Abstract
P. aeruginosa bloodstream infection (BSI) is associated with high hospital mortality. Empirical combination therapy is commonly used, but its benefit remains debated. The purpose of this study was to describe in a paediatric population, demographical characteristics and outcome of children treated for P. aeruginosa BSI receiving either a combined or single antibacterial therapy. We performed a retrospective, single-centre, cohort study of hospitalized children with P. aeruginosa BSI from 2007 to 2015. A total of 118 bloodstream infections (BSI) were analysed (102 (86.4%) hospital-acquired, including 52 (44.1%) hospitalized in intensive care unit). In immunocompromised children, 52% of BSI episodes were recorded. Recent medical history revealed that 68% were hospitalized, 31% underwent surgery and 67% had a prior antibiotic therapy within the last 3 months. In-hospital mortality was similar for patients receiving single or combined anti-Pseudomonas therapy (p = 0.78). In multivariate analysis, independent risk factors for in-hospital mortality were neutropenia (OR = 6.23 [1.94–20.01], hospitalization in ICU (OR = 5.24 [2.04–13.49]) and urinary tract infection (OR = 4.40 [1.02–19.25]).
Conclusion: P. aeruginosa BSI mainly occurred in immunocompromised children. Most infections were hospital-acquired and associated with high mortality. Combination therapy did not improve survival.
What is Known: • P. aeruginosa bloodstream infection (BSI) is associated with high hospital mortality. Empirical combination therapy is commonly used but its benefit remains debated. | |
What is New: • This is the largest cohort of Pseudomonas aeruginosa bacteraemia in children ever published. P. aeruginosa Bloodstream mainly occurred in immunocompromised children. Most infections were hospital-acquired and associated with high mortality. Combination therapy did not improve survival. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas aeruginosa, a Gram-negative non-fermenting Bacillus, was first isolated from green pus by Gessard in 1882. Pseudomonas aeruginosa causes severe nosocomial infections, especially in immunocompromised patients, and is often resistant to antibiotics. Therefore Pseudomonas aeruginosa bloodstream infection (BSI) appears as a serious event [1, 2]. In addition, severity of Pseudomonas aeruginosa BSI increased when the source of infection is unknown. The clinical outcomes of severe infections are strongly associated to multiple factors such as underlying host disease or condition, severity of infection (septic shock), antibiotic appropriateness within the first 24 h, the presence of invasive devices and the development of septic metastasis [3]. Pseudomonas aeruginosa BSI is associated with high mortality rate compared to infection due to other Gram-negative organisms reaching 16.2% in Osmon’s study and results in substantial healthcare costs [4]. Indeed, in a recent study among haematological patients, Pseudomonas aeruginosa BSI was associated with an increased risk of death (OR = 3.7 [1.98–6.95]; p < 0.01) compared to that observed for other Gram-negative rods [5]. In a study conducted by Hilf et al., including 200 consecutive patients with Pseudomonas aeruginosa BSI, it has been shown that the prognosis was closely related to the primary site of infection, and mortality seems usually higher when associated to a pulmonary infection [6]. The most recent Surviving Sepsis guidelines [7] recommend empirical combination therapy, particularly for patients with known or suspected Pseudomonas infections, as a means to decrease the likelihood of administering inadequate empirical antimicrobial therapy. However, it has not been clearly established whether appropriate empirical antimicrobial therapy truly improves survival in cases of suspected Pseudomonas aeruginosa bacteraemia [8,9,10,11]. Prior studies on Pseudomonas aeruginosa BSI varied in how they defined appropriate antimicrobial therapy and did not specifically analyse the influence of administering combination antimicrobials agents on prognosis [12]. There have been few studies focusing on P. aeruginosa BSI in paediatric patients. Herein, we describe the clinical and epidemiological features of P. aeruginosa BSI in children and the influence of combined therapy on Pseudomonas aeruginosa BSI outcome.
