Abstract
This study investigated the association between obesity and obstructive sleep apnea (OSA) in preschool and school-age children. Parents of obese and randomly chosen normal weight children completed a questionnaire on sleep-related symptoms, demography, family, and medical history. All subjects were invited to undergo polysomnography (PSG). OSA cases were defined as obstructive apnea hypopnea index (OAHI) ≥1. A total of 5930 children were studied with 9.5 % obese (11.9 % boys/6.1 % girls), 205/2680 preschool and 360/3250 school children. There were 1030 children (535 obese/495 normal weight) who underwent PSG. OSA was higher in obese children and obese school children had higher OAHI, arousal index, and shorter total sleep time. However, there was no positive correlation between OSA and body mass index (BMI). The main risk factors for OSA in preschool children were adenotonsillar hypertrophy and recurrent respiratory tract infection. The main cause for OSA in school children was a history of parental snoring and obesity. Mallampati scores and sleep-related symptoms were found to be associated with OSA in both preschool and school children.
Conclusion: We demonstrated differential risk factors for OSA in obese children, which suggest that a different mechanism may be involved in OSA development in preschool and school-age children.
What is Known: Various risk factors have been reported in obese children with OSA owing to the different age and different study design. • Obese children have a higher prevalence and severity of obstructive sleep apnea (OSA). • OSA risk factors in obese children are affected by different ages and study designs. |
What is New: • A differential prevalence and risk factors for obese preschool and school-age children with OSA has been demonstrated. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a common global health problem and can cause hypertension, diabetes, hyperlipidemia, and cardiovascular disease in children and adults [12, 13, 25]. In the past decades, the prevalence of obesity in children has doubled in the USA and China [26, 27, 32, 42]. Using the same definition, based on the International Obesity Task Force (IOTF) definition, the prevalence of obesity in children varies from 2.2 to 14.2 % [2, 10, 16, 30; Table 7]. However, according to the World Health Organization (WHO) standards, the varying prevalence rate of obesity in children is from 4.9 to 29.9 % [15, 29; Table 8], owing in part to age, ethnic group, and area.
Previous studies have reported a higher prevalence and severity of obstructive sleep apnea (OSA) in children with obesity [7, 8, 17, 36, 41]. Potential contributions of adiposity to the airway collapsibility during sleep in obese children may cause the occurrence and severity of OSA. Frequent fragmentary sleep in children with OSA may lead to short sleep duration, which may increase appetite and body mass index (BMI) [34, 38], leading to a vicious cycle. It has been hypothesized that OSA contributes to obesity and vice versa. However, recent studies have failed to support a positive relationship between the degree of obesity and the severity of OSA. The risk of OSA has not been shown to significantly increase with increased BMI in younger children [19]. Indeed, the validity of an association between obesity and OSA has recently been questioned [20]. Such discrepant findings partly reside in the limitations imposed by the definition of obesity in terms of BMI. It is important to note that some previous studies were limited by their selected patient recruitment and lack of matched control subjects for comparison [8, 17, 36, 41]. In this study, we aimed to identify the prevalence, sleep characteristics, and risk factors associated with obesity and to compare the different risks factors for OSA in preschool and school children with obesity.
Methods
Study population
First phase—sleep questionnaire
This study is part of an epidemiological study to examine the prevalence of obesity in Wenzhou Chinese children. The protocol was approved by the Institutional Ethics Review Committee with support from the public health and disease control center. Parents of all the obese children and an age, sex, and ethnicity-matched normal weight children aged 3–11 years in 13 randomly selected primary schools and kindergartens were selected and invited to participate and attend an education forum where details of the study were explained. A validated OSA screening sleep questionnaire for children completed by parents [22] and a personally addressed letter asking for consent were then distributed with the help of school teachers. Those who failed to return the questionnaire within a week were given another copy with a self-addressed envelope enclosed for ease of return.
