Abstract
Bronchopulmonary dysplasia (BPD) is one of the most common chronic inflammatory lung disease of premature infants, with serious short- and long-term consequences. Early identification of premature infants at risk of BPD is critical to preventing the pathogenesis of disease. Thus, in the present study, we recruited 126 premature infants, collected peripheral blood samples at different time points during early life, and measured the concentration of Th1 (MCP-1, IP-10, and MIG) and Th2 (eotaxin-1, eotaxin-2, and MCP-4) chemokines in serum. We found serum eotaxin-2 levels were significantly higher in the BPD group than in the non-BPD group on day 1 [1662 pg/ml vs. 1221 pg/ml, P < 0.05], day 7 [1533 pg/ml vs. 1089 pg/ml, P < 0.05], and day 14 [1246 pg/ml vs. 704 pg/ml, P < 0.05] after birth, and serum MCP-4 levels were also significantly higher in the BPD group than in the non-BPD group on day 1 [186 pg/ml vs. 128 pg/ml, P < 0.05], day 7 [199 pg/ml vs. 101 pg/ml, P < 0.05], and day 14 [238 pg/ml vs. 106 pg/ml, P < 0.05] of life.
Conclusions: Increased levels of Th2 chemokines, eotaxin-2, and MCP-4, are associated with BPD in premature infants.
What is Known: • The pathogenesis of BPD is multifactorial and it is difficult to predict and prevent. • Previous studies have demonstrated that inflammation plays a major role in the pathogenesis of BPD. | |
What is New: • Increased Th2 chemokines, eotaxin-2 and MCP-4, were associated with BPD in premature infants. • Abnormal Th1/Th2 response in early life maybe associated with the subsequent development of BPD, which provide a new insight to understand the pathogenesis of the disease. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern perinatal medicine has substantially improved the survival of extremely premature infants; however, it has concomitantly facilitated a steadily rising incidence of chronic lung diseases, such as asthma and bronchopulmonary dysplasia (BPD) [8, 21]. BPD is the most common contributing factor for mortality and the long-term morbidity in extremely low birth weight (ELBW) infants [22]. Early identification of premature infants at risk of BPD is critical to preventing the pathogenesis of disease. However, despite extensive research, there are no effective biomarkers for prediction of preterm infants at risk during early life. Previous studies have demonstrated that inflammation plays a major role in the pathogenesis of BPD, which is commonly accompanied by a high degree of inflammatory cell infiltration and inflammatory chemokine expression in the lung [2, 11]. However, conventional antibiotic and anti-inflammatory treatments are ineffective, suggesting that the inflammatory response involved in BPD has distinct features.
T helper cells (Th1 and Th2), defined by their respective functions, are critical for the regulation of inflammatory responses and maintenance of pulmonary homeostasis [14]. Th1 cells are crucial for cell-mediated immune responses and are associated with host defense protection. Th2 cells stimulate humoral immunity, promote B cell differentiation, and contribute to the pathogenesis of allergic diseases, such as allergic asthma, eosinophilic pneumonia, and atopic dermatitis [5, 9, 15]. Previous studies have demonstrated that an immature immune system and polarization towards Th2-mediated immune responses are commonly present at birth [6]; Th1-mediated responses develop soon after birth under extra-maternal circumstances [25]. Thus, whether abnormal Th1/Th2 responses in early life are associated with the subsequent development of BPD remains unclear.
Chemokines, a superfamily of polypeptide mediators, are critical elements for the selective recruitment and activation of various leukocyte subsets in inflammatory processes [3]. It has been suggested that the expression of some chemokines may be preferentially associated with a Th1 or a Th2 immune response. For example, MCP-1, MIG, and IP-10 are chemotactic for Th1 lymphocytes and play an important role in Th1-mediated immune responses [7, 12]. In contrast, eotaxin-1, eotaxin-2, and MCP-4 are mainly implicated in Th2-mediated immune responses [13]. The measurement of both Th1 chemokines (MCP-1, IP-10, and MIG) and Th2 chemokines (eotaxin-1, eotaxin-2, and MCP-4) in serum might reflect Th1 or Th2 responses in lung. Thus, in the present study, we established a clinical cohort of premature infants and collected peripheral blood samples at different time points during the neonatal stage. We investigated dynamic changes in Th1 (MCP-1, IP-10, and MIG) and Th2 (eotaxin-1, eotaxin-2, and MCP-4) chemokines in the early life of premature infants and evaluated whether variations in Th1 or Th2 chemokines during early life are associated with subsequent development of BPD.