Materials and methods
We performed a single-centre retrospective study at Necker-Enfants Malades University Hospital in Paris. Medical and bacteriological data were retrospectively collected from both paper and electronic records covering a 9-year period from 2007 to 2015. Data were extracted from children’s medical records and computerized hospital databases according to a predefined case report form. The following items were collected: demographical, microbiological data (prior hospitalization, antibiotic therapy or surgical procedures during the past 12 weeks, ward of hospitalization, current treatment, source of infection, time for blood culture’s positivity, outcome), and antibiotic therapy data (mono or combined therapy, treatment duration). All children, excluding neonates who underwent Pseudomonas aeruginosa BSI, were included.
Definition
Bloodstream infection
An episode of Pseudomonas aeruginosa BSI was defined as the presence of the microorganism in blood culture along with clinical evidence of infection. Mixed BSI was excluded. Any positive blood culture after completion of antibiotic therapy was considered as a new bacteraemic episode. In case of more than one episode of BSI during the study period, only the first event was included. The setting of infection was classified as hospital-acquired (HA) or community-acquired onset (CA). Among the latter, healthcare-acquired were defined according to Friedman’s definition [13]. Sepsis was defined according to bone criteria [14].
Prior antimicrobial therapy
Prior use of any antimicrobial was retained in case of any administration within the past 3 months before admission whatever its duration and dosage. Moreover, any surgical intervention or hospitalization within the last 3 months was recorded.
Neutropenia was defined as an absolute neutrophil count < 500/mm3 at the onset of the infection.
Sites of infections
Sites of infections were defined according to the CDC surveillance definitions [15]. BSI in children with an unknown source despite extensive work-up (CBEU, pulmonary imaging, catheter-drawn blood samples) were classified as undefined.
Categories of antimicrobial treatment
Monotherapy consisted of treatment with one of the following antipseudomonal antimicrobials: ticarcillin ± clavulanate, piperacilline ± tazobactam, ceftazidime, cefepime, imipenem, meropenem, aztreonam or ciprofloxacin. Combination therapy consisted of the administration of ticarcillin ± clavulanate, piperacilline ± tazobactam, ceftazidime, cefepime, imipenem, meropenem and aztreonam with either an aminoglycoside (gentamicin or amikacin) or ciprofloxacin [16]. Empirical antipseudomonal therapy was defined as treatment that included at least one antipseudomonal agent and that was started no later than 24 h after the index positive blood sample for culture had been drawn. Definitive antipseudomonal therapy was defined as treatment that included at least one antipseudomonal agent and that was continued or commenced on the day that the antibiogram results were reported to the clinicians.
Microbiological cure
In our institution, repeat blood culture is a standard practice for patients with bloodstream infections. Microbiological cure was defined as negative blood culture 48–72 h after start of antibiotic therapy and no relapse after treatment completion.
Statistical analysis
Qualitative variables were presented as numbers (frequencies) and were tested using chi-square tests or Fischer exact tests. Continuous variables were presented as median (interquartile range) and were compared using exact t test, Mann Whitney or Kruskal-Wallis tests when appropriate.
The risk factors associated with mortality were assessed with a logistic regression model, and the Wald test was used to test the significance of coefficient. All variables with p value < 0.3 in univariate analysis were included in a multivariate analysis. The final multivariate model was selected using a backward and forward stepwise selection procedure, based on Akaike Information Criterion (AIC). A difference was considered as significant at a level of 5% (alpha risk). All statistical analyses were performed with R software, version 3.2.2 [17].
