Abstract
In the context of a 3-month extended-spectrum beta-lactamase-producing Klebsiella pneumonia (ESBL-KP) outbreak in a neonatal care center (NCC), hygiene practices and hospital environment were investigated. ESBL-KP strains isolated from patients and environment were compared by molecular typing. The density of incidence of multi-drug-resistant bacteria (MDRB) was calculated from January 2014 to September 2016. The 3-month ESBL-KP outbreak involved 19 patients. Clinical strains from the 19 patients displayed the same molecular profile between them, and with a strain isolated from an incubator after cleaning. Furthermore, 52.4% of incubator mattresses were positive for diverse pathogens. Hygiene practices were acceptable except for external practitioners and parents. In addition to classical infection control (IC) measures, the replacement of mattresses and the improvement of incubators disinfection stopped the outbreak. The protocol of disinfection was revised and microbiological control was implemented. A significant decrease of MDRB incidence was concomitant (p value = 0.03219) but 3 months later, MDRB incidence increased again.
Conclusion: This investigation highlighted incubators and mattresses as critical materials associated to infectious risk in NCC. NCC and IC teams should implement efficient protocol for incubators disinfection and monitoring.
What is Known: • Environment in neonatal intensive care units is often suspected as reservoir for Enterobacteriaceae outbreaks but is scarcely investigated. • Incubators and mattresses offer wet and warm conditions suitable for pathogens multiplication, but microbiological survey is not performed routinely for assessing bacterial contamination. | |
What is New: • Incubators and mattresses serve as reservoir for pathogens and relay in outbreak. • An infection control protocol associating efficient disinfection and microbiology analysis is proposed. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A stay in neonatal care center (NCC) exposes patients to healthcare-associated infections (HAIs) [6, 33] and outbreaks mostly caused by multi-drug-resistant bacteria (MDRB). Premature neonates are particularly susceptible to MDRB colonization and infections because the implantation of gut microbiota is delayed and forms an immature mucosal barrier. Main reasons are birth by cesarean, feeding by formula or pasteurized breast milk, antibiotics, and nursing in incubator with various medical devices [3, 8, 24]. Multi-resistant Enterobacter cloacae [11] and Klebsiella pneumoniae (KP) [23] are frequently involved in NCC outbreaks.
As generally accepted in HAI context, poor compliance to infection control (IC) precautions is the main cause of outbreaks, but antibiotic therapy in neonates and mothers before birth is also a major source of colonization of very low birth-weight infants with ESBL-producing Enterobacteriaceae [15, 16].
Persistent reservoirs in environment or contaminated devices can also be involved, regardless of the species or the medical specialty [28, 35, 38]. While the source of outbreak is not retrieved in almost 40% of nosocomial outbreaks, the environment (including medical devices and care equipment) is identified as more involved in 22.5% of cases, in equivalent proportion than patient reservoir [38]. Very few studies have explored the environmental diffusion of ESBL-producing Enterobacteriaceae, but abiotic surfaces of hospital environment are susceptible to be contaminated in a rate close to 14%, with a part due to K. pneumoniae around 4%, as reported in the study of Dziri et al. in 2016 [17]. In NCC, when found, environmental sources of Enterobacteriaceae spreading were milk and feeding material [31], close patient environment [7], hygiene products [4, 30], medical devices [29, 34], and perfusion solutions [18, 32]. In 2005, an outbreak investigation in neonatal intensive care unit (NICU) identified incubators as the reservoir of vancomycin-resistant Enterococcus sp. involved in the outbreak [20]. The mattresses are described as at risk for patient contamination in adult units [14], especially when their covers are damaged and permeable [2, 25, 39]. Outbreaks linked with mattresses’ contamination by Gram-negative bacteria such as A. baumannii or P. aeruginosa are reported in the literature [2, 10], but to date, none identified mattresses as responsible for Enterobacteriaceae outbreak in NCC.