The sleep questionnaire sought information from participants regarding sleep habits and problems in the previous 12 months. The information obtained included demography data (age, sex, living environment, parental snoring history, parental education, and physical measurements), medical history (adenotonsillar hypertrophy, recurrent respiratory tract infection and allergic rhinitis, sinusitis, asthma), respiratory symptoms (snoring, witnessed apnea, labored breathing, and mouth breathing), nocturnal symptoms (restless sleep, prone position, nocturnal enuresis, night sweats, night terrors, nightmares, somnambulism, sleep talking, bruxism), and daytime symptoms (morning headache, daytime fatigue, difficulty getting up in the morning, hyperactivity). Parents were asked to provide answers using a five-point frequency scale (0 = never, 1 = “rarely” for 0–1 night per month, 2 = “sometimes” for 1–2 nights per month, 3 = “often” for 1–2 nights per week, 4 = “frequently” for 3 nights or more per week.) and “do not know” category. All the obese and matched normal weight children that had completed the sleep questionnaire were invited to undergo an overnight polysomnographic study.
Second phase—polysomnography
All the respondents were invited to undergo an overnight (at least 7 h) polysomnography (PSG) (Alice 5, Respironics Inc., Pittsburgh, PA, USA). The montage included eight channels (F3, F4, C3, C4, T3, T4, O1, and O2) of electroencephalogram; electrooculography; submental electromyography; vibration detector; thoracic and abdominal excursions by inductive plethysmography; air flow, which was measured by a nasal pressure catheter and supplemented by an oral thermistor; finger pulse oximetry; electrocardiography; and leg electromyography [5]. All computerized sleep data were manually edited by experienced PSG technologists and clinicians according to standardized criteria [1]. Hypopnea was defined as a reduction of 50 % or more in the amplitude of the airflow signal. It was only quantified if longer than two baseline breaths and was associated with oxygen desaturation of at least 4 % and/or arousals. Obstructive apnea hypopnea index (OAHI) was defined as the number of apnea and hypopneas per hour of total sleep time (TST). The obstructive apnea index (OAI) was defined as the average number of apnea episodes. The arousal index was the total number of arousals per hour of sleep.
Definition of obesity and OSA
Body mass index (BMI) was calculated as weight (kg) divided by the height squared (m2). Children were classified as being of normal weight, overweight, or obese according to the criteria defined by the international cutoff points for BMI for overweight and obesity by sex between 2 and 18 years [9]. Children were classified as obese or overweight if their BMI was above the 95th or between the 85th and 95th percentile on the sex- and age-specific growth chart, respectively. Those who were classified as being of normal weight were grouped as controls. For children who underwent PSG, they were separated into OSA cases and non-OSA controls using OAHI ≥1 as the cutoff. Adenotonsillar hypertrophy was defined as tonsil swelling over the pharynx arches and adenoid hypertrophy defined as swelling over more than half of the pharyngeal cavity as assessed by X-ray or laryngoscope.
Statistical analysis
Descriptive data were presented as percentages for discrete variables and as means (standard deviation) or medians (inter-quarter range) for continuous variables. Chi-squared test and Mann–Whitney U test were used to compare the variables between children with and without obesity. When performing comparisons between obese and non-obese participants in different age groups, ANOVAs with the factors of obesity and age were performed first. Age differences were only present if the factor age or the interaction was significant. This statistical approach was also used to compare the prevalence of obesity between school and preschool children. Exploratory factor analysis was used to identify the underlying pattern between the various sleep-related symptoms. Correlation coefficients were analyzed by principal component analysis and subsequent rotation according to the standard varimax criterion. With this analysis, the correlation between parameters was attributed to their common dependence on independent entities called “factors.” The coefficients that link parameters to factors were called “factor loadings”; the number of factors was chosen to be as small as possible but large enough to account for most of the variation within the data. It was decided a priori that the number of factors in the varimax rotation would be based on the number of eigenvalues above 1.0 in the principal component analysis. Logistic regression analysis was used to determine the association between obesity and risk factors which were found to be significant in univariate analysis. All analyses were performed using SPSS version 15.0 (SPSS, Inc. Chicago, USA). All p values reported are two tailed with statistical significance set at <0.05.
