, reliable, bedside diagnosis of CDH, which was confirmed by CT scan, since plain radiology offered diagnostic uncertainties. This further supports the recommendation for the protocolized use of LU in newborns with respiratory distress after birth.
A recent publication describes the postoperative use of LU in patients with CDH [10]. This work describes the semiquantitative ultrasound score evolution by means of an adapted score. This score has the advantage of allowing a more objective evaluation of the ultrasound pattern but has some drawbacks: the pattern of “preoperative intra-abdominal content” scores the same as “postoperative pleural effusion,” and pneumothorax is not included as a possible pattern. In addition, they include 12 areas, which can be challenging to scan in patients with extensive dressings or drainages.
The study included 13 patients and performed two preoperative and two postoperative ultrasounds. Most cases showed a normal pattern at 1 week (score 4/48), parallel to a favorable postoperative evolution, with short mechanical ventilation and hospitalization and few complications. In contrast, our sample, despite being smaller, has the advantage of showing four patients with poor postoperative evolution, ECMO support, recurrent pneumothoraces, and pneumonia.
Both studies highlight that LU has a relevant role in the quick and objective evaluation of pulmonary pathology in patients with CDH at the bedside. Therefore, its daily use is highly recommended to analyze pulmonary reexpansion, detect complications, and even potentially guide extubation, since the absence of extended consolidations and low ultrasound scores are predictive of successful extubation in other groups of patients such as preterm infants [22].
The ultrasonographic pattern of the contralateral lung may have prognostic implications. Prenatal correlation has been described between the lung volume of the contralateral lung and mortality, the need for ECMO, and the development of chronic pulmonary pathology in patients diagnosed with CDH (23). In our sample, the 3 patients who presented the most favorable respiratory evolution had a normal ultrasound pattern in the contralateral lung compared to none of the 5 patients who required prolonged MV or NIV, who maintained a homogeneous interstitial or alveolointerstitial pattern for several weeks in that lung.
Previous studies have compared the correlation between LU and simple chest radiography with respect to chest CT scan in various pathologies, showing LU superiority and demonstrating its predictive capacity for death or severe acute respiratory distress syndrome [24, 25]. Comparisons between LU and CT scan are scarce in the neonatal population, as CT scan is rarely performed; our case no. 6 serves as one illustrative example.
In terms of postoperative evolution, this study shows that follow-up by LU is feasible and useful, since it was able to confirm the diagnosis and the complications that may appear (pneumothorax: cases 5 and 6; pleural effusion: case 4; pneumonia: case 6). Furthermore, we found that in patients with uneventful respiratory evolution, ultrasound described the rapid pulmonary reaeration after surgery, in a similar way to the changes evidenced in the simple portable chest X-ray (Fig. 2) which differed between the groups. This finding suggests that ultrasound could identify patients with a larger lung volume, both preoperatively and postoperatively, since these patients present a normal or interstitial ultrasound pattern in a greater number of fields.
LU prediction capacity in ECMO support or MV withdrawal in these patients remains to be elucidated, and the small sample size of this study does not allow us to draw any conclusions in this regard.
Another advantage of LU in the NICU is related to invasive procedures, such as pneumothorax, pleural effusion drainage or monitoring [26]. In our sample, serial LU was able to identify and measure both pathologies, thereby reducing the associated risks.
There are some limitations to consider. Sample size is small, and the patients are very heterogeneous. Likewise, ultrasound follow-up could only be done until discharge from the NICU, resulting in images available from only one patient at the time of home discharge.
It would have been interesting to analyze the correlation between the oxygenation and the lung ultrasound semiquantitative score (LUS); however, technical limitations such as dressings or pneumothorax made it impossible to obtain images of all the fields in some scans and therefore could not be calculated.
As strengths, this work provides valuable evidence regarding the postoperative ultrasound evaluation of a cohort of neonates with CDH and very diverse respiratory evolution, showing the diagnostic capacity and potentially prognostic relevance of bedside lung ultrasound in patients with this complex pathology.