During the IC investigation of a 3-month outbreak involving ESBL-producing KP (ESBL-KP) in an NCC, incubators were suspected to be environmental reservoir or relay for ESBL-KP and other pathogenic bacteria. The impact of mattresses renewing and enhanced disinfection protocols of incubators on the outbreak dynamics and on the incidence of MDRB in the NCC was assessed.
Methods
Settings and patients
The NCC of Montpellier (France) hospital contained three sectors: neonatal resuscitation unit (NRU) (15 beds in 9 rooms); neonatal intensive care unit (NICU) (19 beds in 8 rooms), and a neonatal kangaroo unit (NKU) (9 single-family rooms and 2 nurseries of 3 beds). NRU receives low birth-weight preterm infants (< 1500 g), critically ill newborns with unstable state, and newborns having surgery. Once stabilized, patients are transferred to the NICU and then in the NKU, before going home. In NRU and NICU, patients are cared in incubators, and are transferred between wards in their incubators. Each unit of the NCC has dedicated healthcare workers during the day but there is only one team for the 3 units at night. There is only one medical team for the whole NCC.
Hygiene measures in the NCC
Medical and healthcare workers wear a dedicated white outfit when working within the NCC. When providing cares for patients in incubators, they wear a single-use apron, in order to protect their outfit from a direct contact with patients’ close environment. Parents also wear a single-use over-gown since their entrance in the NCC. They are trained to hand hygiene and the respect of standard precautions when they help for their children’s cares. These hygiene measures were assessed when auditing hygiene practices during the outbreak period and correctly re-adjusted if needed.
Incubators cleaning protocol
Incubators (Dragër, France) were cleaned once a day by wiping inner surfaces with a cleaning-disinfecting agent containing didecyldimethylammonium chloride (Anios ND 7.85 II®, Anios, France). Each patient changed incubator every week and the used incubator is decontaminated before being assigned to another patient. Weekly cleaning-disinfection of mattresses, and of inner and outer incubator surfaces, was performed using both N-(3-aminopropyl)-N-dodécylpropane-1,3-diamine and didecyldimethylammonium chloride (Surfanios®, Anios, France) and steam cleaning (SV 4000A, Sanivap, France) onto the mattress and both inner and outer incubator surfaces. Before outbreak, microbiological monitoring of cleaned incubators was not performed in routine.
HAI surveillance in NCC
HAIs caused by MDRB, including multi-drug-resistant Gram-negative bacilli, vancomycin-resistant Enterococcus (VRE), and methicillin-resistant Staphylococcus aureus (MRSA), are monitored by automatic alert through computerized patients’ data files and weekly multi-disciplinary reviews [2, 10]. Rectal screenings for ESBL-producing Enterobacteriaceae and other MDRB are performed weekly. When a MDRB outbreak alert occurs, retrospective analysis and prospective surveillance for new cases are performed. The cases are defined as patients positive for MDRB in a rectal sample and/or for a clinical sample realized for diagnosis.
Outbreak investigation
Hygiene practice audit
The observance of standard and contact precautions was assessed according to 16 items (Supplementary Table 1) that should reach > 90% of observance rates to comply with IC rules. Concerning the cleaning practices items (Supplementary Table 1), IC rules were complied for a rate of gloves and apron wearing > 80%, and for a rate > 90% for the other items. Specific formation was provided to healthcare workers and/or visitors when practices improvements were needed.
Environmental microbiology and molecular typing of bacterial strains
In the whole NCC, surfaces of patients’ rooms, nursing room, parents’ rest rooms, and common areas were sampled by sterile cotton swabbing (Coppan®, Italia). Sampling included wet and dry surfaces, reusable medical devices, and computers. Among wet surfaces, faucets’ surfaces, tap, and U-bends were sampled in patients’ rooms and care rooms of the NCC in order to investigate hydric reservoirs. Mattresses cover and foam were cut in small pieces for further detection of microorganisms contaminating both surfaces and deep layers. About 10 mattress chips were randomly selected from every mattress. Swabs and mattress chips were incubated 24 h at 37 °C in Tryptone Soy Broth (Oxoid®, France). Then, the broths were streaked onto Mac Conkey agar (BioMérieux®) (24 h at 37 °C) and onto Columbia agar with 5% sheep blood (BioMérieux®) (48 h at 37 °C) media. Every growing colony was identified by Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) (Bruker®, Germany).
