Abstract
Infantile colic is a functional gastrointestinal disease of the infancy that its cause has not yet been properly identified. It leads to severe discomfort in the infants and anxiety in their mothers. Probiotics have recently been recommended as an effective treatment for the improvement of infantile colic. The objective of this study is to determine the role of prenatal administration of Lactobacillus reuteri (L. reuteri) LR92 DSM 26866 on the occurrence of infantile colic. This double-blinded, placebo-controlled, randomized trial was conducted with healthy pregnant women from December 2017 to December 2018 in Isfahan, Iran. A total of 145 patients was included in this study. The case group consisted of 87 pregnant women, who received daily doses of 1 × 108 colony-forming units of live L. reuteri LR92 DSM 26866, and the control group with 88 pregnant women received the placebo (containing 9% glucose solutions) for the last 4 weeks of pregnancy. Mothers and their infants in both groups did not have significant differences in anthropometric indices, and the infants’ feeding pattern. Infants born to both groups of mothers followed for 5 months on signs and symptoms of colic with the repetitive examination by a blinded pediatrics assistant to record the occurrence of colic and its grading. Mothers who received placebo were 2.36 times more likely to have infants exhibiting infantile colic than mothers in the L. reuteri LR92 DSM 26866 group (CI 95%, 1.18–4.73). Using Mann-Whitney U test, the Mean (SD) of colic severity was significantly lower in the intervention group (p = 0.01). The frequency of colic and its higher grades were significantly lower in the intervention group (p = 0.03 for the presence of colic and p = 0.01 for high grades of colic). The frequency of colic presence and its different grades according to mothers’ delivery mode and infant feeding patterns were not different between the two groups (p > 0.05).
Conclusion: Maternal prenatal supplementation with probiotic L. reuteri LR92 DSM 26866 during the last 4 weeks of pregnancy can prevent the occurrence and reduce the severity of infantile colic.
What is Known • Lactobacillus reuteri LR92 DSM 26866 is effective in improving the symptoms of infantile colic. | |
What is New • Prenatal administration of Lactobacillus Reuteri LR92 DSM 26866 can prevent the occurrence of infantile colic or reduce its severity. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wessel et al. [1] defined infantile colic for the first time in 1954, which was revised in ROME IV meeting [2, 3] as “irritable/uncontrollable/compulsive/inconsolable crying” for more than 3 h a day, at least for 3 days a week, and for 1 week or longer in an infant without any distinct cause or failure to thrive [1, 4, 5]. The onset of the symptoms must occur under 5 months of age to be defined as infantile colic [6]. The prevalence of this condition is different between societies. It is assumed that 1 out of every 5 Iranian infants fit the criteria for infantile colic [7]. Unfortunately, no particular underlying mechanism has been determined as the responsible mechanism for this disorder. Some studies have proposed that mothers’ inappropriate eating, consumption of allergen-containing foods, and lactase insufficiency in the infants are responsible [8,9,10]. Attentions have been paid to the role of gastrointestinal tract flora [11, 12]. Probiotics have recently been suggested to ameliorate colicky pain in infants [13]. Probiotics refer to orally administered supplements, which contain an adequate quantity of live microorganisms that can modify the host intestinal flora [14]. Probiotics play a prominent role in the growth and development of the gastrointestinal tract likely through the gut-brain axis [15]. Some studies proposed the valuable impacts of administrating probiotics for mothers in the perinatal period to alter the infant’s intestinal flora on prevention of infantile colic [16,17,18]. Given the acceptable outcome of L. reuteri DSM 17938 for reduction of colicky pain in infants [19, 20], and due to the scarcity of studies in this setting, we decided to administer this supplement at the end of pregnancy for investigation of its effect in the prevention of infantile colic. This study was designed to assess the role of prenatal L. reuteri LR92 DSM 26866 administration in the management of infantile colic.