Conclusion
Lung ultrasound proves to be a valuable tool in the postoperative follow-up of patients with congenital diaphragmatic hernia. The findings are diverse and heterogeneous, parallel to the respiratory evolution. In addition, it allows the performance of ultrasound-guided procedures (pneumothorax drainage, pneumoperitoneum) eliminating the need for patient displacement and reducing the risks associated with ionizing radiation. Further studies are required in a larger number of patients to verify our findings.
Abbreviations
- BW:
-
Birth weight
- CDH:
-
Congenital diaphragmatic hernia
- CT-scan:
-
Computed tomography scan
- ECMO:
-
Extracorporeal membrane oxygenation
- GA:
-
Gestational age
- HFOV:
-
High-frequency oscillator ventilation
- LU:
-
Lung ultrasound
- MV:
-
Mechanical ventilation
- nCPAP:
-
Nasal continuous positive airway pressure
- NICU:
-
Neonatal intensive care unit
- O/E LHR:
-
Observed/expected lung-to-head ratio
- PH:
-
Pulmonary hypertension
- SENeo:
-
Spanish Society of Neonatology
- S/F index:
-
Peripheral saturation/inspired oxygen fraction index
References
Yang MJ, Russell KW, Yoder BA, Fenton SJ (2021) Congenital diaphragmatic hernia: a narrative review of controversies in neonatal management. Transl Pediatr 10(5):1432–47. [cited 2023 Jan 16]. Available from https://pubmed.ncbi.nlm.nih.gov/34189103/
Lichtsinn K, Waltz PK, Azzuqa A, Church J, Graham J, Troutman J et al (2022) Impact of a standardized management guideline for infants with CDH: a single-center experience. J Pediatr Surg 58(3):389–396. [cited 2023 Jan 16]. Available from https://pubmed.ncbi.nlm.nih.gov/35965150/
Mehollin-Ray AR (2020) Congenital diaphragmatic hernia. Pediatr Radiol 50(13):1855–71. [cited 2023 Jan 16]. Available from https://pubmed.ncbi.nlm.nih.gov/33252754/
Jancelewicz T, Brindle ME (2020) Prediction tools in congenital diaphragmatic hernia. Semin Perinatol 44(1):151165. [cited 2022 Nov 18]. Available from: https://pubmed.ncbi.nlm.nih.gov/31676044/
Soni R, Soni N, Chakkarapani A, Gupta S, Yajamanyam PK, Ali SKM, El Anbari M, Alhamad M, Anand D, More K (2023) The utility of serial echocardiography parameters in management of newborns with congenital diaphragmatic hernia (CDH) and predictors of mortality. Pediatr Cardiol 44(2):354–366. https://doi.org/10.1007/s00246-022-03002-y. Epub 2022 Sep 27. [cited 2023 Jan 16]. Available from: https://pubmed.ncbi.nlm.nih.gov/36163300/
Capasso L, Pacella D, Migliaro F, Salomè S, Grasso F, Corsini I, De Luca D, Davis PG, Raimondi F (2023) Can lung ultrasound score accurately predict surfactant replacement? A systematic review and meta-analysis of diagnostic test studies. Pediatr Pulmonol 58(5):1427–1437. [cited 2023 Mar 1]. Available from: https://pubmed.ncbi.nlm.nih.gov/36717970/
Ruoss JL, Bazacliu C, Cacho N, De Luca D. (2021) Lung Ultrasound in the Neonatal Intensive Care Unit: Does It Impact Clinical Care? Children (Basel). 29;8(12):1098. [cited 2023 Mar 1]. Available from https://pubmed.ncbi.nlm.nih.gov/34943297/
Alonso-Ojembarrena A, Lubián-López SP (2019) Lung ultrasound score as early predictor of bronchopulmonary dysplasia in very low birth weight infants. Pediatr Pulmonol 54(9):1404–9. [cited 2023 Mar 24]. Available from https://pubmed.ncbi.nlm.nih.gov/31216121/