Results
A total of 5930 children were analyzed. The data included 2405 girls and 3525 boys aged 3–11 years. The overall prevalence of obesity was 565/5930 (9.5 %), with boys 419/3525 (11.9 %) two times more likely than girls 146/2405 (6.1 %) to be affected. Among them, the prevalence of obesity in school children tended to be higher than that in preschool children (360/3250 [11.1 %] vs. 205/2680 [7.6 %], p < 0.01) (Table 1).
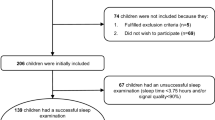
The flow chart for selection of study subjects is shown in Fig. 1. Of all the 1130 subjects, 1030 respondents (91.1 %) completed the questionnaires. There were 535 obese children (94.7 %; 203 preschool children and 332 school children) and 495 normal weight children (87.6 %; 178 preschool children and 317 school children) who agreed to take part in the second phase of the overnight PSG study. The characteristics of the children were shown in Tables 2 and 3.
Based on the PSG study, there was higher prevalence of OSA in obese school children than in normal weight children (normal weight [11.0 %] vs. obesity [41.0 %], p = 0.006), but this was not seen in obese preschool children (normal weight [20.2 %] vs. obesity [28.6 %], p = 0.08) (Table 4). The school children with obesity were found to have higher OAHI (5.8 [2.1–14.8] vs. 1.4 [0.2–3.3], p < 0.01), higher arousal index (9.8 [6.8–15.9] vs. 2.4 [3.2–7.3], p < 0.01), and shorter sleep duration (412 [288–468] vs. 525 [288–468], p < 0.01) than controls. Further analysis showed that wake time after sleep onset (WASO) was prolonged in obese in contrast with normal weight children (22 [12–36] vs. 36 [21–48], p = 0.035). However, children with and without obesity had similar sleep latency and sleep efficiency in all the sleep stages (S1, S2, S3 + 4). In preschool children, the sleep parameter changes in obesity were not seen (Table 4).
Thirteen items in the questionnaire were categorized into three factors by factor analysis, morning or daytime symptoms, nocturnal symptoms, and breathing symptoms (Table 5). The total variance explained by this three-factor model was 52.5 %, and the factor scores were calculated using the regression method. In the first logistic regression model with 203 preschool children with obesity, several risk factors were found to be significantly associated with OSA, including adenotonsillar hypertrophy (odds ratio [OR] 3.52, p < 0.01), recurrent respiratory tract infection (OR 2.57, p < 0.01), nocturnal (including restless sleep, prone position, bruxism, and night sweating) (OR 1.38, p < 0.01), morning and daytime (including morning headache, daytime fatigue, and difficulty getting up in the morning) (OR 2.63, p < 0.01), breathing (including labored breathing witnessed apnea, mouth breathing, and habitual snoring) (OR 1.46, p < 0.01), and Mallampati scores (OR 2.18, p < 0.01) (Table 6).
In the second logistic model that included 351 school children with obesity who had undergone PSG, the risk factors that were found to be significantly associated with OSA were parental snoring history (OR 2.45, p < 0.01), parental obesity (OR 2.12, p < 0.01), nocturnal symptoms (including restless sleep, prone position, bruxism, night sweating, and enuresis) (OR 1.75, p < 0.01), morning or daytime (including morning headache, daytime fatigue, difficulty getting up in the morning, and hyperactivity) (OR 1.53, p < 0.01), breathing (OR 1.33, p < 0.01), BMI z-score (OR 1.15, p = 0.04), and Mallampati scores (OR 2.45, p < 0.01) (Table 6).
Discussion
This study is a large community-based study on the association of obesity with OSA and other sleep-related symptoms. The prevalence of obesity in our cohort of 3- to 11-year-old children was 9.5 %, and the prevalence of OSA was greater in obese than non-obese children with higher OAHI and sleep arousal. A novel finding was the differential age prevalence and risk factors for OSA in children with obesity. The main risk factors for OSA in preschool children were adenotonsillar hypertrophy and recurrent respiratory tract infection, but in school children the parental snoring history and obesity were the main causes for OSA.
