Abstract
Evidence from randomized controlled trials (RCTs) suggests that three-hourly feeding is safe and might help achieve full feeds earlier in preterm infants. We systematically compared the benefits and harms of three-hourly and two-hourly feeding schedules in preterm infants. We searched electronic databases (MEDLINE, CINAHL, Embase, Web of Science, and Scopus) and trial registries until November 16, 2021, for RCTs comparing the two feeding schedules. We did a random-effects meta-analysis using RevMan 5.4 software. The primary outcome was the incidence of stage II or III necrotizing enterocolitis (NEC). Other outcomes were the incidence of any stage NEC, mortality, time to full enteral feeds, and hospital stay. Six trials (872 participants) are included. There was no significant difference in the incidence of stage II/III NEC (3 trials; 530 participants; RR 1.39; 95% CI: 0.53, 3.65; I2 − 0%, low certainty), and any stage NEC (5 studies; 767 participants; RR 0.98; 95% CI: 0.53, 1.82; I2 -0%, very low certainty) between three and two-hourly feeding groups. There was no difference in achieving full feeds (5 trials; 755 participants; MD: − 0.0 days; 95% CI: − 0.32, 0.31, low certainty) or other outcomes. On subgroup analysis, neonates with birthweight < 1000 g and in the three-hourly feeding regime achieved full enteral feeds slower than those in the two-hourly feeding group (1 trial; 84 participants; MD: 2.9 days, 95% CI: 1.16, 4.64, low certainty).
Conclusion: In stable preterm infants (1000–1500 g), three-hourly feeding can be followed safely. In infants < 1000 g, there is insufficient evidence to advise on an optimal feeding interval, although a 2-h interval might be preferable.
Prospero Registration Number: CRD42021246568.
What is Known: • Most of the units follow two-hourly feeding schedules without any evidence. • Recent trials suggest that the three hourly feeding schedules can be safely followed in stable preterm infants. | |
What is New: • Low certainty evidence suggests that three-hourly feeding is safe in stable preterm infants (1000-1500 grams). • In infants with birthweight <1000 grams, two-hourly feeding may be considered as it was associated with a shorter time to full feeds in subgroup analysis. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bolus feeding is considered more physiological than continuous feeding in preterm infants [1, 2]. As a convention, most units prescribe enteral feeding at an arbitrary interval of 2 h irrespective of the infant’s weight or physiological maturity and continue until demand breastfeeding is achieved [3, 4]. A short interval (1–2 h) feeding schedule delivers a smaller volume per feed that may be more easily tolerated. However, frequent bolus feeding might lead to persistently high superior mesenteric artery blood flow, which might not be physiological [5]. Also, frequent feeding stresses nurses (frequent feed administration), mothers (frequent milk expression leading to reduced rest time), and infants (less sleep, shorter kangaroo mother care duration) [3].
On the other hand, a longer feeding interval (3 h or more) translates to a higher volume per feed, which might not be well-tolerated in extremely preterm infants. However, longer feeding intervals might improve postprandial blood flow and gut motility and help achieve full enteral feeds earlier [5]. It might have an impact on reducing nursing time spent on feeding and improving mother-infant attachment.
Due to the lack of scientific basis for a two-hourly feeding schedule and perceived advantages of extended feeding schedules, three-hourly feedings have been used successfully [6,7,8]. Some studies observed that three-hourly feeding schedules might help achieve full enteral feeds earlier, therefore, decreasing the duration of parenteral nutrition and central venous catheter [6]. A previous systematic review concluded that low-quality evidence shows that the three-hourly feeding schedule is safe and helps regain birth weight earlier than a 2-hourly feeding schedule [9]. However, these conclusions are limited by the small sample size and failed to change the clinical practice, thereby meriting further exploration [10, 11]. Moreover, these results were limited to achieving full gavage feeds and did not assess the impact on the transition to oral feeds and time to discharge. The most recent Cochrane review of four RCTs did not find a significant difference between the two- and three-hourly feeding intervals [10]. However, recently, two large trials (one of them is largest until now) comparing the two feeding schedules have been published, which were not included in the Cochrane review [12, 13], warranting a systematic relook into the evidence.