Corsini I, Parri N, Coviello C, Leonardi V, Dani C (2019) Lung ultrasound findings in congenital diaphragmatic hernia. Eur J Pediatr 178(4):491–5. [cited 2022 Nov 18]. Available from https://pubmed.ncbi.nlm.nih.gov/30666398/
Maddaloni C, De Rose DU, Ronci S, Bersani I, Martini L, Caoci S, Capolupo I, Conforti A, Bagolan P, Dotta A, Calzolari F (2023) Lung ultrasound score in neonates with congenital diaphragmatic hernia (CDH-LUS): a cross-sectional study. Diagnostics (Basel) 27;13(5):898. [cited 2023 Mar 23]. Available from https://pubmed.ncbi.nlm.nih.gov/36900042/
Zeballos Sarrato G, Ávila-Álvarez A, Escrig Fernández R, Izquierdo Renau M, Ruiz Campillo CW, Gómez Robles C, Iriondo Sanz M (2022) Neonatal Resuscitation Group of the Spanish Society of Neonatology (GRN-SENeo). Spanish guide for neonatal stabilization and resuscitation 2021: Analysis, adaptation and consensus on international recommendations. An Pediatr (Engl Ed) 96(2):145.e1–145.e9. https://doi.org/10.1016/j.anpede.2021.06.011. Epub 2022 Feb 23
Snoek KG, Reiss IKM, Greenough A, Capolupo I, Urlesberger B, Wessel L et al (2016) Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO Consortium Consensus - 2015 Update. Neonatology 110(1):66–74. [cited 2022 Nov 18]. Available from https://pubmed.ncbi.nlm.nih.gov/27077664/
Raimondi F, Yousef N, Migliaro F, Capasso L, De Luca D (2021) Point-of-care lung ultrasound in neonatology: classification into descriptive and functional applications. Pediatr Res 90(3):524–31. [cited 2023 Jun 7]. Available from https://pubmed.ncbi.nlm.nih.gov/30127522/
Puligandla PS, Skarsgard ED, Offringa M, Adatia I, Baird R, Bailey JAM et al (2018) Diagnosis and management of congenital diaphragmatic hernia: a clinical practice guideline. CMAJ 190(4):E103–12. [cited 2022 Nov 18]. Available from https://pubmed.ncbi.nlm.nih.gov/29378870/
Ito M, Terui K, Nagata K, Yamoto M, Shiraishi M, Okuyama H, et al. Clinical guidelines for the treatment of congenital diaphragmatic hernia. Pediatr Int [ 2021 Apr 1cited 2022 Nov 18];63(4):371–90. Available from https://pubmed.ncbi.nlm.nih.gov/33848045/
Kirby E, Keijzer R (2020) Congenital diaphragmatic hernia: current management strategies from antenatal diagnosis to long-term follow-up. Pediatr Surg Int 36(4):415–29. [cited 2022 Nov 18]. Available from https://pubmed.ncbi.nlm.nih.gov/32072236/
Montero-Gato J, Rodeño-Fernández L, Serna-Guerediaga I, Aguirre-Unceta-Barrenechea A, Aguirre-Conde A, Perez-Legorburu A (2022) Ultrasound of pneumothorax in neonates: diagnostic value of the anterior transverse plane and of mirrored ribs. Pediatr Pulmonol 57(4):1008–14
Ma H, Deng B, Liu J, Jiang P, Xu Y, Song X et al (2023) Lung ultrasound to diagnose infectious pneumonia of newborns: a prospective multicenter study. Pediatr Pulmonol 58(1):122–129
Rodeño Fernández L, Gregorio Hernández R, Serna Guerediaga I, Montero Gato J, Rodríguez Fanjul J, Aldecoa Bilbao V et al (2022) Utilidad de la ecografía pulmonar en el diagnóstico y seguimiento de la patología respiratoria neonatal. An Pediatr (96) 252.e1–252.e13. Available from https://www.analesdepediatria.org/es-utilidad-ecografia-pulmonar-el-diagnostico-articulo-S1695403322000054
Wang J, Wei H, Chen H, Wan K, Mao R, Xiao P, Chang X (2022). Application of ultrasonography in neonatal lung disease: An updated review. Front Pediatr 25;10:1020437.[cited 2023 Mar 7]. Available from https://pubmed.ncbi.nlm.nih.gov/36389379/