The prevalence of obesity in children has dramatically increased worldwide in the last few decades. In the USA, the prevalence of childhood obesity doubled among children aged 6–11 years [26, 27]. According to the multi-center survey for Chinese children aged 6–18 years, the average prevalence of obesity in China had increased from 0.2 % in 1985 to 8.1 % in 2010 [32, 42]. For 3–5-year-old children, the prevalence of obesity is seldom reported. The present study found a prevalence of 9.5 %, which is comparable to studies that used the same definition and involved the same ethnic Chinese children of a similar age and geographical region. However, the prevalence of obesity was considerably lower than that in developed countries [26, 27]. A study involving Chinese children reported a rate of 14.2 % in Shanghai city, which is higher than in our study [16]. The differences in the results may be due to subject age differences or to different cutoffs for BMI, which in previous studies may also give rise to a wide range in the reported prevalence rate. A consistent finding in most studies is that boys have a higher prevalence of obesity, and the prevalence increases with increasing age [2, 10, 15, 16, 29, 30; Tables 7 and 8].
Obese children may be at increased risk of OSA. The proportion of sleep-disordered breathing (SDB) has been found to be markedly increased among obese children [8, 36]. It has also been reported that a BMI of 1 kg/m2 beyond the mean BMI for age and gender can increase the risk of OSA by 12 %. Similar trends demonstrating an increased risk of OSA among obese and overweight children have been reported from all over the world [7, 17, 41]. Our study showed a higher proportion of OSA in school children with obesity, which is consistent with most published studies. A 4-year follow-up study from Hong Kong reported that obesity is the most important risk factor for the development of OSA in children with primary snoring [24].
Because not all obese children will suffer from OSA, the question is which obese children with snoring will have OSA. The results from studies have shown that the risk factors for OSA are variable due to differences in age and study design. It is accepted overall that the primary pathophysiologic mechanism involved in childhood OSA consists of adenotonsillar hypertrophy in the upper airway [14, 35], but several studies [4, 11, 21, 23] have failed to demonstrate the anticipated result. In our study, we demonstrated differential age risk factors for obese children in a large sample population including preschool and school children. School children with obesity were found to have higher OAHI than those without obesity (p < 0.01), but there was no significant differences in preschool children. However, we did not find a significant linear correlation between BMI and OAHI in school children. Logistic regression models confirmed that the main risk factors for OSA in preschool children were adenotonsillar hypertrophy and recurrent respiratory infection, a result in agreement with most studies. It might be that the rapidly developed size of tonsils and adenoids in preschool-age children predisposed them to having complications with respiratory infections because of an immature immune function. In school children, we found the main risk factors were a history of parental snoring and obesity. This finding suggests that genetic factors might play a more important role in school children than in preschool children. This result is consistent with that from a previous study in which adenotonsillar hypertrophy played a smaller role for OAHI in obese children compared with non-obese children. Furthermore, a significant association between BMI z-score and Mallampati scores emerged for the whole cohort (p < 0.01). The results suggest that Mallampati scores can reflect the true volume of pharyngeal cavity of the interactions between the multiple factors contributing to the upper airway collapsibility during sleep, such as neuromuscular response and other important anatomic and genetic factors [18, 28].
Several studies have reported the sleep characteristic in obese children with OSA, but because of different study designs and subjects, it is difficult to make comparisons. Our study randomly selected obese children aged 3–11 years and matched them with normal weight children by age, sex, and ethnicity. All the participants agreed to undergo the whole night PSG study. This sleep study demonstrated a higher prevalence of OSA in obese school children, and also found that they have higher OAHI and arousal as well as shorter total sleep time. This finding is compatible with published studies that suggest obese children with OSA tend to have worse nighttime sleep and excess daytime sleep. However, some studies did not show that children with OSA presented higher rates of obesity, or that obesity influenced its presentation clinically [19, 20, 22]. These studies with different results had probably been influenced by the characteristics of the studied population [3]. While most obese children appeared to have frequent arousal and short sleep duration, it has been reported that these are markers of emotional stress rather than a reflection of true sleep loss [39]. In our study, those obese children with greater sleep apnea and poor sleep may be the result of psychological distress and the interaction of the hypothalamic-pituitary-adrenal (HPA) axis and the pro-inflammatory cytokine response [40].