Materials and methods
Study design and population
This was a prospective cohort study conducted between 2011 and 2014 at the Neonatal Department of West China Second University Hospital. Approval was obtained from the Ethics Committee at West China Second University Hospital, Sichuan University. Informed consent was obtained from the parents/caregivers of the infants for their participation in the study. BPD was defined as oxygen dependency for at least 28 days postnatal days and graded at 36 weeks of postmenstrual age according to the fraction of inspired oxygen (FIO2) and the requirement of respiratory support at this time [10]. Infants with birth gestational age (GA) of < 32 weeks or birth weight of < 1500 g, who survived beyond the first 24 h after birth, were eligible for enrollment in the study. Infants who died before 28 days of life were considered not eligible for a diagnosis of BPD. In addition, infants for whom parental/caregiver informed consent was not available, and infants with congenital/chromosomal anomalies, were excluded from the study. Relevant clinical data were collected by trained research staff; these included birth weight (BW), gestational age (GA), sex, Apgar score, prenatal/postnatal steroid administration, respiratory distress syndrome (RDS), requirement for supplemental oxygen, ventilator days, and presence of sepsis.
Sample collection and chemokine analysis
Samples of peripheral blood (1 ml) were collected from enrolled infants at the following time points: 1 day (within 24 h of birth), 7 days, and 14 days. Serum was separated from blood samples and stored at − 80 °C until the time of analysis. The concentrations of Th1 chemokines (MCP-1, MIG, and IP-10) and Th2 chemokines (eotaxin-1, eotaxin-2, and MCP-4) were measured with Luminex Liquichip assay kit (mouse cytokine/chemokine panel; Merck Millipore, Burlington, MA, USA), following the manufacturer’s instructions.
Statistical analysis
All data were analyzed with SPSS/17.0 software (SPSS, Chicago, IL, USA). Distributions of continuous variables were assessed by histograms and box plots. Mean ± SD was used to present normally distributed data. Median and interquartile ranges (IQRs) were used to present data that were not normally distributed. Categorical variables were described by relative frequency (%). Comparisons between groups of continuous variables were assessed by Student’s t tests for normally distributed data, or by Mann-Whitney U tests for abnormally distributed data. Categorical variables were compared between groups by using chi-squared tests. Covariance analysis was performed to compare of the concentration of cytokines between BPD group and non-BPD group. Receiver operating characteristic (ROC) curves were used to evaluate the diagnostic performance of chemokines for predicting the development of BPD. P < 0.05 was considered statistically significant.
Results
Clinical characteristics of the study infants
Of 215 eligible infants, informed consent was obtained from parental/caregivers of 167; these 167 infants were then enrolled in the study and followed to 36 weeks’ postmenstrual age. Excluding infants who died before diagnosis of BPD, 126 premature infants were ultimately analyzed, including 22 who developed BPD (BPD group) and 104 who did not (non-BPD group). The characteristics of the study infants are displayed in Table 1. Gestational age and birth weight were significantly lower (P < 0.001 for both) in infants who developed BPD, compared with infants who did not. The incidences of premature rupture of membranes and postnatal steroids usage were both significantly higher in infants who developed BPD (P = 0.038 and P < 0.001, respectively). In addition, infants who developed BPD had longer duration of oxygen usage (P < 0.001), nasal-continuous positive airway pressure (n-CPAP; P < 0.001), and ventilatory support (P = 0.012). Our results are consistent with prior reports in the literature.
Dynamic changes in chemokines during early life
To explore how Th1/Th2 responses change in premature infants during early life, the levels of Th1 chemokines (MCP-1, MIG, and IP-10) and Th2 chemokines (eotaxin-1, eotaxin-2, and MCP-4) of premature infants in the non-BPD group were analyzed on days 1, 7, and 14, which are displayed in Table 2. Of the six chemokines detected from the peripheral blood samples of infants in the non-BPD group, the median eotaxin-2 levels on days 1, 7, and 14 were 1221, 1089, and 704 pg/ml, respectively; this represented a statistically significant decreasing trend (Fig. 1e). The median MCP-1, MIG, IP-10, eotaxin-1, and MCP-4 levels on days 1, 7, and 14 are shown in Fig. 1a–d, f, respectively. However, there were no dynamic changes (similar to those observed in eotaxin-2) among MCP-1, MIG, IP-10, eotaxin-1, or MCP-4 during early life.
