Abstract
Testicular adrenal rest tumors (TARTs) are common cause of infertility in males with congenital adrenal hyperplasia (CAH). We studied the role of genotype and disease regulation on TART development, their impact on gonadal function, and frequency in 47 21-hydroxylase deficiency (21-OHD) and four 11-hydroxylase deficiency (11-OHD) male patients. Testicular ultrasound (TU), genotype, hormonal measurement in 51, and spermiogram in five patients were performed. TARTs were detected in 14 SW21-OHD and one 11-OHD patient: 1/8 patients aged <7 years (1.8 years old is the youngest), 1/8 patients aged <12 years, 5/17 patients aged <18 years, and in 8/18 adults. All 21-OHD TART patients had exclusively severe mutations of CYP21A2 gene. Poor hormonal control in 8/15 patients with and 12/36 patients without TART indicates correlation of tumor development with poor disease control. None of the TART patients fathered a child. Low inhibin-B was found in 7/15 TART patients. Azoospermia was found in four and oligoasthenozoospermia in one patient.
Conclusion: TART was detected exclusively in patients with severe CYP21A2 mutations. Disease regulation plays a role in development of TART that impairs testicular function and increases the risk of infertility. Screening for TART by TU is indicated from early childhood.
What is Known: • Due to improved diagnostic and therapeutic possibilities, majority of the male patients with congenital adrenal hyperplasia nowadays reach adulthood and screening for long-term complications is becoming more important. • Testicular adrenal rest tumors (TARTs) are common cause of infertility and impaired gonadal function in males with CAH. |
What is New: • A 1.8-year-old boy described in this paper is the youngest reported patient with TART. • Screening for TART by testicular ultrasound from early childhood, especially in patients with severe CYP21A mutations, is recommended. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital adrenal hyperplasia (CAH) is a group of autosomal recessive disorders affecting steroid synthesis in the adrenal cortex. In about 95% of the patients, CAH is caused by defect of CYP21A2 gene resulting in 21-hydroxylase deficiency (21-OHD). Classic 21-OHD CAH results in impaired production of cortisol, and in about 75% of patients also of aldosterone. Loss of cortisol negative feedback on hypothalamus and pituitary results in increased pituitary adrenocorticotropic hormone (ACTH) secretion, stimulating hyperplasia of the adrenal glands and hyperproduction of adrenal androgens. The insufficient synthesis of cortisol and aldosterone can lead to life-threatening adrenal crisis with salt wasting occurring already in the neonatal period. Treatment consists of substitution of cortisol and aldosterone, thereby also suppressing ACTH synthesis and hyperproduction of adrenal androgens [29].
Due to improved diagnostic and therapeutic possibilities, majority of the CAH patients nowadays reach adulthood and long-term complications are becoming more important. One of them is the development of benign testicular adrenal rest tumor (TART). Its typical location near rete testes is associated with the risk of the seminal duct obstruction, leading to azoospermia, permanent damage of the surrounding testicular tissue, and infertility in adult male patients. The etiology of TART is still unexplained. One of assumptions is that TARTs are derived from ectopic adrenal cells that migrated to the gonads in fetal life, and their growth is under control of ACTH and angiotensin II [8, 19]. Other assumption is that tumor may originate from pluripotent fetal Leydig cells (FLCs) that have ACTH and LH receptors [28].
The reported prevalence of TART in CAH varies considerably, which can largely be attributed to patient selection (age, hormonal control, severity of CAH) and also to the method of tumor detection [3, 6, 14, 18, 22, 23, 25, 27]. There is ample evidence that with use of testicular ultrasound, these small, nonpalpable lesions (usually <2 cm in size) could be detected already in childhood [2, 4, 10, 20, 21, 32].
The objective of this cross-sectional study was to evaluate the role of genotype and disease regulation on tumor development and their influence on gonadal function, as well as estimate the frequency of TART depending on age, in a group of 51 male patients with CAH aged 1.8–40 years.