Methods and materials
Methods
Study participants
A double-blind randomized clinical trial was conducted on 175 pregnant women at the beginning of the last 4 weeks of pregnancy in Beheshti and Imam Hossein Hospitals affiliated to Isfahan University of Medical Sciences (Isfahan, Iran) in 2018–2019 to evaluate the impact of prenatal L. reuteri LR92 DSM 26866 administration on the severity of infantile colic. Children with the following criteria enrolled in the study (inclusion criteria): 18–49 years old healthy pregnant women at the beginning of the last 4 weeks of pregnancy (at the initiation of the 35th weeks of gestation) who filled out a consent form for entrance to study. The mothers had received the probiotic drop daily. They followed by telephone call weekly and by showing the consumed bottle of drug biweekly. Women with HIV infection, renal and hepatic diseases, diabetes mellitus, anemia antibiotic or probiotic consumptions in the recent 30 days, twin pregnancy, or who smoke 10 or more cigarette drag were not entered into the study.
Women with any kind of hypersensitivity to the drug components, utilization of antibiotic or anti-allergic medications during the intervention, termination of pregnancy before the expected date or infant death before the end of the follow-up were excluded from the study.
Study design
The sample size was calculated by the following formula:
where n is the sample size in each group; Z1 represents the desired level of statistical significance (= 1.96); Z2 represents the desired power (= 0.84 for 80% power); d is the relative frequency difference; and P is the average relative frequency. Thus, n was estimated as 87 individuals for each group (case and control) based on the conditions: (i) with considering P of infantile colic, which has been reported 20% at the significant level of 0.05 [21, 23]; (ii) test power of 80%; and (iii) by considering d of 17%. After randomization, 88 and 87 women were placed on placebo and intervention groups, respectively. For randomization, patients will be handed a closed envelope and asked to deliver it to a Gynecology and Obstetrics assistant who divided them into intervention or placebo group, depending on the number existing in the envelope. In the control group, the package containing the placebo (9% glucose solution made by Gostaresh Miilad Pharmed Co.). In the intervention group, mothers received daily doses of 1 × 108 colony forming units (5 drops) of live L. reuteri LR92 DSM 26866 from Gostaresh Milad Pharmed® Prokid™ drop until delivery time. It should be mentioned that the product of L. reuteri LR92 DSM 26866 from Gostaresh Milad Pharmed® Prokid™ drop (Gostaresh Milad Pharmed Co., Tehran, Iran) is under license of Euro natural of Italy (https://www.euronatural.it). The strain Lactobacillus Reuteri LR92 DSM has been subjected to total DNA extraction and a 16S rDNA sequence analysis. Strain typing was performed by PFGE analysis of DNA.
The mothers’ delivery mode and maternal body mass index (BMI) were recorded due to the possibility of their confounding effect. The infants’ gender distribution and feeding patterns were also documented in the first physician visit after birth.
The primary outcome of this trial was to evaluate the presence and severity of colic in the two studied groups 1 month after birth. The secondary outcome was to compare the presence and/or severity of colic in studied groups based on the mode of delivery (vaginal versus cesarian section) and feeding patterns (breast-feds versus formula-fed and standard formula without probiotic).
The infants had been followed for 5 months. During the follow-up period, infants were repetitively examined by the blinded pediatrics assistant (every 2 weeks up to 60 days of age and every 30 days up to 5 months age). If any of the infants were diagnosed with infantile colic during the follow-up period, the pediatric assistant recorded it and assessed the severity of infantile colic.
The severity of colic was assessed by grading on based on the Rome IV infantile colic criteria, to 4 grades (0–3) (Zeevenhooven) [2].
Grade 0: No significant inconsolable crying
Grade 1: Recurrent inconsolable crying with colicky pain posture unknown cause less than 3 h per day and less than 3 days per week
Grade 2: Recurrent inconsolable crying with colicky pain posture unknown cause more than 3 h per day but less than 3 days per week
Grade 3: Recurrent inconsolable crying with colicky pain posture unknown cause more than 3 h per day and more than 3 days per week
The parents were asked to fill out a questionnaire including the severity and duration of the infant crying, pulled up to the stomach, a flushed face, clenched hands, and a wrinkled brow in the existing questionnaire. Based on the questionnaire filled by mothers and the interview between mothers and a pediatric assistant, the grades of colic were recorded. The physician who examined the infant (a pediatric assistant) and the mothers were blinded from the patients’ study groups.