We aimed to systematically synthesize and update the evidence on clinical benefits and harms associated with a three-hourly feeding schedule compared to a conventional two-hourly feeding schedule in stable preterm infants.
Material and methods
Search strategy
We followed Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines [14] and Cochrane Handbook for Systematic Reviews of Interventions [15]. The protocol was prospectively registered with PROSPERO (CRD42021246568). We searched Medline (by PubMed), Embase, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Scopus for randomized controlled trials (RCTs) published until November 16, 2021. One author (JK) developed the electronic search strategy for individual databases (Supplementary Table S1), which was later independently peer-reviewed by two different authors (JM, PD) using the Peer Review of Electronic Search Strategies (PRESS) checklist [16]. After finalizing the search strategy, two investigators (JK, JM) independently searched the literature using the database-specific subject headings (MeSH/Emtree terms), keywords, free-text, and word variants for the study population (preterm infants), intervention (three-hourly feeding schedule), control (two-hourly feeding schedule), and study design (RCT). The electronic database search was supplemented by a manual search of the bibliography of the relevant guidelines, reviews, and included studies. We also searched various clinical trial registries, namely Clinical Trial Registry India (CTRI), ClinicalTrials.gov, and EU Clinical Trials Register (https://www.clinicaltrialsregister.eu), to identify additional published records (searched on November 16, 2021). We did not use any language restriction, limits, or filters in the literature search.
Study selection
RCTs comparing a three-hourly feeding schedule with a two-hourly feeding schedule among preterm infants (< 37 weeks) were considered eligible for this systematic review. First, two investigators (JK, JM) independently screened the titles and abstracts for eligibility and identified potentially eligible studies for full-text screening. Later, two different authors (JK, PD) independently examined the full text of the above-identified studies and included them in the review if they met all the following criteria: (i) population—stable preterm (< 37 weeks) infants on gavage/spoon/paladai/cup feeds and admitted in hospital; (ii) intervention—three-hourly feeding schedule; (iii) comparison—two-hourly feeding schedule; (iv) outcome—reported one or more of the predefined clinical outcomes (mentioned below); and (iv) study design—RCT only. We excluded studies including (i) term infants or older children, (ii) infants on direct breastfeeds or demand feeding, (iii) infants with structural malformations or surgical gastrointestinal anomalies; studies (iv) comparing other feeding intervals, and (v) not reporting any of the predefined clinical outcomes.
Primary and secondary outcomes
Our primary outcome was the incidence of necrotizing enterocolitis (NEC) ≥ stage 2 as per modified Bell’s staging [17]. We chose this outcome as the primary concern with longer feeding intervals (larger feed volumes) is more time taken for complete gastric emptying, which might predispose the preterm neonates for feed intolerance or NEC. Therefore, the clinicians would like to ensure that the larger intervals do not harm (in the form of increased NEC or feed intolerance). As there is wide variability in defining and managing NEC, we restricted the primary outcome to NEC stage ≥ 2, which has a reasonably objective definition, and is clinically relevant [18].
The secondary outcomes included incidence of any stage NEC, time to reach full enteral feeds (at least 100 mL/kg/day), time to achieve full oral (cup/spoon/paladai) feeds, time to regain birth weight, rate of weight gain (g/kg/day), time to discharge, number of days of total parenteral nutrition (TPN) and central venous catheter (CVC) usage, number of episodes of feed intolerance, all-cause in-hospital mortality, and anthropometry parameters (weight/length/occipitofrontal circumference) at discharge, 40 weeks post-menstrual age, and follow-up.