Subjects and methods
A total of 51 children, adolescent, and adult male CAH patients who have been regularly followed in our outpatient endocrine department were included in this study. Patients’ age ranged from 0 to 6 years and 11 months in 8 patients, 7 to 11 years and 11 months in 8 patients, 12 to 17 years and 11 months in 17 patients, and 18 to 42 years in 18 patients. The patients with 21-OHD were categorized as salt wasting (SW), simple virilizing (SV), and nonclassical (NC). Twenty five had SW, 14 SV, and 8 NC form of disease as defined by medical history and laboratory assessment. In all of the patients, diagnosis was verified by genotyping of the CYP21A2 gene. Four patients had CAH due to 11-hydroxylase deficiency (11-OHD), verified by genotyping of CYP11B1 gene.
All 25 SW children were diagnosed in the first 2 weeks of life, and were treated from the neonatal period with hydrocortisone three times a day (15–20 mg/m2 in first 1–2 months of life and 12–15 mg/m2/day after 2 months of age) and fludrocortisone.
Twelve out of 14 children with SV CAH were diagnosed during childhood, between ages 2 and 5years, after developing symptoms of pseudoprecocious puberty and advanced growth and bone age, while two were diagnosed through family studies. All were treated with hydrocortisone three times a day (10–12 mg/m2/day). Four of eight patients with NC 21-OHD were diagnosed between 6 and 10 years of age, after development of pubic hair, and four were diagnosed through family studies. Four of them have been treated with hydrocortisone three times a day (6–8 mg/m2/day) (nos. 9, 17, 24, and 42). Four patients with 11-OHD were diagnosed between 2 and 4 years of age due to pseudoprecocious puberty and advanced growth and bone age, and all of them have been on therapy with hydrocortisone three times a day (10–12 mg/m2/day).
Furthermore, patient nos. 10, 12, and 14 were on therapy with gonadotropin-releasing hormone analog (GnRHa) due to central precocious puberty, at the time of examination (Table 1).
Blood sampling and testicular ultrasound were performed on the same day. Blood was drawn after an 8–10h overnight fast (2 h after morning medication). Sera were separated and tests were performed immediately or frozen at −20 °C until assayed. Measurement methods were standard recommended, performed on automated or semi-automated platforms by using commercial kits: luteinizing hormone (LH), follicle-stimulating hormone (FSH), and testosterone (T) by Abbott Laboratories (USA); 17-hydroxyprogesterone (17-OHP) and androstenedione (A) by DRG (Germany); inhibin-B by Beckman Coulter Inc. (Ireland); and plasma renin activity (PRA) by DiaSorin (USA).
Adequate hormonal control of CAH was defined by serum androgen levels and PRA being within the normal range, and 17-OHP concentration in serum of 6–30 nmol/L. Possible overtreatment was defined as suppressed androgen levels and normalized to decreased 17-OHP concentrations in serum, and undertreatment if serum androgens and PRA levels were over normal range and 17-OHP was over 30 mmol/L.
Molecular genetic analysis of CYP21A2 gene initially included allele-specific PCR for the seven most common mutations (p.P31L, I2G, Ex3∆8bp, p.I173N, Ex6 cluster (p.I236N, p.V237E, p.M239K), p.V282L, p.Q319X, and p.R357W) and was performed as described previously [34]. If mutations were not found, other widely used diagnostic techniques such as Southern blot, restriction fragment length polymorphism (RFLP), or multiplex ligation-dependent probe amplification (MLPA) were used to detect deletions and large gene conversions. Moreover, Sanger sequencing was performed to detect rare mutations [15, 30]. In four patients with 11-OHD, PCR and sequencing of CYP11B1 gene were performed as previously described [12].
The genotypes were categorized according to predicted severity of mutations: group null (complete enzyme impairment), group A (almost complete impairment), group B (severe impairment), and group C (partial impairment) [33].
Testicular ultrasound was performed by a single experienced radiologist in all 51 patients using GE Healthcare LOGIO S8 and E9 ultrasound systems Aloka scanner equipped with 15-MHz linear transducer probe. Testicular and TART volumes were calculated using the following formula: V (mL) = length (cm) × width (cm) × (depth (cm) × 0.52 [20].
According to Claashen-van der Grinten et al., TARTs are classified in five different stages [6].
-
Stage 1.