Serum Th1/Th2 chemokines levels in non-BPD group at different time of early life. Serum concentrations of MCP-1 (a), MIG (b), IP-10 (c), eotaxin-1 (d), eotaxin-2 (e), and MCP-4 (f) were obtained from non-BPD group on days 1, 7, and 14 of life. Because data were not normally distributed and were presented as median chemokines concentrations (line) and 25th and 75th percentiles (shading). Error bars represent 10th and 90th percentiles (P < 0.05)
Increased serum eotaxin-2 and MCP-4 are associated with the development of BPD in premature infants
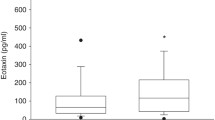
We investigated whether variations of Th1/Th2 chemokines during the early life of premature infants are associated with subsequent development of BPD. As shown in Fig. 2, serum eotaxin-2 levels were significantly higher in the BPD group than in the non-BPD group on day 1 [1662 pg/ml vs. 1221 pg/ml, P < 0.05], day 7 [1533 pg/ml vs. 1089 pg/ml, P < 0.05], and day 14 [1246 pg/ml vs. 704 pg/ml, P < 0.05] after birth (Fig. 2a). Another finding, shown in Fig. 2a, was that the eotaxin-2 levels of both BPD and non-BPD groups exhibited a statistically significant decreasing trend on days 1, 7, and 14. In addition, serum MCP-4 levels were also significantly higher in the BPD group than in the non-BPD group on day 1 [186 pg/ml vs. 128 pg/ml, P < 0.05], day 7 [199 pg/ml vs. 101 pg/ml, P < 0.05], and day 14 [238 pg/ml vs. 106 pg/ml, P < 0.05] of life (Fig. 2b). Thus, levels of both eotaxin-2 and MCP-4 on days 1, 7, and 14 were higher in the BPD group than in the non-BPD group.
Comparisons of eotaxin-2 and MCP-4 concentrations in non-BPD and BPD groups at postpartum days 1, 7, and 14. Serum values of eotaxin-2 (a) and MCP-4 (b) from non-BPD and BPD groups at postpartum days 1, 7, and 14 were measured. The data were not normally distributed. So, the box plots show the median chemokines concentrations (line) and 25th and 75th percentiles (shading). Error bars represent 10th and 90th percentiles (P < 0.05)
Prediction of BPD with eotaxin-2 and MCP-4 in premature infants
By using cutoff values selected by using receiver operating characteristic (ROC) curves, we compared the diagnostic performance of eotaxin-2 and MCP-4 for predicting the development of BPD. Serum eotaxin-2 levels ≥ 1458 pg/ml on the first day had an AUC of 0.855, a sensitivity of 76.2%, and a specificity of 83.7% for predicting BPD in these preterm infants (Fig. 3a). MCP-4 levels on day 14 (cutoff, 200 pg/ml) showed a 71.4% sensitivity, 88.7% specificity, and an AUC of 0.848(Fig. 3b).
Discussion
Identifying effective biomarkers for prediction of preterm infants at risk during early life will be of great benefit for better prevention and treatment of BPD. In the present study, we found that Th2-related chemokines, such as eotaxin-2 and MCP-4, dynamically changed during early life, increased serum eotaxin-2, and MCP-4 were associated with the subsequent development of BPD in premature infants.
A variety of studies have shown that pulmonary inflammation, caused by various insults, is the common final pathway and primary etiological factor in the pathogenesis of BPD [19, 20]. A variety of inflammatory mediators, including chemokines, adhesion molecules, and proinflammatory cytokines, have been associated with BPD and/or an increased risk for an adverse clinical course [17, 18]. For example, Ambalavanan et al. [1] studied the serum concentrations of 25 cytokines and reported that higher concentrations of certain cytokines (IL-1β, IL-6, IL-8, IL-10, and IFN-γ) and lower concentrations of other cytokines (IL-17, RANTES, and TNF-β) were associated with the development of BPD and/or death in ELBW infants. In the present study, we found that serum levels of eotaxin-2 and MCP-4 (Th2-related chemokines) were significantly higher on days 1, 7, and 14 in infants who developed BPD, compared with infants who did not. In addition, by using cutoff values selected with ROC curves, we found that the eotaxin-2 level on day 1 and the MCP-4 level on day 14 both show good predictive effects for BPD risk. These results suggest that increased levels of Th2-related chemokines, eotaxin-2, and MCP-4, are associated with the subsequent development of BPD in premature infants; moreover, these chemokines might help us to understand the pathophysiology of BPD in a different way.