Data extraction and quality assessment
Two investigators (JM, JK) read the full text of all included studies and independently extracted the study data using a predesigned structured performa. The extracted data included, but was not limited to, the first author’s name, year of publication, inclusion and exclusion criteria, study design, and methodology (randomization, allocation concealment, blinding, attrition, protocol deviations, etc.) to assess the risk of bias, demographic details of the study participants of each group, feeding protocol (type of feeds, rate of feed hiking, mode of feeding, TPN details), and predefined clinical outcomes. This data was later entered into RevMan version 5.4 software for analysis. Another investigator (PD) cross-checked the entire extracted data for its completeness and accuracy. Two investigators (JK and JM) independently assessed the risk of bias using the Cochrane Collaboration tool [19]. A senior investigator (PK) intervened in case of disagreement, and his decision was considered final. We also contacted the corresponding authors of the included studies for additional details. Studies with multiple published reports were combined and summarized under the primary study as per PRISMA 2020 recommendations [14].
Statistical analysis
We systematically synthesized evidence for all predefined outcomes. Considering the variability in the study population and feeding protocols, we used random-effects meta-analysis. We calculated the pooled risk ratio (RR) for dichotomous variables and a mean difference (MD) for continuous variables, along with 95% confidence intervals (CI). RevMan calculator or appropriate statistical conversion formulas were used to convert interquartile range (IQR), range, or 95% CI to standard deviation [20]. Study heterogeneity was explored by visual assessment of the forest plots and chi-square test on Cochrane’s Q statistics and was quantified with I2 statistics. As decided a priori, we did subgroup analysis based upon gestational age, birth weight, and intrauterine growth restriction status, wherever feasible. We also did sensitivity analysis to explore the heterogeneity. RevMan 5.4 software was used for quantitative analysis.
We followed standard GRADE recommendations to assess the certainty of the evidence for the clinical outcomes and used GRADE Pro software (https://gdt.gradepro.org) to generate the summary of findings table [21, 22]. Two researchers (JK, JM) independently assessed the certainty of the evidence, and the discrepancy was resolved through discussion with an experienced researcher (PK).
Results
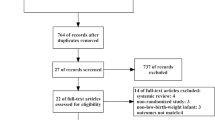
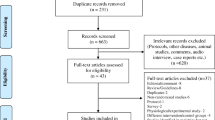
A total of 3060 records were identified, of which 374 duplicates were removed before the screening. We screened through titles and abstracts of 2686 articles, of which 26 were considered potentially relevant for full-text screening. Out of 26, six reports [12, 13, 23,24,25,26] fulfilled the inclusion criteria and were evaluated for qualitative and quantitative synthesis (Fig. 1). We identified one additional study through citation search [8]. Two published reports [13, 26] from one trial were combined under the primary research for analysis. The exact reasons for excluding full-text articles are given in Fig. 1. Details of excluded studies are provided in the supplementary appendix.
Study characteristics and risk of bias assessment
Six studies (872 participants) are included in this systematic review [8, 12, 13, 23,24,25]. The mean gestation varied between 29 and 32 weeks across the trials. The proportion of small for gestational age (birth weight less than 10th centile for that gestational age) infants varied from 16 to 42% across study populations. All except one included very low birthweight (< 1500 g) neonates. Dhingra et al. [23] enrolled neonates up to 1750 g, though most were very low birth weight only (mean birth weight was 1210 g and 1249 g in three and two-hourly feeding groups, respectively). Unal et al. [12] enrolled neonates only after achieving a weight of 1500 g while they were on full gavage feeding, and they are the only ones to provide information on time to transition to full oral feeds. Detailed descriptions of the study participants, eligibility criteria, feeding schedule, and outcomes are provided in Table 1. We used the Cochrane Collaboration tool for assessing the risk of bias of the randomized controlled trials [19]. All trials were open-label trials and were at high risk for performance bias. In addition, blinding of outcome assessment was not possible due to the nature of the intervention. Therefore, they are at increased risk for detection bias for subjective outcomes like feed intolerance, time to full enteral feeds, withholding feeds, and time to discharge (Fig. 2).