Clinical and environmental strains of ESBL-KP were typed by Pulsed Field Gel Electrophoresis (PFGE) after XbaI macrorestriction [21].
Survey of MDRB incidence
A daily computer-assisted surveillance listed all cases of MDRB colonization or infection in the NCC between January 2014 and September 2016. The density of incidence reported monthly new cases per days of hospitalization in the NCC. The mean incidence rates between the different periods of the survey were calculated and compared by Student t test using R64 software. A p value < 0.05 was considered as significant. In the meantime, monthly antibiotic consumption was collected between January 2014 and September 2016 in order to assess its impact onto the density of incidence of MDRB.
Results
Outbreak description
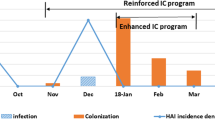
On 27 November 2015, an outbreak alert was emitted because four patients displayed digestive colonization with ESBL-KP. Retrospective analysis showed two cases of colonization and one case of infection prior to the alert (Fig. 1a). Ninety patients were affected among 235 patients hospitalized during the 3-month outbreak period (incidence rate of 8%) (Fig. 1a). For every patient, the onset of digestive colonization was late from 10 to 80 days. For 2 patients, the colonization was followed by infections: sepsis for patient 6 and conjunctivitis for patient 2 (Table 1). NRU, NICU, and NKU were the acquisition units for 4, 6, and 8 patients, respectively (Table 1). The outbreak ended on 13 January 2016.
The 21 ESBL-KP clinical strains isolated from 19 patients shared a same PFGE profile A (Table 1, Fig. 1b), indicating cross-transmission among patients or exposition to a same source. Two additional cases of ESBL-KP colonizing twin patients 20 and 21 occurred later in January 2016. The strains displayed the PFGE profile B and therefore were considered as unrelated to the main outbreak (Table 1).
IC audit and management
From 16 to 23 December 2016, (Fig. 1), 290 observations of hygiene practices highlighted global observance rate of 91.2%, 92%, and 92.4% for standard precautions, contact precautions, and environment cleaning practices, respectively (Supplementary Table 1). Major failures concerned the wearing of external practitioners (radiologist, surgeon, and physiotherapist), the cleaning of shared devices and the parents’ hand hygiene. Moreover, contact precautions were hardly applied in twin rooms. The water point-of-use cleaning protocol includes daily tap flushing and cleaning of tap, sink, and U-bends (Supplementary Table 1). Inappropriate practices were immediately corrected by reminding the good practices to be adopted.
Environmental microbiology investigation and corrective measures
An environmental source for ESBL-KP clone was searched by sampling surfaces (first sampling campaign, Fig. 1) onto 39 sites in the bedroom n°3 in NRU (where the 4 “alert cases” were hospitalized) and 55 sites corresponding to shared objects and care-giving equipment in the whole NCC. Sixteen surfaces out of the 94 were positive for bacteria, including 5 for Enterobacteria. ESBL-KP was retrieved from a swab sampled onto a cleaned incubator (n = 1), and E. cloacae from a swab sampled onto another incubator (n = 1) just after the cleaning process. E. cloacae was also isolated on a breast pump (n = 1) and on a mobile radiograph device (n = 1), and Escherichia vulneris on an electrocardiograph (n = 1). The strain of ESBL-KP isolated from incubator displayed the same PFGE profiles than clinical strains (Fig. 1b), demonstrating the persistence of the epidemic strain in one incubator despite the cleaning.
These results led to a thorough cleaning of all NCC surfaces by a three steps procedure associating detergency, rinsing, and disinfection. An improved cleaning protocol of incubators and mattresses was also implemented on December 24 by adding a cleaning-disinfecting step using Surfanios® after steam cleaning (Fig. 1).