Presence of adrenal rest cells within the rete testis, not detectable by scrotal testicular ultrasound.
-
Stage 2.
The adrenal rest cells might be visible by testicular ultrasound.
-
Stage 3.
Further growth of adrenal rest cells has compressed the rete testis.
-
Stage 4.
Further hypertrophy and hyperplasia of the adrenal rest cells with progressive obstruction of the rete testis.
-
Stage 5.
Chronic obstruction and irreversible damage of testicular parenchyma.
Bone age (BA) was determined according to Greulich-Pyle in all children who did not reach final height. In order to determine BA advancement, the BA was expressed in months in correlation with chronological age (CA) (BA-CA).
Seminal fluid was collected after 3–5 days of self-reported ejaculatory abstinence in four adults, and analysis included assessment of semen volume, sperm concentration, total sperm count, and motile and immotile spermatozoa according to World Health Organization criteria by Cooper et al. [11].
Final adult height (FH) and target height (TH) values were compared with t test.
The study was approved by the Ethics Committee of University Hospital Zagreb, Croatia. Written informed consent for the study was obtained from all adult patients or patients’ parents or guardians.
Results
Radiological evaluation
Testicular adrenal rest tumor was detected in 15 of 51 (29%) investigated patients: in one of 8 (12.5%) patients aged 0 to 6 years and 11 months, one of 8 (12.5%) patients aged 7 to 11 years and 11 months, five of 17 (29.4%) patients aged 12 to 17 years and 11 months, and eight of 18 (44.4%) adult patients ranged 18 to 40 years (Table 1 and Fig. 1).
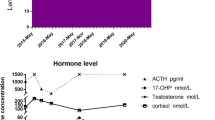
Longitudinal testicular ultrasound and color Doppler images of a 1.8-year-old boy with salt wasting form of congenital adrenal hyperplasia. Arrows indicate bilaterally slightly lobulated, well-defined hypoechoic masses with central hyperechoic part in the area of rete testes measuring 1.1 × 0.6 × 0.4 cm = 0.13 mL (right testis) (1a) and 1.0 × 0.6 × 0.4 cm = 0.12 mL (left testis) (1b). The masses identified as testicular adrenal rest tumors are hypervascular (right testis (2a) and left testis (2b))
In patient no. 32, TART was detected accidentally during ultrasound examination due to testicular torsion, and in patient no. 29, TART was suspected by palpation and confirmed by US examination. In remaining 13 patients, TARTs were discovered during screening by ultrasound. In 12 patients, TARTs were present bilaterally and were described as round lesions (nos. 8, 16, 29, 30, 33, 44–46, and 48–51) and unilaterally in three patients (nos. 31, 32, and 47). Testicular adrenal rest tumors were staged as 2/− in three patients (nos. 31, 32, and 47), 2/2 in three patients (nos. 8, 45, and 46), 2/3 in one patients (no. 16), 3/3 in two patients (nos. 30 and 50), and 4/4 in six patients (nos. 29, 33, 44, 48, 49, and 51).
In 11 patients, the lesions were hypoechoic (nos. 30–33, 44–49, and 51), in two hypoechoic in one and hyperechoic in the other testis (nos. 16 and 29), and in two hypoechoic and hypoechoic lesion were detected in both testes (nos. 6 and 50) (Fig. 1). In one patient without TART (no. 40), microlithiasis was detected.
The volume of both testes in the group of eight adult patients with TART compared to the group of 10 adult patients without TART was very similar—26.7 mL (range 19.7–33.0) and 27.1 mL (range 19.7–33), respectively. Moreover, five 13.3–15.5-year-old pubertal patients with TART and eight pubertal patients without TART aged 13.1–16.0 years also had similar testicular volume—22.7 mL (range 17.3–28.5) and 23.8 mL (15.5–30.3), respectively.
Testicular volumes were not compared in 16 prepubertal children with and without TART due to small number of patients with TART (2/14).
Clinical evaluation
TART was found in 14 SW 21-OHD patients and one 11-OHD patient. One patient (no. 1) had Tanner stage 1, one patient (no. 16) had Tanner stage 3, five patients (nos. 29–33) had Tanner stage 4, and eight patients were adults (nos. 44–51).