The relationship between obesity and sleep-related symptoms has also been reported in previous studies. Studies reporting both positive and negative associations have been published [31, 33]. This discrepancy may be explained by the differences in study design and sample size. In our study, a large sample of children underwent PSG, the current gold standard for diagnosing OSA, allowing us to accurately classify the subjects into OSA and non-OSA groups. We used factor analysis on various sleep-related symptoms, which were divided into three common factors. This study showed that the obese children with OSA were associated with morning or daytime symptoms, night symptoms, and respiratory symptoms. Further analysis found that the BMI z-score and the Mallampati scores were associated with OSA in school children, but not in preschool children. Nocturnal enuresis and hyperactivity during the daytime were also found to be important risk factors of OSA. These findings are consistent with our previous studies, as well as results reported in the literature [37].
There are certain limitations in our study. First, only the selected obese children and matched normal weight children completed our questionnaire survey. This could have introduced a degree of selection bias because parents whose children had sleep symptoms or problems with sleep were more likely to return the questionnaire, than parents whose children did not exhibit these symptoms or problems. Another potential selection bias is that a greater number of obese subjects with high risk factors for OSA underwent PSG in the second phase, despite our strict inclusion criteria. By comparing the demographic and socio-economic data of respondents and non-respondents, we could not find a significant difference between the two groups. Nonetheless, we believe that our study cohort still showed a good overall representation of the sample population. Second, the survey questionnaire was completed by parents, and very often, they did not sleep in the same room as their children, and therefore, would not know their actual sleep behavior. This is a known intrinsic problem associated with the use of questionnaire surveys. Despite this, a recent publication on preschool children showed a significant and independent association between parentally reported and objectively measured sleep symptoms [6]. Furthermore, the reliability of our screening questionnaire has been previously demonstrated [5].
The strength of this study is that it included a large number of subjects and used a validated, reliable, and locally applicable questionnaire. Furthermore, all subjects who agreed to take part in the second phase of this study underwent PSG to ascertain their OSA status, thus allowing an accurate comparison of the proportion of OSA between obese and normal weight children.
In summary, we found an obesity prevalence rate similar to that reported in studies carried out in ethnic Chinese children. We found a higher proportion of OSA in obese children, and a differential risk factor for OSA in preschool and school-aged children. The results suggest that different mechanisms may be involved in the development of OSA in preschool and school-age children with obesity.
Abbreviations
- BMI:
-
Body mass index
- OAHI:
-
Obstructive apnea hypopnea index
- OSA:
-
Obstructive sleep apnea
- PSG:
-
Polysomnography
- REM:
-
Rapid eye movement
References
American Thoracic Society (1999) Cardiorespiratory sleep studies in children. Establishment of normative data and polysomnographic predictors of morbidity. Am J Respir Crit Care Med 160:1381–1387
Bingham DD, Varela-Silva MI, Ferrão MM, Augusta G, Mourão MI, Nogueira H, Marques VR, Padez C (2013) Socio-demographic and behavioral risk factors associated with the high prevalence of overweight and obesity in Portuguese children. Am J Hum Biol 25:733–742
Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A (2005) Excessive daytime sleepiness in a general population sample. The role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90:4510–4515
Brooks LJ, Stephens BM, Bacevice AM (1998) Adenoid size is related to severity but not the number of episodes of obstructive apnea in children. J Pediatr 132:682–686