Results
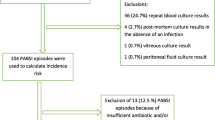
During the 9-year period, 118 children developed Pseudomonas aeruginosa BSI at Necker-Enfants Malades University Hospital, representing 3% of BSI. Patients’ characteristics are detailed in Table 1. Antimicrobial susceptibility changes of Pseudomonas aeruginosa from 2007 to 2015 are presented in Tables 2 and 3. Briefly, the median age of the population was 1.5 year [0.4–4.3], and 64 (54%) patients were boys. Almost half BSI episodes were recorded in immunocompromised children (62; 52%). Among them, 43 (36%), 19 (16%) and 5 (4%) had immunosuppressive treatment, neutropenia or malignancy, respectively. A patient could have multiple immunosuppression. Regarding the underlying conditions, 9 (8%) presented chronic lung disease, and 4 (3%) had urinary malformation. Most infections occurred in intensive care units (ICU) and medical departments (52, 44%), followed by surgical departments for 14 patients (12%). A total of 102 (86%) children had a hospital-acquired infection, and the median time between admission and infection was 15 days [5.25–52]. Among patients with a hospital-acquired infection, 47 (40%), 43 (36%) and 12 (10%) were hospitalized in ICU, medical and surgical unit, respectively. In the 16 (14%) community onset infections, 14 (12%) were considered healthcare-associated infections. Regarding prior medical history, 80 (68%) patients were hospitalized within the last 3 months, 37 (31%) underwent surgery, and 79 (67%) had a previous antibiotic therapy. Among 118 cases, 84 (71%) and 34 (29%) cases presented with sepsis and septic shock, respectively. Catheter-related infection was the most frequent source of infection (31%), followed by primary bloodstream infection (22%), respiratory tract infection (17%) and skin and soft tissue infections (15%).
When referring to treatment, we observed that antibiotic treatment against Pseudomonas aeruginosa was started within an average of 12 ± 20.64 h after the first positive blood culture.
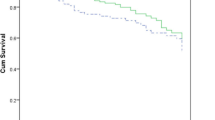
A total of 113 children (96%) were given appropriate empirical therapy, which included combination therapy in 93 (79%) and single therapy in 20 (17%). Among the 108 children (92%) who were treated with appropriate definitive targeted treatment according to antibiotic test susceptibility, 75 (69%) were given combination therapy, and 33 (31%) were given single therapy (data presented as supplemental data in Table S1). Among the 93 patients who received combined antibiotic therapy, the most frequent combination consisted in a β-lactam with an aminoglycoside (79, 85%) followed by a β-lactam with a fluoroquinolones (10, 11%). The mean duration of combination antibiotic therapy was 5.9 days (± 6.1 days). The overall clinical and microbiological cure rates were 69 (81/118) and 96% (113/118), respectively. Blood culture became negative within a median of 2 days (1–3 days) after appropriate treatment. Median duration of treatment was 14 days [10–15 days], and median duration of hospitalization was 39 days [18–139 days]. Mortality rate was 31.4% (37 patients) without differences between patients treated with single antibiotic therapy (75.6%) and those treated with combined antibiotic therapy (80.2%; p = 0.62). Furthermore, there was no statistical difference in hospital mortality between patients receiving appropriate initial empirical antimicrobial treatment with a single anti-Pseudomonas drug and patients treated with a combined antibiotic therapy in multivariate analysis. Similarly, for appropriate definitive antimicrobial treatment, there was no statistical difference in hospital mortality among patients receiving monotherapy or combined antibiotic therapy in multivariate analysis (Table 1).
We also attempted to identify risk factors for mortality, which are summarized in Table 4. In univariate analysis admission department, septic shock and neutropenia were associated with poor outcome. The following variables are as follows: admission department, septic shock, neutropenia and urinary tract infection as source of infection and age were integrated in multivariate model. In multivariate analysis after logistic regression, neutropenia (OR = 6.23 IC 95% [1.94–20.01]; p = 0.002) hospitalization in intensive care unit (OR = 5.24 IC 95% [2.04–13.49]; p = 0.006) and urinary tract infection as source of bacteraemia (OR = 4.40 IC 95% [1.02–19.25]; p = 0.04) were associated with higher mortality. Interestingly, severity of infection and healthcare-associated status were not statistically different among patients receiving combination antimicrobial therapy directed against Pseudomonas aeruginosa compared to those receiving monotherapy.