Primary outcome
Our primary outcome was the incidence of NEC ≥ stage 2 as per modified Bell’s staging [17]. Three trials (530 participants) reported the primary outcome and did not find any statistically significant difference between the two groups (RR: 1.39; 95% CI: 0.53 to 3.65; I2-0%) (Fig. 3a). Also, the incidence of any stage NEC did not differ significantly (5 studies, 767 participants, RR 0.98; 95% CI: 0.53 to 1.82, I2-0%) (Table 2).
Secondary outcomes
There was variability in the definition of full enteral feeds. One study determined 100 mL/kg/d as full feed, while the rest five considered 150 mL/kg/d to be full enteral feed (Table 1). We have presented them as two separate outcomes (Table 2). Irrespective of the threshold used, there was no significant difference in time to achieve full enteral feeds (Fig. 3b).
There was no significant difference between the groups in the incidence of all-cause mortality, duration of hospital stay, daily weight gain (g/kg/day), time to regain birth weight, weight at discharge, duration of intravenous fluids, and length of hospitalization (Table 2). In addition, there was no significant difference in the incidence of hypoglycemia, apnea, feed intolerance, or GER requiring medical therapy between the two feeding schedules (Table 2).
Three-hourly feeding schedules were associated with a shorter duration of nursing time spent on feeding (1 trial, 87 participants, MD − 22.00 min/day, 95% CI − 23.9 to − 20.1 min/day). However, one trial [12] also compared the time to reach full oral feeding and time taken for transitioning from tube to oral feeding and did not observe any significant differences (Table 2).
None of the trials reported TPN duration, CVC duration, anthropometry at 40 weeks, or during follow-up.
Subgroup analysis
We aimed to perform subgroup analysis based upon gestation (< 28 weeks, 28–32 weeks, and more than 32 weeks), birth weight (≤ 1000 g, 1001–1500 g, > 1500 g), and intrauterine growth restriction status (SGA). However, we could do it only for birth weight < 1000 g vs. 1001–1500 g, and appropriate for gestational age (AGA) vs small for gestational age (SGA) with the available data.
Birth weight (extremely low birth weight infants)
Five trials (772 participants) reported enrollment of 99 (12.8%) extremely low birth weight (ELBW) neonates; however, only three trials [13, 24, 25] provided data for subgroup comparison (< 1000 g vs. 1001–1500 g). Only one trial (84 ELBW infants) reported the outcomes in the ELBW subgroup [25]. This trial did not report NEC; however, they showed that ELBW neonates fed three-hourly achieved full enteral feeds (150 mL/kg/d) slower than those fed at two-hourly intervals (14.14 vs. 11.24 days; MD: 2.90 days, 95% CI: 1.16 to 4.64). There was a significant subgroup difference in comparison to 1001–1500 g infants (p-0.0009). There was no significant difference in feed intolerance, apnea, and hypoglycemia. As the other four trials enrolled only 15 ELBW neonates (2.3% of all participants), the results are unlikely to be affected by the missing outcome information.
Small for gestational age
Out of 872 participants, 245 (28.1%) were SGA; however, only two trials (166 participants) provided separate data on outcomes in them. There was no significant difference in NEC (stage 2 or more), feed intolerance, hypoglycemia, time to full enteral feeds, and time to regain birth weight between the two feeding schedules among SGA infants (Table 3). On subgroup analysis, there were no significant differences between SGA and AGA neonates for feed intolerance and hypoglycemia (low to very low certainty evidence).
None of the trials provides separate data for variables like gestational age, type of feeds (formula/human milk), probiotics use, and antenatal doppler abnormalities (absent/reversed end-diastolic flow), which can potentially affect the outcomes [27, 28]. Therefore, we could not do subgroup analysis for these variables.
Due to fewer trials, we could not do meta-regression to assess the effect of gestational age or birth weight on various outcomes; or identify meaningful subgroup interaction effects.