No TART was found in 36 patients (11 of the 24 SW, 3 of 4 the 11-OHD, 14 SV, and 8 NC patients). Seven patients had Tanner stage 1, six had Tanner stage 2, three had Tanner stage 3, four had Tanner stage 4, six had Tanner stage 5, and 10 were adults.
Eight patients with TART reached FH (nos. 44–51) and TH was 2.2–3.5 SD lower in all of them. In all of 14 patients without TART who reached FH, FH was also lower than TH for 2.5–3.5. There was not statistically significant difference in FH and TH between groups of adult patients with and without TART.
From seven patients with TART who did not reach FH, CA was identical with BA in two (nos. 8 and 31), and in five other BA-CA was calculated and was between 12 and 48 months (nos. 16, 29, 30, 32, and 33). In three out of 22 patients who had no TART and did not reach FH, CA was identical with BA, and in 19 other BA-CA was calculated and was between 6 and 48 months. The mean BA-CA in group of five pubertal patients with TART aged 13.3–15.5 years (nos. 29–33) was 20.4 months (range 0–48) and 12 months (range 0–24) in eight patients without TART aged 13.1–16.0 years.
BA-CA, in these two groups of patients, were not compared in prepubertal children due to small number of patients with TART in proportion those without TART (2/14).
Parameters of short-term and long-term hormonal regulation
At the time of ultrasound examination, six of 15 patients with TART (nos. 30, 33, 45, 47, 49, and 51) showed signs of poor hormonal control (undertreatment), and all of them showed significant difference between BA-CA or lower FH compared with TH indicating poor long-term regulation. Nine of patients with TART were treated adequately at the time of ultrasound examination (nos. 8, 16, 29, 31, 32, 44, 46, 48, and 50), but in one of them, differences between BA-CA and lower FH compared with TH in other were observed (nos. 16 and 29).
At the time of ultrasound examination, four of 36 patients without TART (nos. 5, 6, 19, and 34) showed signs of poor hormonal control (undertreatment), and in all of them, differences between BA-CA and lower FH compared with TH were found. Thirty two were treated adequately, but in eight of them (nos. 9, 10, 11, 12, 14, 20, 21, and 35), advanced BA or decreased FH compared to TH was noted. All eight of them were SV or NC 21-OHD or 11-OHD patients with late established diagnosis.
Four patients with TART had body mass index (BMI) 90% or higher for age (nos. 16, 29, 45, and 49) as well as three patients without TART (nos. 10, 12, and 14), but hormonal results at the time of examination did not imply overtreatment in any of 51 patients (Table 1).
Molecular genetic analysis
None of the 14 CAH 21-OHD patients with TART had milder mutation (groups B and C), but exclusively from group null and A. One of the detected mutations was deletion or conversion in 10 of 14 CAH 21-OHD patients with TART (nos. 8, 16, 30–33, 45, 46, 48, and 49) (Table 1).
Fertility
Three of 15 patients with TART are married (nos. 48, 50, and 51). They attempted to father a child over the course of 2 to 3.5 years, but have not succeeded (possible female factors of infertility were excluded). After discovering that they have TART, low levels of inhibin-B and high levels of FSH, semen analysis was performed and azoospermia was discovered.
Among remaining 12 patients, five were adults (nos. 44–47 and 49) but reported no current or past cohabitation with female partner, and seven were children or adolescents (nos. 8, 16, 29–33). One adolescent and one adult patient had low levels of inhibin-B and high levels of FSH (nos. 33 and 49), one adult had low inhibin-B level and suppressed gonadotropins (no. 45), one adult had low level of inhibin-B and normal level of FSH (no. 44), and two adults had normal levels of inhibin-B and FSH (nos. 46 and 47) (Table 1). Semen analysis performed in patient nos. 45 and 49 revealed oligoasthenozoospermia and azoospermia, respectively. Three adults (nos. 44, 46, and 47) were not motivated to perform it.
Among 36 patients without TART, lower levels of inhibin-B but normal levels of FSH at the time of ultrasound examination were found in one poor regulated patient (no. 6), three adequately regulated patients (nos. 22, 28, and 43), and two patients with significantly advanced BA (nos. 9 and 10).