Chemokines play a central role in regulating inflammation in pulmonary diseases by recruiting inflammatory cells to the lung [3]. Eotaxin-2 and MCP-4 are both Th2-related chemokines [13] that can recruit eosinophils [16, 23]. Brostrom et al. [4] investigated the role of eosinophils in the pathogenesis of BPD in premature infants and found that eosinophil activation was linked to BPD. Another study by Yang et al. [24] included 261 infants born at < 34 weeks gestation; the investigators found that eosinophils reached peak levels in the fourth postnatal week and were significantly higher in BPD patients beginning in the first postnatal week. In our study, we found that higher concentrations of eotaxin-2 and MCP-4, which can recruit greater numbers of eosinophils, were associated with the development of BPD. Indeed, eosinophils are known for their roles in asthma and are widely studied in its pathogenesis [23]. Previous studies have reported that BPD in the neonatal period may serve as a risk factor for asthma in childhood and adolescence [4, 24]. Therefore, eosinophils should be further studied to explore the relationship between asthma and BPD.
A major limitation of our study is that serum concentrations of chemokines could be influenced by a variety of conditions, such that they may not accurately reflect concentrations in the lung. BPD is primarily an alveolar-interstitial disease; thus, measurement of tracheal aspirate or bronchoalveolar lavage fluid, in addition to serum chemokine levels, might be more suitable to study the impact of chemokine-associated inflammation on BPD. Our study also was limited by a small sample size and short follow-up period. Additionally, the infants included in our study often had gestational ages of 28–32 weeks on account of the birth rate of gestational age less than 28 week is relatively low in our country and it is usually hard for them to survive. These differences may have an impact on the results. Therefore, more scientific, standardized analyses, with adequate sample sizes and long follow-up periods, are needed to facilitate identification of biomarkers for early diagnosis of BPD risk in premature infants. But, it is important to clarify that these marker studies can only show an association with BPD. To establish a causal relationship, studies in developmentally appropriate animal models and human lungs with BPD need to be conducted.
In conclusion, increased serum concentrations of Th2-related chemokines, eotaxin-2, and MCP-4 were associated with subsequent development of BPD in premature infants in this study, which enable us to better understand the pathophysiology of BPD development and to study it in a different way.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- ELBW:
-
extremely low birth weight
- IP:
-
interferon (IFN) gamma-induced protein
- MCP:
-
monocyte chemotactic protein
- MIG:
-
monokine induced by interferon-γ
- Th:
-
T helper type
References
Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, Thorsen P, Higgins RD (2009) Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics 123(4):1132–1141
Balany J, Bhandari V (2015) Understanding the impact of infection, inflammation, and their persistence in the pathogennesis of bronchopulmonary dysplasia. Front Med 2:90
Bonecchi R, Graham GJ (2016) Atypical chemokine receptors and their roles in the resolution of the inflammatory response. Front Immunol 7:224
Brostrom EB, Katz-Salamon M, Lundahl J, Hallden G, Winbladh B (2007) Eosinophil activation in preterm infants with lung disease. Acta Paediatr 96(1):23–28
Cosmi L, Maggi L, Santarlasci V, Liotta F, Annunziato F (2014) T helper cells plasticity in inflammation. Cytometry A 85(1):36–42
Frezza S, Gallini F, Palazzo R, Carollo M, De Carolis MP, D’Andrea V, Catenazzi P, Romagnoli C, Ausiello CM (2016) T-cell polarization: potential serological markers in preterm and term infants. Early Hum Dev 101:69–71
Hodge DL, Reynolds D, Cerban FM, Correa SG, Baez NS, Young HA, Rodriguez-Galan MC (2012) MCP-1/CCR2 interactions direct migration of peripheral B and T lymphocytes to the thymus during acute infectious/inflammatory processes. Eur J Immunol 42(10):2644–2654
Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE (2015) Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med 192(2):134–156