Then, a systematic microbiological control (second sampling campaign, Fig. 1) of incubators and mattresses was performed by swabbing to evaluate new practices and residual contamination before their reuse. During 1 month, 48 incubators including 26 mattresses were sampled (Supplementary Table 2). Most of the mattress covers appeared cracked and remained wet long time after steam cleaning. Microbial analysis showed human skin–associated bacteria (57.1% for incubator and 34.6% for mattresses) and environmental bacteria (39.3% for incubator and 53.8% for mattresses) (Supplementary Table 2). Four bacteria pathogenic to neonates (Enterococcus faecalis, E. cloacae, Bacillus cereus, Staphylococcus capitis) were retrieved on the outer surfaces of 4 incubators.
Finally, given visual aspect and microbiology data, all the mattresses were renewed on 19 January 2016. Since this date, steam cleaning was limited to Plexiglas surfaces of incubators excluding mattresses to avoid persistent moisture, and Surfanios ® was used before and after steam process to eliminate potential re-contamination from aerosolized bacteria. Mattresses are cleaned out of the steam-dedicated room by a 2-step whipping using Surfanios®. The traceability of the link between patients and incubators was initiated. The occurrence of new cases caused by the ESBL-KP outbreak strain (PFGE type A) ended just after the mattresses changing (Fig. 1).
Before discarding mattresses, the cover and foam were analyzed for 42 mattresses. The global rate of contamination reached 100%, confirming their potential role as bacterial reservoir. Twenty-two mattresses (52.4%) were positive for pathogenic bacteria, mainly methicillin-susceptible Staphylococcus aureus (MSSA) (23.8% of mattresses), S. capitis, and B. cereus (7.1% each). E. cloacae, E. coli, E. faecalis, and Pseudomonas aeruginosa were each isolated from 1 mattress (2.4% each) (Supplementary Table 2).
Evaluation of new practices for incubator and mattress managing
In the first month after mattress change, 56 incubator surfaces and 49 mattresses were tested after cleaning and before their reuse for a new patient (third sampling campaign, Fig. 1a, Supplementary Table 2). Non-pathogenic bacteria were retrieved from incubator surfaces of respectively 25% (human origin) and 11% (environmental origin) of the incubators. Out of the 49 mattresses, 2 were positive for MSSA and B. cereus, and 17 (34.7%) and 13 (26.5%) were positive for human and environmental non-pathogenic bacteria, respectively (Supplementary Table 2). Compared to the second sampling campaign, the rate of contamination decreased markedly from 79.2 to 35.6% for the external surfaces, but more slightly from 76.9 to 61.2% for the mattresses (Supplementary Table 2). The contamination remained significant during the first month, probably due to implementation of new practices without thorough supervision.
During the following 7-month period, formation and audit to new practices were implemented. Then, 61 incubators and their mattresses were sampled (outside and inside) (fourth sampling campaign, Supplementary Table 2). Escherichia coli was the sole pathogen isolated while skin-associated bacteria were frequent contaminants. Compared to the previous campaign of incubator sampling, the overall contamination decreased markedly from 35.6 to 4.9% for incubators and from 61.2 to 9.8% for mattresses (Supplementary Table 2). A positive sample for bacteria, pathogenic or not, conduced to redo the disinfection before incubator reuse. A routine microbiological surveillance was then implemented by random sampling of cleaned incubators and mattresses.
Elusive impact of IC measures on the whole MDRB density of incidence in the NCC
The density of incidence of MDRB and the mean rates per period in the NCC from January 2014 to September 2016 are represented on Fig. 2, also presenting the monthly consumption of antibiotic. Before the ESBL-KP clonal outbreak (January 2014 to September 2015), the density of incidence varied between 0.67 and 8.50/00 (mean value of 5.950/00), excepted just before the outbreak in July 2015 when it increased up to 140/00 and remained significantly higher during the outbreak than during the pre-outbreak period (11.360/00 in mean, p value = 0.0127, Fig. 3). In February, after the bundle of IC measures including mattresses replacement, the rates deeply decreased to 20/00, and remained significantly lower during 8-month in post-outbreak period than during the outbreak (p value = 0.03219) but reached a mean of 6.130/00, which was similar to the pre-outbreak period (Fig. 3). In the meantime, the antibiotic consumptions remained stable before, during, and after the outbreak period, suggesting that the evolution density of incidence of MDRB was not linked to a selective pressure exerted by antibiotics.