Discussion
This single-centre, retrospective study sought to determine clinical characteristics and influence of antibacterial strategy on mortality of children with Pseudomonas aeruginosa BSI. Literature describing risk factors for mortality in children with BLSI is scarce. As previously described, in adult population, Pseudomonas aeruginosa BSI in our study occurred mostly in immunocompromised children and were mostly healthcare-acquired and associated with a high mortality [18,19,20,21]. We observed that neutropenia, hospitalization in ICU and urinary tract infection were independent risk factors for in-hospital mortality. In addition, there was no difference in hospital mortality between appropriate single therapy and combination therapy.
Most of Pseudomonas aeruginosa BSI were severe late hospital-acquired infections. Only 16 patients had a community onset infection including 62% (10/16) that were healthcare-acquired. As previously reported in patients with neutropenia, more than 20% of Pseudomonas aeruginosa episodes were primary BSI. The latter is mainly due to pulmonary infections and bacteraemia during periods of neutropenia [22]. The overall mortality rate among our patients was 37%. This relatively high death rate was similar to those reported in the literature for Pseudomonas aeruginosa BSI in adult populations. Indeed Kang et al. [23] and Chamot et al. [24] each reported a comparable 30-day mortality rate of 39%. This high mortality might be related to the severity of the infections with septic shock (29%), intensive care unit hospitalization (44%) and the high rate of severely immunocompromised hosts (53%). Moreover 4 patients died from Pseudomonas aeruginosa bacteraemia, among 6 patients with mixed immunosuppression (cellular and humoral).
In the present study, independent risk factors for poor outcome were neutropenia and initial clinical severity. Despite the severity of the infection, combination therapy for the treatment is still discussed. Surprisingly, we did not find a beneficial effect of combination therapy on the outcome of our patients. However, host factors and severe ill situations are confounding factors that may influence physician’s choice for combination therapy or single agent therapy. This phenomenon could partially explain the absence of positive effect of combination therapy in our study. Our data suggest that combination therapy tended to be administered to patients with more severe clinical presentation. In addition, antibiotic susceptibility data in our centre show a low proportion of strains resistant to conventional antibiotics. This may lead to discuss the value of combination therapy in centres with higher levels of antibiotic resistance. Combination therapy could increase the proportion of initial antibiotic therapy adequacy, and a switch to monotherapy could be justified after receipt of antibiotic susceptibility tests. From previous studies, combination antibiotics as empirical therapy had been associated with higher appropriateness [25]; however, in case of definitive therapy, it seems that combination therapy does not add any benefit [10, 24, 26,27,28].
Furthermore, combination therapies have been put forward to decrease the rate of resistance evolution, but few studies have investigated it, and experimental data on the beneficial effect of combination therapy are conflicting [29]. A recent study showed that combination therapy with ceftazidime and ciprofloxacin selected for mutants displayed broad-spectrum resistance by mutational inactivation of the repressor gene mexR that regulates the multidrug efflux operon mexAB–oprM [30]. This is a key point of our study: With the emergence of multidrug-resistant Pseudomonas aeruginosa, it is a challenge to all physicians in choosing the best strategy for their children. As suggested by Martinez [31], combination therapy reduces the risk of inadequate initial antibiotic therapy.
In conclusion, in this single-centre study, treatment with combined antibiotic therapy did not reduce overall mortality compared with single-drug antibiotic therapy in children, even those with severe initial clinical presentation and/or underlying disease(s). The results are in agreement with previous studies in adults and suggest that combination therapy as empiric treatment increased the chance of therapeutic adequation but is not a decisive factor in the prognosis or BSI-PA after susceptibility test results [32]. This information is important in a period of increase of global antibiotic resistance among Pseudomonas aeruginosa isolates.