Seven patients without TART are married and living with female partner. All of them fathered at least one child naturally. One SW patient (no. 43) had one child, three SV patients (nos. 37, 38, and 40) had two children each, one NC patient (no. 42) had two children, one of 11-OHD patients (no. 41) had two children, and other (no. 35) had one child. The remaining 29 patients without children were under aged or are not living with female partner. None of the 51 patients reported homosexuality or bisexuality.
Discussion
Frequency of TART in our study that comprised 51 CAH patients aged 1.8 to 40 years was 29%. Testicular adrenal rest tumor was found exclusively in SW patients due to 21-OHD and 11-OHD CAH patients. This is in concordance with other reports of TART findings mainly in SW 21-OHD and 11-OHD patients, rarely in SV 21-OHD patients [2, 10, 27, 32] but only in two NC 21-OHD patients [14]. Although less often TARTs can be already present in childhood [13,14,15,16,17,18], its prevalence increases during and after puberty, and in adulthood, they can be found in 34–70% of patients [3, 14, 18].
Except in 1.8-year-old patient (no. 1), to the best of our knowledge, the youngest patient reported with TART detected by ultrasound examination, and the 10.2-year-old patient (no. 16), TART in our group of CAH patients was discovered only during puberty (nos. 29–33) and adulthood (nos. 44–51). However, since in three of five pubertal patients (nos. 29, 30, and 33) TARTs were already stage 3 or 4 at the time of first ultrasound examination, it is highly likely that the onset of tumor growth was in prepubertal period. This indicates that ultrasound screening of TART should be initiated already in early childhood, particularly keeping in mind that in only one of our patients TART was suspected by clinical examination.
In all of patients, TARTs were located within the rete testes (bilaterally in 12 and unilaterally in 3) and those were predominately hypoechoic lesions what is in concordance with other reports [6, 8, 10, 17, 26, 27]. Poyrazoglu et al. reported higher prevalence of testicular microlithiasis in CAH patients with TART [24] , but in this study, it was detected only in one patient without TART (no. 40). The volume of testes with TART can be increased [21], but mostly remains normal or below normal [16, 27], as we also ascertained in our patients.
There are different opinions regarding the role of disease control in the development of TART. Some investigators suggest positive association between levels of ACTH and 17-OHP, frequency, and size of TARTs [18, 22, 23]. Other studies demonstrated no correlation of hormone control with TART volume and association between long-term parameters of disease control and presence of TART [3, 6, 27].
In our group of 15 patients with TART, eight (53%) showed signs of poor long-term control (advanced BA and significant difference between FH and TH), and in five of them, signs of poor hormonal regulation at the time of investigation were established. The remaining seven patients were well controlled. Among 36 patients without TART, 12 (33%) showed signs of poor long-term regulation. Moreover, signs of poor hormonal regulation at the time of investigation were ascertained in four of them. More frequent discovering TART in poorly controlled CAH patients suggests that control of disease plays role in TART development, but considering number of inadequately regulated patients without TART, and adequately regulated patients without TART, the regulation of the disease is not the only factor that influences tumor development.
All of 14 21-OHD patients with TART had exclusively mutations from group null and A, and in 10 of them (77%), deletion or conversion was detected (the frequency of deletion or conversion in classical 21-OHD patients in Croatian population is 18.8%) [13]. Our results are in accordance with results of Stikkelbroeck et al. [31] and Mouritsen et al. [21], which found that TART is most frequently detected in patients with severe CYP21A2 mutations, and in such patients, TART may occur already in early childhood.
Many recent investigations have shown low fertility rates in male CAH patients as a result of hypergonadotropic or hypogonadotropic hypogonadism [3, 25, 28].
The most common cause of hypergonadotropic hypogonadism in these patients is TART, which can cause infertility by compression of the rete testis and seminiferous tubules leading to obstructive azoospermia. It may be reversible in early stages of development when intensification of treatment might decrease tumor size and improve testicular function and fertility, indicating early screening of TART by ultrasonography to prevent testicular dysfunction [2, 18, 31]. Further growth can lead to irreversible damage of surrounding testicular tissue affecting endocrine and exocrine testicular function [7]. Particularly affected is secretion of hormones involved in FSH feedback control such as inhibin-B, and prevalence of low levels of inhibin-B and high levels of FSH was higher in CAH patients with TART than in those without tumor [3, 25].