Statistical analysis
Data were documented and analyzed in the Statistical Package for the Social Sciences (SPSS for windows 16.0. SPSS Inc., Chicago, IL) using a chi-square test for categorical data, independent samples t test and Mann-Whitney U test for numerical data. Estimating the odds ratio of using probiotics for preventing infantile colic was calculated using the logistic regression test. The p values < 0.05 were considered statistically significant.
Ethics statement
This project was approved by the ethics committee of the Isfahan University of Medical Sciences (IR.MUI.MED.REC.1397.248). It was also registered at the site of the Iranian Registry of clinical trials (IRCT) with code of IRCT20131004014882N7. All parents were provided information regarding the research method in simple language. The children were included in the study after their parents agreed and signed the informed consent form.
Results
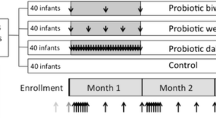
Initially, 145 pregnant women completed the trial (71 in the placebo and 74 in the probiotics groups) from 175 pregnant women, who were enrolled in the study (Fig. 1). Table 1 shows the demographic characteristics of mothers and their infants. There were no significant differences regarding maternal age, infant gender, gestational age, BMI, the mode of delivery, and the infants’ feeding pattern (p > 0.05).
Since colic grades were not normally distributed, we ran Mann-Whitney U test as the nonparametric alternative for independent samples t test. Mean (SD) of colic severity (colic grades), frequency of colic existence, and different grades are presented in Table 2. Mean (SD) of colic severity was significantly lower in the intervention group (p = 0.01). The frequency of colic and its high grades were significantly lower in the intervention group, i.e., p = 0.03 and 0.01 for the presence of colic and high grades of colic, respectively.
The frequency of colic existence and its different grades according to mothers’ delivery mode and infants’ feeding patterns are presented in Table 3. There was no significant difference between groups (p > 0.05). Figure 2 shows the frequency of different grades of colic in the infants of pregnant women in probiotic and placebo groups.
A dichotomous logistic regression was performed to ascertain the effects of feeding mode, delivery mode, gestational age, and intervention in the patient (on the likelihood of infantile colic in infants). Mothers who received placebo were 2.36 times more likely to have infants exhibiting infantile colic than mothers in the L. reuteri LR92 DSM 26866 group (OR = 2.36; CI 95%, 1.18–4.73). The other three mentioned variables were not associated with an increased likelihood of exhibiting colic symptoms.
Discussion
This study aimed to evaluate the efficacy of L. reuteri LR92 DSM 26866 for pregnant women at the beginning of the last 4 weeks of pregnancy on the prevention of infantile colic or reducing its severity. We found that L. reuteri LR92 DSM 26866 (108 CFUs) is more beneficial than the placebo in the prevention of infantile colic as the means of reducing both the frequency of the disease and the severity of the symptoms.
Several studies have shown the relatively favorable impacts of probiotic administration during the perinatal period [4, 16,17,18]. Some of these have specifically focused on L. reuteri to evaluate its effect on ameliorating infantile colic [19, 21,22,23]. Furthermore, Sung et al. [24] through a meta-analysis showed that L. reuteri could be effective for improving infantile colic, especially in breastfed infants.
Some researches were designed concerning the safety of probiotic administration during pregnancy. They have not shown a significant adverse effect. Investigations revealed that probiotic supplementation during pregnancy is not only safe but also effective in protection against preeclampsia, gestational diabetes, vaginal infections, maternal and infant weight gain, and later childhood diseases [18]. They may also facilitate the reduction of waist circumference in mothers [25, 26]. The investigation of Allen et al. [27] on two species of probiotics (lactobacilli and bifidobacteria) showed that a daily intake of 1 × 1010 colony-forming units during the last month of pregnancy was safe. While several studies suggested that oral probiotic administration in early life can significantly reduce the severity and incidence of infantile colic, only a few investigations were conducted for the assessment of the efficacy of perinatal supplementation of L. reuteri LR92 DSM 26866 on prevention of infantile colic [5, 13, 19]. They perceived that maternal consumption of probiotics in late pregnancy may exert an impressive effect on the reduction of infantile colic by various mechanisms. Hoffman et al. [28] revealed that the administration of multispecies probiotic to mother in pregnancy can improve her flatulence and constipation and reduce symptoms of infantile colic, diarrhea, and constipation.