Abbreviations
- BSI:
-
Bloodstream infections
- CA:
-
Community-acquired
- CBEU:
-
Cytobacteriological examination of the urine
- HA:
-
Hospital-acquired
- ICU:
-
Intensive care unit
- OR:
-
Odd ratio
References
Solís Y, Álvarez AM, Fuentes D, de la Barra D, Avilés CL, Becker A, Salgado C, Silva P, Topelberg S, Tordecilla J, Varas M, Villarroel M, Viviani T, Zubieta M, Aedo S, Santolaya ME (2012) Bloodstream infections in children with cancer and high risk fever and neutropenia episodes in six hospitals of Santiago, Chile between 2004 and 2009. Rev Chil Infectologia Organo Of Soc Chil Infectologia 29:156–162. https://doi.org/10.4067/S0716-10182012000200006
Zhang Q, Smith JC, Zhu Q, Guo Z, MacDonald NE (2012) A five-year review of Pseudomonas aeruginosa bacteremia in children hospitalized at a single center in southern China. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 16:e628–e632. https://doi.org/10.1016/j.ijid.2012.03.014
Bisbe J, Gatell JM, Puig J, Mallolas J, Martinez JA, Jimenez de Anta MT et al (1988) Pseudomonas aeruginosa bacteremia: univariate and multivariate analyses of factors influencing the prognosis in 133 episodes. Rev Infect Dis 10:629–635
Osmon S, Ward S, Fraser VJ, Kollef MH (2004) Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest 125:607–616
Cattaneo C, Antoniazzi F, Casari S, Ravizzola G, Gelmi M, Pagani C, D'Adda M, Morello E, Re A, Borlenghi E, Manca N, Rossi G (2012) P. aeruginosa bloodstream infections among hematological patients: an old or new question? Ann Hematol 91:1299–1304. https://doi.org/10.1007/s00277-012-1424-3
Hilf M, Yu VL, Sharp J, Zuravleff JJ, Korvick JA, Muder RR (1989) Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med 87:540–546
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R et al (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377. https://doi.org/10.1007/s00134-017-4683-6
Bodey GP, Jadeja L, Elting L (1985) Pseudomonas bacteremia. Retrospective analysis of 410 episodes. Arch Intern Med 145:1621–1629
Vidal F, Mensa J, Almela M, Martínez JA, Marco F, Casals C, Gatell JM, Soriano E, Jimenez de Anta MT (1996) Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch Intern Med 156:2121–2126
Kuikka A, Valtonen VV (1998) Factors associated with improved outcome of Pseudomonas aeruginosa bacteremia in a Finnish university hospital. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 17:701–708
Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD (1998) The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 244:379–386
Vardakas KZ, Tansarli GS, Bliziotis IA, Falagas ME (2013) β-Lactam plus aminoglycoside or fluoroquinolone combination versus β-lactam monotherapy for Pseudomonas aeruginosa infections: a meta-analysis. Int J Antimicrob Agents 41:301–310. https://doi.org/10.1016/j.ijantimicag.2012.12.006
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP et al (2002) Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA et al (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 2009 136:e28
CDC. CDC/NHSN Surveillance definitions for specific types of infections 2016
EUCAST: Clinical breakpoints n.d. http://www.eucast.org/clinical_breakpoints/ (accessed August 18, 2015)
R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. n.d.