Cai XH, Li XC, Hu QQ, Yu CY, Zhou YH, Su MS, Zhao YP, Hu YL, Wang LX (2013) Multiple system morbidities associated with children with snore symptom. Pediatr Pulmonol 48:381–389
Castronovo V, Zucconi M, Nosetti L, Marazzini C, Hensley M, Veglia F, Nespoli L, Ferini-Strambi L (2003) Prevalence of habitual snoring and sleep-disordered breathing in preschool-aged children in an Italian community. J Pediatr 142:377–382
Cauter EV, Knutson KL (2008) Sleep and the epidemic of obesity in children and adults. Eur J Endocrinol 159:59–66
Chay OM, Goh A, Abisheganaden J, Tang J, Lim WH, Chan YH, Wee MK, Johan A, John AB, Cheng HK, Lin M, Chee T, Rajan U, Wang S, Machin D (2000) Obstructive sleep apnea syndrome in obese Singapore children. Pediatr Pulmonol 29:284–290
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Decelis A, Fox K, Jago R (2013) Prevalence of obesity among 10-11-year-old Maltese children using four established standards. Pediatr Obes 8:e54–58
Erdamar B, Suoglu Y, Cuhadaroglu C, Katircioglu S, Guven M (2001) Evaluation of clinical parameters in patients with obstructive sleep apnea and possible correlation with the severity of the disease. Eur Arch Otorhinolaryngol 258:492–495
Freedman DS, Dietz WH, Srinivasan SR, Berenson GS (1999) The relation of overweight to cardiovascular risk factors among children and adolescents: The Bogalusa Heart Study. Pediatrics 103:1175–1182
Gozal D, Capdevila OS, Kheirandish - Gozal L (2008) Metabolic alterations and systemic inflammation in obstructive sleep apnea among non-obese and obese pre-pubertal children. Am J Respir Crit Care Med 177:1142–1149
Greenfeld M, Tauman R, Sivan A, DeRowe Y (2003) Obstructive sleep apnea syndrome due to adenotonsillar hypertrophy in infants. Int J Pediatr Otorhinolaryngol 67:1055–1060
Hernández-Herrera RJ, Mathiew-Quirós Á, Díaz-Sánchez O, Reyes-Treviño NO, Álvarez-Álvarez C, Villanueva-Montemayor D, de la Garza-Salinas LH, González-Guajardo E (2014) Prevalence of overweight and obesity in children from Monterrey. Nuevo León Rev Med Inst Mex Seguro Soc 52(Suppl 1):S42–47
Jiang XX, Hardy LL, Baur LA, Ding D, Wang L, Shi HJ (2014) High prevalence of overweight and obesity among inner city Chinese children in Shanghai, 2011. Ann Hum Biol 41:469–472
Kaditis AG, Alexopoulos EI, Hatzi F, Karadonta I, Chaidas K, Gourgoulianis K, Zintzaras E, Syrogiannopoulos GA (2008) Adiposity in relation to age as predictor of severity of sleep apnea in children with snoring. Sleep Breath 12:25–31
Katz ES, D’Ambrosio CM (2008) Pathophysiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 5:253–262
Kohler MJ, Thormaehlen S, Kennedy J, Pamula Y, van den Heuvel CJ, Lushington K, Martin AJ (2009) Differences in the association between obesity and obstructive sleep apnea among children and adolescents obstructive sleep apnea in children. J Clin Sleep Med 5:506–511
Kohler MJ, van den Heuvel CJ (2008) Is there a clear link between overweight/obesity and sleep disordered breathing in children? Sleep Med Rev 12:347–361
Lam YY, Chan EY, Ng DK, Chan CH, Cheung JM, Leung SY, Chow PY, Kwok KL (2006) The correlation among obesity, apnea-hypopnea index, and tonsil size in children. Chest 130:1751–1756
Li AM, Cheung A, Chan D, Wong E, Ho C, Lau J, Wing YK (2006) Validation of a questionnaire instrument for prediction of obstructive sleep apnea in Hong Kong Chinese children. Pediatr Pulmonol 41:1153–1160
Li AM, Wong E, Kew J, Hui S, Fok TF (2002) Use of tonsil size in the evaluation of obstructive sleep apnea. Arch Dis Child 87:156–159
Li AM, Zhu Y, Au CT, Lee DL, Ho C, Wing YK (2013) Natural history of primary snoring in school-aged children: a 4-year follow-up study. Chest 143:729–735
Lu X, Shi P, Luo CY, Zhou YF, Yu HT, Guo CY, Wu F (2013) Prevalence of hypertension in overweight and obese children from a large school-based population in Shanghai, China. BMC Public Health 13:24
Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM (2010) Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 303:242–249
Ogden CL, Flegal KM, Carroll MD, Johnson CL (2002) Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732