Discussion
The occurrence of an outbreak in a hospital unit leads to implement bundle of IC measures acting in synergy towards outbreak resolution. Consequently, outbreak investigation rarely identifies single cause and source of transmission. In our NCC, IC measures were basically well observed but some IC procedures cannot be fully improved in the outbreak period, such as the isolation of MDRB-carrying patients in single room. The parents hand hygiene, already described as involved in pediatric ward contamination by enteric virus [19], was perfectible in the present case. Besides, environment is increasingly described to amplify and relay epidemic cycle of Enterobacteria in hospital [9, 26]. Several studies demonstrate that Gram-negative bacteria can persist for several months on hospital surfaces [1, 12], and outbreaks’ reports identifying environment as relaying pathogens transmission are published in the literature. Considering herein the outbreak persistence despite rather good compliance to IC precautions and the late-onset of patient’s contamination, an environmental reservoir was likely, urging to investigate deeper the NCC environment. At least one incubator was involved in the ESBL-KP outbreak, as proved by molecular typing. Even if incubators or mattresses cannot be identified as sources for all cases, this result suggests that incubators can act as a relay in the outbreak describes herein. The presence of other NCC-associated pathogens (i.e., S. aureus [36], E. cloacae [11] and B. cereus [27], and S. capitis [5]) on cleaned incubators surfaces is a strong argument for considering incubators as reservoirs for pathogens in NCC. In the outbreak reported herein, the delay for an infant to get colonized is coherent with incubators as sources of transmission. Indeed, every patient was put weekly in a new incubator with a new mattress while his previous incubator was cleaned and given to another patient. If cleaning-disinfecting process was not fully efficient, neonates could be exposed to pathogens from other patients. Microbiological data showed sporadic contamination of incubators, which may explain the low outbreak dynamics observed herein. Finally, the replacement of incubators mattresses and the implementation of new procedures of disinfection immediately stopped the outbreak, supporting their critical role. Improved incubators and mattresses management proposed herein confirmed their efficiency to limit contamination. Concomitantly, we observed the significant decrease of MDRB colonization in the ward, directly after having replaced all mattresses. However, this was a short-term decrease suggesting that MDRB endemicity in the NCC was multi-factorial despite the critical role that incubators might play.
The warm and humid ambiance in incubators probably facilitates bacterial growth [13] in close contact with neonates. Therefore, efficient decontamination before accommodating a new patient is critical to minimize HAI risk [10]. Since 2009, steam cleaning procedures was implemented for disinfecting incubators according to the French recommendations, in order to limit exposure of neonates to residual chemical products [37]. Its efficiency to reduce HAIs risk without using chemical products is especially appreciated in NCC but is conditioned by the contact time on surfaces. Furthermore, aerosolized pathogens that can then settle on cleaned surfaces [22] require using steam systems in a dedicated well-ventilated room in order to limit air-mediated recontamination and environmental bioburden. In our experience, the use of steam on mattress’s waterproof cover increased the residual moisture in the foam, and probably the risk of bacterial persistence or growth. For these reasons, we now recommended to use steam cleaning only onto Plexiglas surfaces of incubators while mattresses are disinfected by biocide, Surfanios®.
Conclusion
In NCC outbreaks, environmental reservoir is frequently suspected but scarcely characterized, i.e., in about one-third of published cases for KP outbreaks. The HAI pathogens detected beside ESBL-KP urges to consider incubators and mattresses as critical medical materials, to exert constant vigilance on them, to monitor their microbial contamination, and to question their decontamination. Incubators and mattresses management and monitoring should be included in the bundle of measures implemented to control HAI in NCC.