Tang P-C, Lee C-C, Li C-W, Li M-C, Ko W-C, Lee N-Y (2015) Time-to-positivity of blood culture: an independent prognostic factor of monomicrobial Pseudomonas aeruginosa bacteremia. J Microbiol Immunol Infect Wei Mian Yu Gan Ran Za Zhi. https://doi.org/10.1016/j.jmii.2015.08.014
Kwee F, Walker SAN, Elligsen M, Palmay L, Simor A, Daneman N (2015) Outcomes in documented Pseudomonas aeruginosa bacteremia treated with intermittent IV infusion of ceftazidime, meropenem, or piperacillin-tazobactam: a retrospective study. Can J Hosp Pharm 68:386–394
Sligl WI, Dragan T, Smith SW (2015) Nosocomial gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities, and outcomes. Int J Infect Dis IJID Off Publ Int Soc Infect Dis 37:129–134. https://doi.org/10.1016/j.ijid.2015.06.024
Dantas RC, Ferreira ML, Gontijo-Filho PP, Ribas RM (2014) Pseudomonas aeruginosa bacteraemia: independent risk factors for mortality and impact of resistance on outcome. J Med Microbiol 63:1679–1687. https://doi.org/10.1099/jmm.0.073262-0
Blot S, Vandewoude K, Hoste E, Colardyn F (2003) Reappraisal of attributable mortality in critically ill patients with nosocomial bacteraemia involving Pseudomonas aeruginosa. J Hosp Infect 53:18–24
Kang C-I, Kim S-H, Kim H-B, Park S-W, Choe Y-J, Oh M-D et al (2003) Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin Infect Dis Off Publ Infect Dis Soc Am 37:745–751. https://doi.org/10.1086/377200
Chamot E, Boffi El Amari E, Rohner P, Van Delden C (2003) Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 47:2756–2764
Morata L, Cobos-Trigueros N, Martínez JA, Soriano A, Almela M, Marco F, Sterzik H, Núñez R, Hernández C, Mensa J (2012) Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 56:4833–4837. https://doi.org/10.1128/AAC.00750-12
Bowers DR, Liew Y-X, Lye DC, Kwa AL, Hsu L-Y, Tam VH (2013) Outcomes of appropriate empiric combination versus monotherapy for Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother 57:1270–1274. https://doi.org/10.1128/AAC.02235-12
Leibovici L, Paul M, Poznanski O, Drucker M, Samra Z, Konigsberger H et al (1997) Monotherapy versus beta-lactam-aminoglycoside combination treatment for gram-negative bacteremia: a prospective, observational study. Antimicrob Agents Chemother 41:1127–1133
Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH (2005) Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49:1306–1311. https://doi.org/10.1128/AAC.49.4.1306-1311.2005
Tamma PD, Cosgrove SE, Maragakis LL (2012) Combination therapy for treatment of infections with gram-negative bacteria. Clin Microbiol Rev 25:450–470. https://doi.org/10.1128/CMR.05041-11
Vestergaard M, Paulander W, Marvig RL, Clasen J, Jochumsen N, Molin S et al (2015) Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2015.09.014
Kim YJ, Jun YH, Kim YR, Park KG, Park YJ, Kang JY, Kim SI (2014) Risk factors for mortality in patients with Pseudomonas aeruginosa bacteremia; retrospective study of impact of combination antimicrobial therapy. BMC Infect Dis 14:161. https://doi.org/10.1186/1471-2334-14-161
Peña C, Suarez C, Ocampo-Sosa A, Murillas J, Almirante B, Pomar V et al (2013) Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post hoc analysis of a prospective cohort. Clin Infect Dis Off Publ Infect Dis Soc Am 57:208–216. https://doi.org/10.1093/cid/cit223
Author information
Authors and Affiliations
Contributions
BP: collect data, data analysis, writting manuscript. FAL: collect data, review manuscript. MLF: collect data, review manuscript. VS: data analysis, review manuscript. RG: review manuscript. HGR: microbiological analysis, review manuscript. MP: collect data, review manuscript. JT: review manuscript. EB: microbiological analysis, review manuscript. OL: project supervision, review manuscript. JRZ: project supervision, review manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Nicole Ritz
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Pilmis, B., Alby-Laurent, F., Fasola, M.L. et al. Pseudomonas aeruginosa bloodstream infections in children: a 9-year retrospective study. Eur J Pediatr 179, 1247–1254 (2020). https://doi.org/10.1007/s00431-020-03598-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03598-4