Sensitivity analysis
We also did a fixed-effect meta-analysis, but the results were almost similar for all outcomes (Supplementary Table S2). There was no significant heterogeneity for any outcome except time to regain birth weight (I2-56%, p-0.08). Finally, we did a sensitivity analysis to explore this heterogeneity and observed that the heterogeneity is mainly contributed by Yadav et al.’s [13] study. In this trial, the mean gestational age, birth weight, and proportion of SGA neonates were higher than others. After excluding this study, the pooled analysis of 3 studies (350 participants) showed that the infants in the three-hourly feeding group regained birth weight earlier than the two-hourly feeding group (MD −1.11 days; 95% CI −2.16 to −0.06 days; I2-0%). Most neonates in the Tali et al.’s [25] study were ELBW, and the authors provided separate data for some outcomes; we did sensitivity analysis by excluding the ELBW population. The effect size and direction were similar for all results, though the confidence interval widened due to the loss of sample size. The funnel plot (Supplementary Fig. 1) and Egger’s test (coefficient 2.11 [95% CI: −2.51 to 6.74]; p-0.2) do not suggest publication bias.
Discussion
In this systematic review of six RCTs, three-hourly feedings were not associated with an increased incidence of NEC (stage 2 or more) or all-cause mortality compared to two-hourly feeding in stable preterm infants (low certainty evidence for both the outcomes). In addition, the time to reach full enteral feeds, duration of hospitalization, and anthropometric parameters at discharge in the three-hourly feeding group were not different from the two-hourly feeding group (low to moderate certainty evidence). The incidence of feed intolerance, regurgitation of feeds, and apnea were also similar (low to very low certainty evidence). Furthermore, the moderate certainty evidence suggests that the risk for hypoglycemia with three-hourly feeding schedules is not different from a two-hourly feeding schedule. In addition, a three-hourly plan saved the nursing time spent on feeding (very low certainty evidence).
SGA neonates are at a higher risk for NEC and feed intolerance. On subgroup analysis, there were no significant differences between SGA and AGA neonates for feed intolerance and hypoglycemia (low to very low certainty evidence). The SGA infants in the three-hourly feeding group took significantly longer to reach full enteral feeds (low to very low certainty evidence).
As 85% of the study population was between 1000 and 1500 g, these results can be generalized to this birth weight group. However, the safety data in ELBW neonates was based on a single small trial. Therefore, due to the small sample size and wider confidence intervals, we are uncertain about the outcomes in this subpopulation. Moreover, two-hourly feeding schedules helped achieve full feeds earlier in the ELBW infants, though the evidence is low quality. Therefore, a two-hourly feeding schedule might be followed in ELBW infants until more evidence is available. There is marked variability in feeding practices among neonatal units worldwide [29]. Most neonatal units practice two-hourly feeding schedules for stable preterm infants. This practice of two-hourly feeding is based on tradition rather than evidence. Two-hourly feeding increases the nursing workload and involves more frequent handling of the neonates, which might be stressful [3, 23]. Physiologically, frequent bolus feeding is not desirable as it leads to persistently increased superior mesenteric artery blood flow [5, 10].
Previous observational studies showed variable results precluding meaningful conclusions [6, 7, 30]. Initial small RCTs suggested three-hourly feedings can be safely followed in stable very low birth weight infants [20, 21]. Based on limited evidence, Dutta et al. [4] suggested using three-hourly feedings in infants weighing > 1250 g; however, they advised continuing a 2-hourly schedule for infants < 1250 g. On the other hand, Binchy et al. [3] suggested 2-hourly feeding in all VLBW infants. The World Health Organization (WHO) feeding guidelines advocate bolus feeding over continuous feeding but do not recommend any feeding interval [31]. To some extent, these conflicting recommendations are a result of the pooling of heterogeneous observational studies. Therefore, we restricted ourselves to RCTs only. Considering the physiological differences between ELBW infants (< 1000 g) and those between 1000 and 1500 g, it may not be wise to club them under a single umbrella. Therefore, we attempted subgroup analysis for ELBW infants.