Abbreviations
- ESBL-KP:
-
Beta-lactamase-producing Klebsiella pneumonia
- HAI:
-
Healthcare-associated infection
- IC:
-
Infection control
- KP:
-
Klebsiella pneumonia
- MALDI-TOF MS:
-
Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry
- MDRB:
-
Multi-drug-resistant bacteria
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MSSA:
-
Methicillin-susceptible Staphylococcus aureus
- NCC:
-
Neonatal care center
- NICU:
-
Neonatal intensive care unit
- NKU:
-
Neonatal kangaroo unit
- NRU:
-
Neonatal resuscitation unit
- PFGE:
-
Pulsed field gel electrophoresis
- VRE:
-
Vancomycin-resistant Enterococcus
References
APR W (2018) The role of the environment in the spread of healthcare associated infections. J Hosp Infect 100(3):363–364
Bradbury SL, Mack D, Crofts T, Ellison RT III (2014) Potential bloodborne pathogen exposure from occult mattress damage. Am J Infect Control 42(4):421–422
Brooks B, Firek BA, Miller CS, Sharon I, Thomas BC, Baker R, Morowitz MJ, Banfield JF (2014) Microbes in the neonatal intensive care unit resembl those found in the gut of premature infants. Microbiome 2:1
Buffet-Bataillon S, Rabier V, Bétrémieux P, Beuchée A, Bauer M, Pladys P, le Gall E, Cormier M, Jolivet-Gougeon A (2009) Outbreak of Serratia marcescens in a neonatal intensive care unit: contaminated unmedicated liquid soap and risk factors. J Hosp Infect 72(1):17–22
Butin M, Martins-Simões P, Picaud JC, Kearns A, Claris O, Vandenesch F, Laurent F, Rasigade JP (2015) Adaptation to vancomycin pressure of multiresistant Staphylococcus capitis NRCS-A involved in neonatal sepsis. J Antimicrob Chemother 70(11):3027–3031
Carey AJ, Saiman L, Polin RA (2008) Hospital-acquired infections in the NICU: epidemiology for the new millennium. Clin Perinatol 35(1):223–249
Casolari C, Pecorari M, Della Casa E, Cattani S, Venturelli C, Fabio G, Tagliazucchi S, Serpini GF, Migaldi M, Marchegiano P, Rumpianesi F, Ferrari F (2013) Serratia marcescens in a neonatal intensive care unit: two long-term multiclone outbreaks in a 10-year observational study. New Microbiol 36(4):373–383
Cilieborg MS, Boye M, Sangild PT (2012) Bacterial colonization and gut development in preterm neonates. Early Hum Dev 88(Supplement 1):S41–S49
Clarivet B, Grau D, Jumas-Bilak E, Jean-Pierre H, Pantel A, Parer S, Lotthé A (2016) Persisting transmission of carbapenemase producing Klebsiella pneumoniae due to an environmental reservoir in a university hospital, France, 2012 to 2014. Euro Surveill 21(17). https://doi.org/10.2807/1560-7917.ES.2016.21.17.30213
Creamer E, Humphreys H (2008) The contribution of beds to healthcare-associated infection: the importance of adequate decontamination. J Hosp Infect 69(1):8–23
Dalben M, Varkulja G, Basso M, Krebs VLJ, Gibelli MA, van der Heijden I, Rossi F, Duboc G, Levin AS, Costa SF (2008) Investigation of an outbreak of Enterobacter cloacae in a neonatal unit and review of the literature. J Hosp Infect 70(1):7–14
Dancer SJ (2014) Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev 27(4):665–690
de Goffau MC, Bergman KA, de Vries HJ et al (2011) Cold spots in neonatal incubators are hot spots for microbial contamination. Appl Environ Microbiol 77(24):8568–8572
de Oliveira AC, Viana REH, Damasceno QS (2012) Contamination of hospital mattresses by microorganisms of epidemiological relevance: an integrative review. J Nurs UFPE on line 7(1):236–245
Denkel LA, Schwab F, Kola A, Leistner R, Garten L, von Weizsacker K, Geffers C, Gastmeier P, Piening B (2014) The mother as most important risk factor for colonization of very low birth weight (VLBW) infants with extended-spectrum β-lactamase producing Enterobacteriaceae (ESBL-E). J Antimicrob Chemother 69(8):2230–2237