In addition to mechanical obstruction, toxic effect of local adrenal steroids or metabolites derived from adrenal rest can impair Sertoli and/or germ cells [18].
King et al. [18] found that suppressed LH (which is often reversible) was the strongest predictor of severe oligospermia, although they did not find an association with the presence of TART or with reduced fertility outcome.
Among 15 patients with TART in our study, low levels of inhibin-B were found in six of them. Three of these 15 patients (nos. 48, 50, and 51) had attempted to father a child, but never succeeded. Presumable cause of azoospermia in these three patients, similar to patient no. 49, was TART, since all of them had low inhibin-B levels and high levels of FSH. This is in concordance with results of Reisch et al., who found positive correlation of inhibin-B with sperm concentration considering that inhibin-B is a reliable marker of Sertoli cell function in adult CAH patients with TART. Oligoasthenozoospermia discovered in patient no. 45 with low inhibin-B level, suppressed level of gonadotropins, and small TARTs (stage 2) probably is primarily caused by hypogonadotropic hypogonadism.
Among 36 patients without TART, lower levels of inhibin-B were found in six of them, but without elevated FSH levels, which according to King et al. [18] is exclusively associated with the presence of TART. Five of these six patients were children or adolescents (nos. 6, 9, 10, 22, and 28), while one adult (no. 43) fathered one child. It is interesting that Martinez-Aquajo et al. [20] found lower levels of inhibin-B in prepubertal boys with CAH in the absence of TART, supporting the hypothesis of impaired Sertoli cell development in CAH patients independent of TART presence. On the other hand, Claaashen-van dr Grinten H.L. et al. found normal levels of inhibin-B in 15 of 16 CAH patients with TART aged 10 to 19 years [5].
Considering the size of TARTs, restoration of spermatogenesis in azoospermic patient nos. 48–50 and 51 cannot be expected irrespective of intensification of glucocorticoid therapy (patient nos. 48 and 50 even show adequate regulation) and surgical intervention was considered. Since removal of TART by testis sparing surgery in order to eliminate obstruction of the seminiferous tubules was found to have no effect on improvement of semen quality and can even contribute to further testicular damage, this option was abolished [7].
As possibility of sperm cryopreservation was missed due to late discovering of TARTs, real treatment option for three of them could be a combination of testicular sperm extraction and intracytoplasmic sperm injection. Sperm retrieval, simultaneous with tumor resection, recently performed in two CAH patients with TART, is offered as a new treatment approach [17].
On the other side, optimization of glucocorticoid treatment in the patient no. 45 with hypogonadotropic hypogonadism and small TARTs leading to decrease in adrenal androgen production and their conversion to estrogens and normalizing suppressed gonadotropins can result in improving of spermatogenesis.
In conclusion, this study demonstrated that presence of TART is associated with severity of disease and disease control is one of the factors influencing tumor development.
One of the reasons of very low frequency of TART in younger age groups can be delay of tumor screening during childhood (in some of our puberty patients, TART was already grade ¾ at the time of first examination). As our youngest patient with TART was 1.8 years old, we recommend screening by TU already from early childhood. TART impairs gonadal function and significantly rises the risk of infertility.