This systematic review suggests a three-hourly feeding schedule is as safe as two-hourly feeding in stable preterm infants with a birth weight between 1000 to 1500 g. A three-hourly feeding schedule might be considered in this subpopulation, considering the potential benefits of lower nursing workload, lesser handling of the neonate, and less mother-infant dyad stress. Most studies limited their follow-up till the infant achieved full tube feeds. So, there may be a concern whether three-hourly feeding with relatively larger volumes per feed would be suitable for transitioning from tube to oral feeding by spoon/cup. There is low-quality evidence that a three-hourly feeding schedule performs like two-hourly feeding in transitioning from tube to full oral feeds. This review also provides evidence that the same feeding schedule might be followed in SGA neonates.
Recent Cochrane review of four RCTs (n = 417 participants) by Ibrahim et al. [10] have similar conclusions except that they found a longer time to regain birth weight in infants receiving two-hourly feeds. However, we did not find any significant difference in this outcome. This difference is due to the inclusion of the recent trial by Yadav et al. [13] (n = 350) in the index review. Also, we were able to get additional data from Yadav et al. [13] for SGA infants who are at higher risk for feed intolerance and other complications (hypoglycemia, NEC, Doppler abnormalities). Therefore, we could do separate subgroup analyses to make results more generalizable. Also, we analyzed data on transition from tube to oral feeds, which is an essential milestone in feeding transition in these neonates.
Previous retrospective studies reported variable results with two feeding schedules [6, 7, 30]. For example, Chu et al. [6] and Rudiger et al. [7] compared 2-hourly vs. 3-hourly feeding schedules in ELBW infants and reported shorter (not statistically significant) time to reach full enteral in the 3-hourly feeding group. Also, the 3-hourly feeding group had a significantly shorter TPN duration and central catheter use but was associated with a more extended period of phototherapy and continuous positive airway pressure (CPAP) use. On the contrary, DeMauro et al. [30] reported that infants fed at the two-hourly interval had better tolerance, achieved full enteral feeds earlier, and required less TPN. However, all three studies reported a similar incidence of NEC with two feeding schedules. These variations might be due to differences in the feeding practices and are subject to potential limitations of retrospective studies.
This review of RCTs found that two-hourly feeding in ELBW infants helps to reach full enteral feeds by 2.9 days earlier (low certainty evidence). Our review also suggests that three-hourly feeding does not increase the risk for feed intolerance, hypoglycemia, and apnea in this subpopulation (very low certainty evidence). The authors did not report the incidence of NEC in this subpopulation. Thus, there is a definite need for further RCTs focused on ELBW infants. However, considering the benefits of 2-hourly feeding to achieve full enteral feeds earlier and no information about NEC, we suggest continuing 2-hourly feeding in ELBW neonates till further evidence.
Limitations
Although we followed standard guidelines for this review and used the GRADE framework to assess the certainty of the evidence, this review has many limitations. Most of the included trials were small and not adequately powered for critical outcomes like mortality and NEC. Of the six trials, four are from India and one each from Turkey and Malaysia; therefore, the interpretations may not be generalizable to the NICUs in the developed world. The number of ELBW neonates was too low to make robust conclusions. Also, they were at high risk for performance and detection bias for many outcomes due to lack of blinding. All trials used mixed feeding but did not analyze the effect of the type of feeds; therefore, we cannot rule out the impact of formula milk on adverse outcomes like NEC or feed intolerance. There was wide variation in the volume and rate of advancement of feeds and the definition of full enteral feeds across the studies. Non-availability of data according to gestation, type of feed, and antenatal doppler abnormalities precluded specific subgroup analyses. Also, the trials did not study the effect of feeding intervals on maternal and infants stress levels, quality and duration of sleep, kangaroo mother care, anthropometry parameters at follow-up, and neurodevelopmental outcome. Due to a limited number of studies, a possible publication bias cannot be excluded.
Conclusions
Based on limited low to moderate certainty evidence, we suggest three-hourly feedings in stable preterm infants with a birth weight of 1000 g and more. However, we suggest continuing two-hourly feeding until further evidence is available for extremely low birth weight (< 1000 g) infants.