Dubois V, De Barbeyrac B, Rogues A-M et al (2010) CTX-M-producing Escherichia coli in a maternity ward: a likely community importation and evidence of mother-to-neonate transmission. J Antimicrob Chemother 65(7):1368–1371
Dziri D, Klibi N, Alonso CA, Ben Said L et al (2016) Characterization of extended-spectrum β-lactamase (ESBL)-producing Klebsiella, Enterobacter, and Citrobacter obtained in environmental samples of a Tunisian hospital. Diagn Microbiol Infect Dis 86(2):190–193
Fabbri G, Panico M, Dallolio L, Suzzi R, Ciccia M, Sandri F, Farruggia P (2013) Outbreak of ampicillin/piperacillin-resistant Klebsiella pneumoniae in a neonatal intensive care unit (NICU): investigation and control measures. Int J Environ Res Public Health 10(3):808–815
Gallimore CI, Taylor C, Gennery AR, Cant AJ, Galloway A, Xerry J, Adigwe J, Gray JJ (2008) Contamination of the hospital environment with gastroenteric viruses: comparison of two pediatric wards over a winter season. J Clin Microbiol 46(9):3112–3115
Golan Y, Doron S, Sullivan B, Snydman DR (2005) Transmission of vancomycin-resistant Enterococcus in a neonatal intensive care unit. Pediatr Infect Dis J 24(6):566–567
Gouby A, Neuwirth C, Bourg G, Bouziges N, Carles-Nurit MJ, Despaux E, Ramuz M (1994) Epidemiological study by pulsed-field gel electrophoresis of an outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a geriatric hospital. J Clin Microbiol 32(2):301–305
Griffith CJ, Dancer SJ (2009) Hospital cleaning: problems with steam cleaning and microfibre. J Hosp Infect 72(4):360–361
Gupta A (2002) Hospital-acquired infections in the neonatal intensive care unit-Klebsiella pneumoniae. Semin Perinatol 26(5):340–345
Hartz LE, Bradshaw W, Brandon DH (2015) Potential NICU environmental influences on the neonate’s microbiome: a systematic review. Adv Neonatal Care 15(5):324–335
Health C for D and R. Safety Communications - Damaged or Worn Covers for Medical Bed Mattresses Pose Risk of Contamination and Patient Infection: FDA Safety Communication. Available at: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm348016.htm. Accessed 12 Nov 2016
Hendrik TC, Voor in ‘t holt AF, Vos MC (2015) Clinical and molecular epidemiology of extended-spectrum beta-lactamase-producing Klebsiella spp.: a systematic review and meta-analyses. PLoS One 10(10):e0140754
Hosein IK, Hoffman PN, Ellam S, Asseez TM, Fakokunde A, Silles J, Devereux E, Kaur D, Bosanquet J (2013) Summertime Bacillus cereus colonization of hospital newborns traced to contaminated, laundered linen. J Hosp Infect 85(2):149–154
Lin R, Wu B, Xu X-F, Liu X-C, Ye H, Ye GY (2012) Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae infection in a neonatal intensive care unit. World J Pediatr 8(3):268–271
Macdonald TM, Langley JM, Mailman T et al (2011) Serratia marcescens outbreak in a neonatal intensive care unit related to the exit port of an oscillator. Pediatr Crit Care Med 12(6):e282–e286
Madani TA, Alsaedi S, James L, Eldeek BS, Jiman-Fatani AA, Alawi MM, Marwan D, Cudal M, Macapagal M, Bahlas R, Farouq M (2011) Serratia marcescens-contaminated baby shampoo causing an outbreak among newborns at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. J Hosp Infect 78(1):16–19
Nakamura K, Kaneko M, Abe Y, Yamamoto N, Mori H, Yoshida A, Ohashi K, Miura S, Yang TT, Momoi N, Kanemitsu K (2016) Outbreak of extended-spectrum β-lactamase producing Escherichia coli transmitted through breast milk sharing in a neonatal intensive care unit. J Hosp Infect 92(1):42–46