The exact underlying mechanism of action of probiotics is unknown, but some investigations were accomplished in this setting. The study of Baldassure et al. [16] demonstrated that probiotic use in mothers can raise the TGF-B, which in turn stimulates the gut maturity and IgA secretion, controls inflammation, and improves gastrointestinal functions in infants [29]. Since Lactobacillus rhamnosus GG can stabilize tight junction and stimulate mucin with IgA synthesis, it improves the performance of the gut permeability barrier [30]. It is also shown that Lactobacillus rhamnosus promotes the production of gamma aminobutyric acid (GABA), which is the major inhibitory neurotransmitter [30], and influences on individual behavior [31, 32]. Other probiotics including L. reuteri LR92 DSM 26866 also may alleviate colic through the same mechanism.
On the other hand, another survey directed in Finland and Germany to compare the effect of perinatal probiotic administration on breastfed infants (Finland) with formula supplementation after birth (Germany) with analyzing infantile gastrointestinal flora [31]. It was suggested that probiotic treatment had different effects on gut microorganisms in Finnish and German infants due to different types of feeding. Although L. reuteri LR92 DSM 26866 administration can significantly reduce the severity of infantile colic in our study, the infant feeding pattern was not associated with a difference in the incidence of infantile colic (Table 3). Although formula-fed infants received standard formulas without probiotics in the present study, it should be noted that the number of formula-fed infants was not sufficient for judgment.
In the case of comparing delivery mode between two groups, despite a decrease in the existence and severity of colic by L. reuteri LR92 DSM 26866, we did not found a significant difference between infants born by vaginal delivery with the cesarian section (Table 3). Nonetheless, the study of Sirilun [32] ensured that infants who were born by cesarean section have had a significant paucity of bifidobacteria in their feces. For exclusively breastfed infants, intestinal bifidobacteria counts were significantly affected by vaginal delivery and high amount of bifidobacterial copy in their mothers’ gastrointestinal tract. Lundgren et al. [33] also observed that global gut microbial community structure and specific microorganisms differed in relation to maternal dietary issues, often concerning the delivery route, in the exclusively breastfed infants.
Collado et al. [34] investigated the effect of maternal microorganisms on infant gastrointestinal tract flora, and the result of their investigation is consistent with our research. They examined the maternal stool, placenta, amniotic fluid, colostrum, meconium, and infant stool samples from 15 mother candidates and their babies for the cesarian section and the composition of gut microorganisms, respectively. They resulted that the placenta and amniotic fluid had different microorganisms with lower counts and variability but the predominance of proteobacteria. The findings of the study implicated for maternal to fetal microbial transfer before delivery. Four days after birth, the composition of gut microbiota was similar to breast milk. This resembles our findings that the effect of probiotics on the human body may not be related to the delivery route.
Some researchers have suggested that the effect of probiotics may be through the transfer of bacteria components from mothers’ intestine via lymphatic channels by dendritic cells amid tight junctions into the mother’s mammary gland [3, 34] then into the breast milk in the future. This situation can modify the gastrointestinal composition of microorganisms in infants and consequently enhance the maturation of the immune system (3–36). Moreover, investigators showed that the ingestion of probiotics by mother could significantly increase the measures of anti-inflammatory mediators such as IL6 levels in the colostrum and IL10 and TGF-B levels in the breast milk [33]. Further, increasing TGF-B in breast milk facilitates intestinal maturity and production of IgA, which leads to ameliorate functional gastrointestinal disorders in the infant [17, 35]. Thus, colonization of the gut may not be necessary for the beneficial effect of probiotics and modifying the cytokine profiles in breast milk would be the main mechanism [35].
In the present work, there was no difference between breast and formula-fed infants; however, the number of formula-fed infants was insufficient. More clinical trials are needed to compare breast-fed infants with formula-fed infants in this setting. One of the limitations of the present study was the paucity of formula-fed infants for more reliable comparison with the breast-feds. It is suggested that a study with a similar design can be executed to measure the probiotic colony counts in the infantile fecal specimen. Moreover, a similar clinical trial may be conducted with a longer follow-up period to quantify the impact of these products on mothers’ depression and quality of life.
Conclusion
Consumption of probiotic L. reuteri LR92 DSM 26866 by the pregnant mother during the last month of pregnancy can prevent the occurrence of infantile colic or reduce the severity.