Ronen O, Malhotra A, Pillar G (2007) Influence of gender and age on upper-airway length during development. Pediatrics 120:e1028–e1034
Rudnick EF, Walsh JS, Hampton MC, Mitchell RB (2007) Prevalence and ethnicity of sleep-disordered breathing and obesity in children. Otolaryngol Head Neck Surg 137:878–882
Salehi-Abargouei A, Abdollahzad H, Bameri Z, Esmaillzadeh A (2013) Underweight, overweight and obesity among zaboli adolescents: a comparison between international and Iranians’ national criteria. Int J Prev Med 4:523–530
Snell EK, Adam EK, Duncan GJ (2007) Sleep and the body mass index and overweight status of children and adolescents. Child Dev 78:309–323
Song Y, Wang HJ, Ma J, Wang Z (2013) Secular trends of obesity prevalence in urban Chinese children from 1985 to 2010: gender disparity. PLoS One 8:e53069
Sogut A, Altin R, Uzun L, Ugur MB, Tomac N, Acun C, Kart L, Can G (2005) Prevalence of obstructive sleep apnea syndrome and associated symptoms in 3-11-year-old Turkish children. Pediatr Pulmonol 39:251–256
Spiegel K, Tasali E, Penev P, Van Cauter E (2004) Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 141:846–850
Strauss SG, Lynn AM, Bratton SL, Nespeca MK (1999) Ventilatory response to CO2 in children with obstructive sleep apnea from adenotonsillar hypertrophy. Anesth Analg 89:328–332
Sulit LG, Storfer-Isser A, Rosen CL, Kirchner HL, Redline S (2005) Associations of obesity, sleep-disordered breathing, and wheezing in children. Am J Respir Crit Care Med 171:659–664
Su MS, Li AM, So HK, Au CT, Ho C, Wing YK (2011) Nocturnal enuresis in children: prevalence, correlates, and relationship with obstructive sleep apnea. J Pediatr 159:238–242
Verhulst SL, Schrauwen N, Haentjens D, Suys B, Rooman RP, Van Gaal L, De Backer WA, Desager KN (2007) Sleep-disordered breathing in overweight and obese children and adolescents: prevalence, characteristics and the role of fat distribution. Arch Dis Child 92:205–208
Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, Chrousos GP, Bixler EO (2008) Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 32:801–809
Vicennati V, Pasquali R (2000) Abnormalities of the hypothalamicpituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab 85:4093–4098
Wing YK, Hui SH, Pak WM, Ho CK, Cheung A, Li AM, Fok TF (2014) A controlled study of sleep related disordered breathing in obese children. Arch Dis Child 88:1043–1047
Yu Z, Han S, Chu J, Xu Z, Zhu C, Guo X (2012) Trends in overweight and obesity among children and adolescents in China from 1981 to 2010: a meta-analysis. PLoS One 7:e51949
Acknowledgments
The authors thank all the staff at the Center for Disease Control and Prevention in Wenzhou City, Sleep Center of Hong Kong Chinese University for their logistic support, and the cooperation and participation of all the schools, children, and their parents.
Contributors
M-SS was the guarantor of integrity and conceived the study, participated in its design, carried out sleep studies, and prepared the manuscript; H-LZ participated in the literature research, performed the statistical analysis, and helped draft the manuscript; X-HC helped coordinate and design the study; YL participated in data acquisition and data analysis; P-NL selected the epidemiology survey and clinic data; Y-BZ and W-ZH carried out sleep questionnaire; C-CL and Y-FX were mentors for M-SS and helped the design, data analysis, and final revision of the manuscript. All authors read and approved the final manuscript.
Conflict of interest
The authors have no financial relationship with the organization that sponsored the research.
Funding
This study was supported by funding from the Natural Science Foundation of China (81172689), Zhejiang Province Health Department Project (2013RCA037), Science and Technology Department Project (2013 C33174), and Wenzhou City Science and Technology Bureau (Y 20140502).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by Peter de Winter
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Su, MS., Zhang, HL., Cai, XH. et al. Obesity in children with different risk factors for obstructive sleep apnea: a community-based study. Eur J Pediatr 175, 211–220 (2016). https://doi.org/10.1007/s00431-015-2613-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2613-6