Availability of data and material
The data is given in the manuscript. The primary source of information is already available in the public domain.
Code availability
Not applicable.
Abbreviations
- CI:
-
Confidence interval
- CVC:
-
Central venous catheter
- ELBW:
-
Extremely low birth weight
- GRV:
-
Gastric residual volume
- LBW:
-
Low birth weight
- MD:
-
Mean difference
- NEC:
-
Necrotizing enterocolitis
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- RCT:
-
Randomized controlled trial
- RR:
-
Risk ratio
- RD:
-
Risk difference
- SMD:
-
Standardized mean difference
- TPN:
-
Total parenteral nutrition
References
Davis TA, Fiorotto ML, Suryawan A (2015) Bolus vs. continuous feeding to optimize anabolism in neonates. Curr Opin Clin Nutr Metab Care 18:102–108. https://doi.org/10.1097/MCO.0000000000000128
Wang Y, Zhu W, Luo B-R (2020) Continuous feeding versus intermittent bolus feeding for premature infants with low birth weight: a meta-analysis of randomized controlled trials. Eur J Clin Nutr 74:775–783. https://doi.org/10.1038/s41430-019-0522-x
Binchy Á, Moore Z, Patton D (2018) Feeding intervals in premature infants ≤1750 g: an integrative review. Adv Neonatal Care 18:168–178. https://doi.org/10.1097/ANC.0000000000000486
Dutta S, Singh B, Chessell L et al (2015) Guidelines for feeding very low birth weight infants. Nutrients 7:423–442. https://doi.org/10.3390/nu7010423
Lane AJ, Coombs RC, Evans DH, Levin RJ (1998) Effect of feed interval and feed type on splanchnic haemodynamics. Arch Dis Child Fetal Neonatal Ed 79:F49-53. https://doi.org/10.1136/fn.79.1.f49
Chu E, Freck S, Zhang L et al (2019) Three-hourly feeding intervals are associated with faster advancement in very preterm infants. Early Hum Dev 131:1–5. https://doi.org/10.1016/j.earlhumdev.2019.01.021
Rüdiger M, Herrmann S, Schmalisch G et al (2008) Comparison of 2-h versus 3-h enteral feeding in extremely low birth weight infants, commencing after birth. Acta Paediatr 97:764–769. https://doi.org/10.1111/j.1651-2227.2008.00774.x
Anushree N, Shaw S, Negi V (2018) 2 hourly versus 3 hourly feeding schedule in very low birth weight preterm neonates. J Mar Med Soc 20:96. https://doi.org/10.4103/jmms.jmms_18_18
Razak A (2019) Two-hourly versus three-hourly feeding in very low-birth-weight infants: a systematic review and meta-analysis. Am J Perinatol 37:898–906. https://doi.org/10.1055/s-0039-1691767
Ibrahim NR, Van Rostenberghe H, Ho JJ, Nasir A (2021) Short versus long feeding interval for bolus feedings in very preterm infants. Cochrane Database Syst Rev 8:CD012322. https://doi.org/10.1002/14651858.CD012322.pub2
Ibrahim NR, Van Rostenberghe H, Ho JJ (2016) Short versus long feeding interval for bolus feedings in very preterm infants. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012322
Unal S, Demirel N, Bas AY et al (2019) Impact of feeding interval on time to achieve full oral feeding in preterm infants: a randomized trial. Nutr Clin Pract 34:783–788. https://doi.org/10.1002/ncp.10244
Yadav A, Siddiqui N, Debata PK (2021) Two-hourly versus three-hourly feeding in very low birthweight neonates: a randomized controlled trial. Indian Pediatr 58:320–324
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ n71. https://doi.org/10.1136/bmj.n71
Higgins JPT, Thomas J, Chandler J et al (2021) Cochrane Handbook for Systematic Reviews of Interventions version 6.2. (updated February 2021). Cochrane Available from www.training.cochrane.org/handbook.