Raimondi F, Rodriguez Fanjul J, Aversa S, Chirico G, Yousef N, De Luca D et al (2016) Lung ultrasound for diagnosing pneumothorax in the critically Ill neonate. J Pediatr 175:74–78.e1. [cited 2023 Mar 27]. Available from https://pubmed.ncbi.nlm.nih.gov/27189678/
El Amrousy D, Elgendy M, Eltomey M, Elmashad AE (2020) Value of lung ultrasonography to predict weaning success in ventilated neonates. Pediatr Pulmonol 55(9):2452–6. [cited 2023 Mar 24]. Available from https://pubmed.ncbi.nlm.nih.gov/32609928/
Hagelstein C, Burger-Scheidlin S, Weis M, Weiss C, Schoenberg SO, Schaible T et al (2016) Separate evaluation of the ipsilateral and contralateral MR fetal lung volume in patients with congenital diaphragmatic hernia. AJR Am J Roentgenol 207(2):415–23. [cited 2023 Mar 23]. Available from https://pubmed.ncbi.nlm.nih.gov/27249543/
Aguersif A, Sarton B, Bouharaoua S, Gaillard L, Standarovski D, Faucoz O et al (2022) Lung ultrasound to assist ICU admission decision-making process of COVID-19 patients with acute respiratory failure. Crit care Explor 4(6):e0719. [cited 2023 Mar 23]. Available from https://pubmed.ncbi.nlm.nih.gov/35765373/
Tierney DM, Huelster JS, Overgaard JD, Plunkett MB, Boland LL, St Hill CA et al (2020) Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med 48(2):151–7. [cited 2023 Mar 7]. Available from https://pubmed.ncbi.nlm.nih.gov/31939782/
Gregorio-Hernández R, Pérez-Pérez A, Alonso-Ojembarrena A, Arriaga-Redondo M, Ramos-Navarro C, Sánchez-Luna M (2022) Neonatal pneumothoraces with atypical location: the role of lung ultrasound. Eur J Pediatr 181(4):1751–6. [cited 2023 Mar 27]. Available from https://pubmed.ncbi.nlm.nih.gov/34845527/
Acknowledgements
We would like to thank patients and their families. We are also in debt with NICU nurses and assistants who helped us performing the studies.
Author information
Authors and Affiliations
Contributions
Rebeca Gregorio-Hernández designed the study, performed the acquisition and analysis of the data and draft the text. Sara Vigil Vázquez, María Arriaga-Redondo, Alba Pérez-Pérez and Cristina Ramos-Navarro collected data during the study period. Elena Rodriguez-Corrales performed statistical analysis, Manuel Sánchez-Luna made contributions to the conception of the work and revised the work critically. All the authors revised and approved the final version of the text.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee (Gregorio Marañón Hospital, reference number 379/22) and the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
Consent to participate
Informed consent was obtained from all legal guardians of the patients included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary material 1: Lung ultrasound at diagnosis (case nº3) (MP4 1628 KB)
Supplementary material 2: Pneumothorax lung point in high frequency oscillatory ventilation (case nº5) (MP4 3367 KB)
Supplementary material 3: Right posterolateral consolidation with dynamic bronchogram (case nº6) (MP4 3345 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gregorio-Hernández, R., Ramos-Navarro, C., Vigil-Vázquez, S. et al. Lung ultrasound and postoperative follow-up of congenital diaphragmatic hernia. Eur J Pediatr 182, 3973–3981 (2023). https://doi.org/10.1007/s00431-023-05074-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-023-05074-1