Abbreviations
- BMI:
-
Body mass index
- ICS:
-
Infantile colic severity score
- IRCT:
-
Iranian registry of clinical trials
References
Wessel MA, Cobb JC, Jackson EB, Harris GS, Detwiler AC (1954) Paroxysmal fussing in infancy, sometimes called" colic". Pediatrics. 14(5):421–435
Zeevenhooven J, Koppen IJ, Benninga MA (2017) The new Rome IV criteria for functional gastrointestinal disorders in infants and toddlers. Pediatr Gastroenterol Hepatol Nutr 20(1):1–13
Zeevenhooven J, Browne PD, L’Hoir MP, de Weerth C, Benninga MA (2018) Infant colic: mechanisms and management. Nat Rev Gastroenterol Hepatol 15(8):479–496
Sung V, Hiscock H, Tang M, Mensah FK, Heine RG, Stock A et al (2012) Probiotics to improve outcomes of colic in the community: protocol for the baby biotics randomised controlled trial. BMC Pediatr 12(1):135
Savino F, Cordisco L, Tarasco V, Palumeri R, Calabrese E, Oggero R et al (2010) Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 126(3):e526–ee33
Urbańska M, Szajewska H (2014) The efficacy of lactobacillus reuteri DSM 17938 in infants and children: a review of the current evidence. Eur J Pediatr 173(10):1327–1337
Talachian E, Bidari A, Rezaie MH (2008) Incidence and risk factors for infantile colic in Iranian infants. World J Gastroenterol: WJG 14(29):4662
Ståhlberg M, Savilahti E (1986) Infantile colic and feeding. Arch Dis Child 61(12):1232–1233
Kanabar D, Randhawa M, Clayton P (2001) Improvement of symptoms in infant colic following reduction of lactose load with lactase. J Hum Nutr Diet 14(5):359–363
Ahmed M, Billoo AG, Iqbal K, Memon A (2018) Clinical efficacy of lactase enzyme supplement in infant colic: a randomised controlled trial. JPMA J Pak Med Assoc 68(12):1744–1747
Weerth Cd, Fuentes S, Puylaert P, de Vos WM (2013) Intestinal microbiota of infants with colic: development and specific signatures
Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R et al (2010) Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics. 126(3):e526
Szajewska H, Dryl R (2016) Probiotics for the management of infantile colic. J Pediatr Gastroenterol Nutr 63(1S):S22–SS4
Praveen V, Praveen S, Deshpande G, Patole SK(2014) Oral probiotics for infantile colic. (3):Art. No.: CD010986. DOI: https://doi.org/10.1002/14651858.CD010986
Di Mauro A, Neu J, Riezzo G, Raimondi F, Martinelli D, Francavilla R et al (2013) Gastrointestinal function development and microbiota. Ital J Pediatr 39(1):15
Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M (2008) Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics. 122(1):8–12
Baldassarre M, Di Mauro A, Mastromarino P, Fanelli M, Martinelli D, Urbano F et al (2016) Administration of a multi-strain probiotic product to women in the perinatal period differentially affects the breast milk cytokine profile and may have beneficial effects on neonatal gastrointestinal functional symptoms. A randomized clinical trial. Nutrients 8(11):677
Sohn K, Underwood MA (2017) Prenatal and postnatal administration of prebiotics and probiotics. Semin Fetal Neonatal Med 22(5):284–289
Chau K, Lau E, Greenberg S, Jacobson S, Yazdani-Brojeni P, Verma N et al (2015) Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. The Journal of pediatrics 166(1):74–78 e1
Szajewska H, Gyrczuk E, Horvath A (2013) Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr 162(2):257–262
Mi G-L, Zhao L, Qiao D-D, Kang W-Q, Tang M-Q, Xu J-K (2015) Effectiveness of lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie Van Leeuwenhoek 107(6):1547–1553
Xiao L, Ding G, Ding Y, Deng C, Ze X, Chen L et al (2017) Effect of probiotics on digestibility and immunity in infants: a study protocol for a randomized controlled trial. Medicine 96(14):e5953
Xu M, Wang J, Wang N, Sun F, Wang L, Liu X-H (2015) The efficacy and safety of the probiotic bacterium lactobacillus reuteri DSM 17938 for infantile colic: a meta-analysis of randomized controlled trials. PLoS One 10(10):e0141445
Sung V, D’Amico F, Cabana MD, Chau K, Koren G, Savino F et al (2018) Lactobacillus reuteri to treat infant colic: a meta-analysis. Pediatrics. 141(1):e20171811