Abbreviations
- 11-OHD:
-
11-h ydroxylase deficiency
- 17-OHP:
-
17-h ydroxyprogesterone
- 21-OHD:
-
21-h ydroxylase deficiency
- A:
-
Androstenedione
- ACTH:
-
Adrenocorticotropic hormone
- BA:
-
Bone age
- BMI:
-
Body mass index
- CA:
-
Chronological age
- CAH:
-
Congenital adrenal hyperplasia
- FH:
-
Final height
- FHSDS:
-
Final height standard deviation score
- FLC:
-
Fetal Leydig cells
- FSH:
-
Follicle-stimulating hormone
- GnRHa:
-
Gonadotropin-releasing hormone analog
- L:
-
Left
- LH:
-
Luteinizing hormone
- MLPA:
-
Multiplex ligation-dependent probe amplification
- NC:
-
Nonclassical
- PRA:
-
Plasma renin activity
- R:
-
Right
- RFLP:
-
Restriction fragment length polymorphism
- SV:
-
Simple virilizing
- SW:
-
Salt wasting
- T:
-
Testosterone
- TART:
-
Testicular adrenal rest tumor
- TH:
-
Target height
- THSDS:
-
Target height standard deviation score
- TU:
-
Testicular ultrasound
References
Andersson AM, Juul A, Petersen JH, Muller J, Groome NP, Skakkebaek NE (1997) Serum inhibin B in healthy pubertal and adolescent boys: relation to age, stage of puberty, and follicle-stimulating hormone, luteinizing hormone, testosterone, and estradiol levels. J Clin Endocrinol Metab 82:3976–3981
Aycan Z, Bas VN, Cetinkaya S, Yilmaz Agladioglu S, Tiryaki T (2013) Prevalence and long-term follow-up outcomes of testicular adrenal rest tumours in children and adolescent males with congenital adrenal hyperplasia. Clin Endocrinol 78:667–672
Bouvattier C, Esterle L, Renoult-Pierre P et al (2015) Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French National Survey. J Clin Endocrinol Metab 100:2303–2313
Cakir ED, Mutlu FS, Eren E, Pasa AO, Saglam H, Tarim O (2012) Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia. J Clin Res Pediatr Endocrinol 4:94–100
Claahsen-van der Grinten HL, Dehzad F, Kamphuis-van Ulzen K, de Korte CL (2014) Increased prevalence of testicular adrenal rest tumours during adolescence in congenital adrenal hyperplasia. Horm Res Paediatr 82:238–244
Claahsen-van der Grinten HL, Hermus AR, Otten BJ (2009) Testicular adrenal rest tumours in congenital adrenal hyperplasia. Int J Pediatr Endocrinol 624823
Claahsen-van der Grinten HL, Otten BJ, Hermus AR, Sweep FC, Hulsbergen-van de Kaa CA (2008) Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril 89:597–601
Claahsen-van der Grinten HL, Otten BJ, Sweep FC et al (2007) Testicular tumors in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency show functional features of adrenocortical tissue. J Clin Endocrinol Metab 92:3674–3680
Claahsen-van der Grinten HL, Otten BJ, Takahashi S et al (2007) Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J Clin Endocrinol Metab 92:612–615
Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR, Otten BJ (2007) Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol 157:339–344
Cooper TG, Noonan E, von Eckardstein S et al (2010) World Health Organization reference values for human semen characteristics. Hum Reprod Update 16:231–245
Dumic K, Wilson R, Thanasawat P et al (2010) Steroid 11-beta hydroxylase deficiency caused by compound heterozygosity for a novel mutation in intron 7 (IVS 7 DS+4A to G) in one CYP11B1 allele and R448H in exon 8 in the other. Eur J Pediatr 169:891–894
Dumic KK, Grubic Z, Yuen T (2017) Molecular genetic analysis in 93 patients and 193 family members with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency in Croatia. J Steroid Biochem Mol Biol 165:51–56
Falhammar H, Nystrom HF, Ekstrom U, Granberg S, Wedell A, Thoren M (2012) Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol 166:441–449
Jang JH, Jin DK, Kim JH et al (2011) Multiplex ligation-dependent probe amplification assay for diagnosis of congenital adrenal hyperplasia. Ann Clin Lab Sci 41:44–47
Jedrzejewski G, Ben-Skowronek I, Wozniak MM, Brodzisz A, Budzynska E, Wieczorek AP (2013) Testicular adrenal rest tumors in boys with congenital adrenal hyperplasia: 3D US and elastography—do we get more information for diagnosis and monitoring? J Pediatr Urol 9:1032–1037
Kavoussi PK, Summers-Colquitt RB, Odenwald KC et al (2016) Sperm retrieval and concomitant tumor resection in azoospermic men with congenital adrenal hyperplasia and bilateral testicular adrenal rest tumors: a case report. J Assist Reprod Genet 33:545–548