Jimenez-Alvarez L, Zuniga J, Gaxiola M, Checa M, Becerril C, Mendoza F, Pardo A, Selman M (2010) Inflammatory response and dynamics of lung T cell subsets in Th1, Th2 biased and Th2 deficient mice during the development of hypersensitivity pneumonitis. Exp Mol Pathol 88(3):407–415
Jobe AH, Bancalari E (2001) Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163(7):1723–1729
Koksal N, Kayik B, Cetinkaya M, Özkan H, Budak F, Kiliç Ş, Canitez Y, Oral B (2012) Value of serum and bronchoalveolar fluid lavage pro- and anti-inflammatory cytokine levels for predicting bronchopulmonary dysplasia in premature infants. Eur Cytokine Netw 23(2):29–35
Manicone AM, Burkhart KM, Lu B, Clark JG (2008) CXCR3 ligands contribute to Th1-induced inflammation but not to homing of Th1 cells into the lung. Exp Lung Res 34(7):391–407
Meyer-Hoffert U, Lezcano-Meza D, Bartels J, Montes-Vizuet AR, Schröder JM, Teran LM (2003) Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol 131(4):264–271
Odaka M, Matsukura S, Kuga H et al (2007) Differential regulation of chemokine expression by Th1 and Th2 cytokines and mechanisms of eotaxin_CCL-11 expression in human airway smooth muscle cells. Int Arch Allergy Immunol 143(1):84–88
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 74(1):5–17
Rojas-Ramos E, Avalos AF, Perez-Fernandez L, Cuevas-Schacht F, Valencia-Maqueda E, Teran LM (2003) Role of the chemokines RANTES, monocyte chemotactic proteins-3 and -4, and eotaxins-1 and -2 in childhood asthma. Eur Respir J 22(2):310–316
Ryan RM, Ahmed Q, Lakshminrusimha S (2008) Inflammatory mediators in the immunobiology of bronchopulmonary dysplasia. Clin Rev Allergy Immunol 34(2):174–190
Schneibel KR, Fitzpatrick AM, Ping XD, Brown LA, Gauthier TW (2013) Inflammatory mediator patterns in tracheal aspirate and their association with bronchopulmonary dysplasia in very low birth weight neonates. J Perinatol 33(5):383–387
Shahzad T, Radajewski S, Chao CM, Bellusci S, Ehrhardt H (2016) Pathogenesis of bronchopulmonary dysplasia: when inflammation meets organ development. Mol Cell Pediatr 3(1):23
Speer CP (2009) Chorioamnionitis, postnatal factors and proinflammatory response in the pathogenetic sequence of bronchopulmonary dysplasia. Neonatology 95(4):353–361
Stocks J, Hislop A, Sonnappa S (2013) Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med 1(9):728–742
Stoll BJ, Hansen NI, Bell EF et al (2010) Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126(3):443–456
White JR, Lee JM, Dede K et al (2000) Identification of potent, selective non-peptide CC chemokine receptor-3 antagonist that inhibits eotaxin-1, eotaxin-2, and monocyte chemotactic protein-4-induced eosinophil migration. J Biol Chem 275(47):36626–36631
Yang JY, Cha J, Shim SY, Cho SJ, Park EA (2014) The relationship between eosinophilia and bronchopulmonary dysplasia in premature infants at less than 34 weeks’ gestation. Korean J Pediatr 57(4):171–177
Zhang B, Ohtsuka Y, Fujii T, Baba H, Okada K, Shoji H, Nagata S, Shimizu T, Yamashiro Y (2005) Immunological development of preterm infants in early infancy. Clin Exp Immunol 140(1):92–96
Acknowledgments
The authors thank the study participants and their parents. In addition, the authors gratefully acknowledge the support from the National Key R&D Program of China (No. 2017YFC0211705) and the National Natural Science Foundation of China (No. 81601326).
Author information
Authors and Affiliations
Contributions
Participated in research design: Hanmin Liu, Huajing Wan, and Dan Zhou.
Conducted experiments: Dan Zhou and Fang Shi.
Performed data analysis: Fang Shi, Ying Xiong, and Min Zhou.
Wrote or contributed to the writing of the manuscript: Dan Zhou, Fang Shi, and Hanmin Liu.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants’ parents included in the study.
Additional information
Communicated by Peter de Winter
Rights and permissions
About this article
Cite this article
Zhou, D., Shi, F., Xiong, Y. et al. Increased serum Th2 chemokine levels are associated with bronchopulmonary dysplasia in premature infants. Eur J Pediatr 178, 81–87 (2019). https://doi.org/10.1007/s00431-018-3266-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-018-3266-z