Ilmonen J, Isolauri E, Poussa T, Laitinen K (2011) Impact of dietary counselling and probiotic intervention on maternal anthropometric measurements during and after pregnancy: a randomized placebo-controlled trial. Clin Nutr 30(2):156–164
Luoto R, Laitinen K, Nermes M, Isolauri E (2010) Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr 103(12):1792–1799
Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor M, Garaiova I, Plummer SF, Wang D, Morgan G (2010) Dietary supplementation with lactobacilli and bifidobacteria is well tolerated and not associated with adverse events during late pregnancy and early infancy. J Nutr 140(3):483–488
Hofmann H (2015) Probiotikum gegen Verdauungsbeschwerden in der Schwangerschaft und Säuglingskoliken. Gyn-Aktiv;
Sitarik AR, Bobbitt KR, Havstad SL, Fujimura KE, Levin AM, Zoratti EM et al (2017) Breast milk TGFβ is associated with neonatal gut microbial composition. J Pediatr Gastroenterol Nutr 65(3):e60
Mu Q, Tavella VJ, Luo XM (2018) Role of Lactobacillus reuteri in human health and diseases. Front Microbiol 9:757
Grześkowiak Ł, Grönlund M-M, Beckmann C, Salminen S, von Berg A, Isolauri E (2012) The impact of perinatal probiotic intervention on gut microbiota: double-blind placebo-controlled trials in Finland and Germany. Anaerobe. 18(1):7–13
Sirilun S, Takahashi H, Boonyaritichaikij S, Chaiyasut C, Lertruangpanya P, Koga Y et al (2015) Impact of maternal bifidobacteria and the mode of delivery on Bifidobacterium microbiota in infants. Benefic Microbes 6(6):767–774
Lundgren SN, Madan JC, Emond JA, Morrison HG, Christensen BC, Karagas MR, Hoen AG (2018) Maternal diet during pregnancy is related with the infant stool microbiome in a delivery mode-dependent manner. Microbiome. 6(1):109
Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S (2016) Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Sci Rep 6:23129
Baldassarre M, Palladino V, Amoruso A, Pindinelli S, Mastromarino P, Fanelli M et al (2018) Rationale of probiotic supplementation during pregnancy and neonatal period. Nutrients. 10(11):1693
Acknowledgments
This survey was conducted as a thesis with the support of the Child Growth and Development Research Center, Research Institute for Primordial Prevention of Noncommunicable Disease and Beheshti hospital obstetric ward, Isfahan University of Medical Sciences. The authors appreciate the Gostaresh Milad Poya Co. for their excellent cooperation in the preparation of probiotic and placebo drops, also we thank for the best contribution of pregnant women and their family.
Author information
Authors and Affiliations
Contributions
Mohammad Ali Pourmirzaiee: principal investigator, supervisor, contributed to the conception of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript, and agreed to all aspects of the work.
Fatemeh Famouri: principal investigator, supervisor, corresponding author, contributed to the conception of the work, conducting the study, drafting and revising the draft, approval of the final version of the manuscript, and agreed to all aspects of the work.
Wida Moazeni: resident of pediatrics, contributed to the conception of the work, conducting the study, revising the draft, and agreed to all aspects of the work.
Akbar Hassanzadeh: statistics consultant, contributed to the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed to all aspects of the work.
Maryam Hajihashemi: contributed to the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed to all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This project was approved by the ethics committee of the Isfahan University of Medical Sciences (IR.MUI.MED.REC.1397.248).
Informed consent
Consent was obtained from all individual participants included in the study.
Additional information
Communicated by Peter de Winter
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pourmirzaiee, M.A., Famouri, F., Moazeni, W. et al. The efficacy of the prenatal administration of Lactobacillus reuteri LR92 DSM 26866 on the prevention of infantile colic: a randomized control trial. Eur J Pediatr 179, 1619–1626 (2020). https://doi.org/10.1007/s00431-020-03641-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03641-4