King TF, Lee MC, Williamson E, Conway GS (2016) Experience in optimising fertility outcomes in men with congenital adrenal hyperplasia due to 21 hydroxylase deficiency. Clin Endocrinol 84:830–836
Lottrup G, Nielsen JE, Skakkebaek NE, Juul A, Rajpert-De Meyts E (2015) Abundance of DLK1, differential expression of CYP11B1, CYP21A2 and MC2R, and lack of INSL3 distinguish testicular adrenal rest tumours from Leydig cell tumours. Eur J Endocrinol 172:491–499
Martinez-Aguayo A, Rocha A, Rojas N et al (2007) Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J Clin Endocrinol Metab 92:4583–4589
Mouritsen A, Jorgensen N, Main KM et al (2010) Testicular adrenal rest tumours in boys, adolescents and adult men with congenital adrenal hyperplasia may be associated with the CYP21A2 mutation. Int J Androl 33:521–527
Nermoen I, Rorvik J, Holmedal SH et al (2011) High frequency of adrenal myelolipomas and testicular adrenal rest tumours in adult Norwegian patients with classical congenital adrenal hyperplasia because of 21-hydroxylase deficiency. Clin Endocrinol 75:753–759
Pierre P, Despert F, Tranquart F et al (2012) Adrenal rest tissue in gonads of patients with classical congenital adrenal hyperplasia: multicenter study of 45 French male patients. Ann Endocrinol (Paris) 73:515–522
Poyrazoglu S, Saka N, Agayev A, Yekeler E (2010) Prevalence of testicular microlithiasis in males with congenital adrenal hyperplasia and its association with testicular adrenal rest tumors. Horm Res Paediatr 73:443–448
Reisch N, Flade L, Scherr M et al (2009) High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab 94:1665–1670
Reisch N, Rottenkolber M, Greifenstein A et al (2013) Testicular adrenal rest tumors develop independently of long-term disease control: a longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J Clin Endocrinol Metab 98:E1820-6
Reisch N, Scherr M, Flade L et al (2010) Total adrenal volume but not testicular adrenal rest tumor volume is associated with hormonal control in patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab 95:2065–2072
Smeets EE, Span PN, van Herwaarden AE et al (2015) Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. J Clin Endocrinol Metab 100:E524–E530
Speiser PW, Azziz R, Baskin LS et al (2010) Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95:4133–4160
Speiser PW, New MI (1994) Prenatal diagnosis and treatment of congenital adrenal hyperplasia. J Pediatr Endocrinol 7:183–191
Stikkelbroeck NM, Otten BJ, Pasic A et al (2001) High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab 86:5721–5728
Wang Z, Yang Z, Wang W et al (2015) Diagnosis of testicular adrenal rest tumors on ultrasound: a retrospective study of 15 cases report. Medicine (Baltimore) 94:e1471
Wedell A, Thilen A, Ritzen EM, Stengler B, Luthman H (1994) Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J Clin Endocrinol Metab 78:1145–1152
Wilson RC, Wei JQ, Cheng KC, Mercado AB, New MI (1995) Rapid deoxyribonucleic acid analysis by allele-specific polymerase chain reaction for detection of mutations in the steroid 21-hydroxylase gene. J Clin Endocrinol Metab 80:1635–1640
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
MD designed the study, collected and analyzed the data, and wrote the manuscript; VD performed the ultrasounds and helped with writing the manuscript; ZG performed the DNA analysis, reviewed the study findings, and wrote the manuscript; SKO performed the hormonal analysis and reviewed the manuscript; VS contributed to the clinical aspect of the trial and reviewed the manuscript; and VK performed the hormonal analysis and wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Communicated by Peter de Winter
Rights and permissions
About this article
Cite this article
Dumic, M., Duspara, V., Grubic, Z. et al. Testicular adrenal rest tumors in congenital adrenal hyperplasia—cross-sectional study of 51 Croatian male patients. Eur J Pediatr 176, 1393–1404 (2017). https://doi.org/10.1007/s00431-017-3008-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-017-3008-7