McGowan J, Sampson M, Salzwedel DM et al (2016) PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol 75:40–46. https://doi.org/10.1016/j.jclinepi.2016.01.021
Kliegman RM, Walsh MC (1987) Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 17:213–288. https://doi.org/10.1016/0045-9380(87)90031-4
Valpacos M, Arni D, Keir A et al (2018) Diagnosis and management of necrotizing enterocolitis: an international survey of neonatologists and pediatric surgeons. Neonatology 113:170–176. https://doi.org/10.1159/000484197
Higgins JPT, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Higgins JP, Li T, Deeks JJ (2019) Choosing effect measures and computing estimates of effect. Cochrane handbook for systematic reviews of interventions 23:143-76. Available from www.training.cochrane.org/handbook.
GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2020 (developed by Evidence Prime, Inc.). https://gradepro.org/
Schünemann H, Brożek J, Guyatt G, Oxman (2013). A GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group. https://gdt.gradepro.org/app/handbook/handbook.html
Dhingra A, Agrawal SK, Kumar P, Narang A (2009) A randomised controlled trial of two feeding schedules in neonates weighing <or=1750 g. J Matern Fetal Neonatal Med 22:198–203. https://doi.org/10.1080/14767050802385749
Ibrahim NR, Kheng TH, Nasir A et al (2017) Two-hourly versus 3-hourly feeding for very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 102:F225–F229. https://doi.org/10.1136/archdischild-2015-310246
Tali S, Kabra N, Ahmad J, et al Effect of feeding schedule on time to reach full feeds in ELBW and VLBW neonates: a randomized trial. Perinatology 17:95–102. https://www.perinatology.in/effect-of-feeding-schedule-on-time-to-reach-full-feeds-in-elbw-and-vlbw-neonates
Debata PK (2021) Feeding schedule in preterm infants: two hourly versus three hourly: authors’ reply. Indian Pediatr 58:495–496
Neu J (2020) Necrotizing enterocolitis: the future. Neonatology 117:240–244. https://doi.org/10.1159/000506866
Kumar J, Yadav A (2021) Feeding schedule in preterm infants: two hourly versus three hourly. Indian Pediatr 58:495
Klingenberg C, Embleton ND, Jacobs SE et al (2012) Enteral feeding practices in very preterm infants: an international survey. Arch Dis Child Fetal Neonatal Ed 97:F56-61. https://doi.org/10.1136/adc.2010.204123
DeMauro SB, Abbasi S, Lorch S (2011) The impact of feeding interval on feeding outcomes in very low birthweight infants. J Perinatol 31:481–486. https://doi.org/10.1038/jp.2010.153
World Health Organization (2012) Guidelines on optimal feeding of low birth weight infants in low- and middle-income countries. https://www.who.int/maternal_child_adolescent/documents/9789241548366.pdf
Acknowledgements
A portion of this work was done to develop Clinical Practice Guidelines on “Feeding of low birthweight neonates” for the National Neonatology Forum of India. We want to extend our sincere gratitude to all the guideline development group members for their valuable inputs.
Author information
Authors and Affiliations
Contributions
JK—conceived idea, did literature search, retrieved and analyzed the data, and drafted the manuscript. JM and PD—peer-reviewed search strategy, literature search, analyzed the data, and drafted the manuscript. PK—conceived idea, supervised data synthesis and analysis, and critically reviewed the manuscript. JS and AS—provided critical inputs in data analysis and critically reviewed the manuscript. All the authors approved the final version of the manuscript and shall be accountable for all aspects of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not required.
Consent to participate
Not required.
Consent for publication
All authors consented to publication.
Conflict of interest
The authors declare no competing interests.
Additional information
Communicated by Daniele De Luca
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, J., Meena, J., Debata, P. et al. Three-hourly versus two-hourly feeding interval in stable preterm infants: an updated systematic review and meta-analysis of randomized controlled trials. Eur J Pediatr 181, 2075–2086 (2022). https://doi.org/10.1007/s00431-022-04405-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-022-04405-y