Narayan SA, Kool JL, Vakololoma M, Steer AC, Mejia A, Drake A, Jenney A, Turton JF, Kado J, Tikoduadua L (2009) Investigation and control of an outbreak of Enterobacter aerogenes bloodstream infection in a neonatal intensive care unit in Fiji. Infect Control Hosp Epidemiol 30(8):797–800
Pessoa-Silva CL, Meurer Moreira B, Câmara Almeida V, Flannery B, Almeida Lins MC, Mello Sampaio JL, Martins Teixeira L, Vaz Miranda LE, Riley LW, Gerberding JL (2003) Extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit: risk factors for infection and colonization. J Hosp Infect 53(3):198–206
Pestourie N, Garnier F, Barraud O, Bedu A, Ploy MC, Mounier M (2014) Outbreak of AmpC β-lactamase-hyper-producing Enterobacter cloacae in a neonatal intensive care unit in a French teaching hospital. Am J Infect Control 42(4):456–458
Rastogi V, Nirwan PS, Jain S, Kapil A (2010) Nosocomial outbreak of septicaemia in neonatal intensive care unit due to extended spectrum β-lactamase producing Klebsiella pneumoniae showing multiple mechanisms of drug resistance. Indian J Med Microbiol 28(4):380–384
Romano-Bertrand S, Filleron A, Mesnage R, Lotthé A, Didelot M, Burgel L, Bilak E, Cambonie G, Parer S (2014) Staphylococcus aureus in a neonatal care center: methicillin-susceptible strains should be a main concern. Antimicrob Resist Infect Control 3:21
Sexton JD, Tanner BD, Maxwell SL, Gerba CP (2011) Reduction in the microbial load on hightouch surfaces in hospital rooms by treatment with a portable saturated steam vapor disinfection system. Am J Infect Control 39(8):655–662
Vonberg RP, Weitzel-Kage D, Behnke M, Gastmeier P (2011) Worldwide outbreak database: the largest collection of nosocomial outbreaks. Infection 39(1):29–34
Yu M, Cross K, Petrich A, Fish J (2016) Crib mattress investigation: a quality improvement study to assess mattress cover permeability and bacterial growth in crib mattresses. Am J Infect Control 44(7):837–839
Funding
This work was supported by the University Hospital of Montpellier and the association ADEREMPHA, Sauzet, France.
Author information
Authors and Affiliations
Contributions
LC wrote the first draft of the manuscript and provided environmental sampling and bacteriological analyses; HB made the audit observations and analyzed their results; EJB helped for the interpretation of results from bacteriological analyses; MND contributed to the survey of cases in the NCC wards; AM performed molecular typing on strains; GDB provided antibiotic consumption reports; GC diagnosed cases and contributed to their survey in the NCC wards; SP managed the audit study and the application of preventive measures; SRB managed the environmental investigation, the application of preventive measures, and the writing of the manuscript. All authors have seen and approved the submission of this version of the manuscript and take full responsibility for the manuscript. No authors received any grant or honorarium or payment for producing this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Additional information
Communicated by Patrick Van Reempts
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 84 kb)
Rights and permissions
About this article
Cite this article
Cadot, L., Bruguière, H., Jumas-Bilak, E. et al. Extended spectrum beta-lactamase-producing Klebsiella pneumoniae outbreak reveals incubators as pathogen reservoir in neonatal care center. Eur J Pediatr 178, 505–513 (2019). https://doi.org/10.1007/s00431-019-03323-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-019-03323-w