Abstract
Subclinical cardiac abnormalities represent predisposing factors for cardiovascular disease (CVD) in obese subjects. The aim of this study was to evaluate early cardiac abnormalities in obese youth and the potential association with insulin resistance (IR). Thirty obese (12 males (M)/18 females (F); age = 11.5 ± 2.4 years; body mass index (BMI)-standard deviation score (SDS) = +2.1 ± 0.5) and 15 normal weight (10 M/5 F; age = 12.8 ± 3.1 years; BMI-SDS = +0.3 ± 0.9) children and adolescents underwent Doppler two-dimensional echocardiographic assessments of left atrial (LA) and ventricular (LV) geometry and LV diastolic function (peak early [E] and late waves, E wave deceleration time, myocardial flow velocities). Homeostasis model assessment of IR (HOMA-IR) was used as an IR index. LA size was increased in obese children, as indicated by higher LA diameter (4.9 ± 0.5 vs 4.1 ± 0.4 cm, p < 0.001), area (14.3 ± 2.5 vs 10.7 ± 2.0 cm2, p < 0.001), and volume (33.8 ± 10.6 vs 23.7 ± 6.4 ml, p = 0.003). LV mass was also increased in obese children (87.0 ± 16.6 vs 68.8 ± 13.2 g, p = 0.003), who also showed subtle diastolic dysfunctions, as indicated by higher values of E (97.1 ± 14.3 vs 86.2 ± 11.9 cm/s, p = 0.02). All the above parameters were significantly associated with BMI-SDS (p < 0.05). In addition, HOMA-IR was independently associated with LA diameter, area, and volume (β = 0.314, p = 0.040; β = 0.415, p = 0.008; β = 0.535, p = 0.001).
Conclusion: Obese children feature increased LA size, which emerged to be mainly correlated to, and possibly driven by IR, suggesting an increased CVD risk.
What is Known: • Left atrial and ventricular alterations have been reported in obese adults, and they represent predisposing factors for cardiovascular disease. • There is some evidence suggesting that obese children show increased left ventricular mass and also increased atrial size, although with conflicting results. |
What is New: • Obese normotensive children showed a moderately increased atrial size, subtle alterations in left cardiac diastolic function, and ventricular mass. • An association between insulin resistance and left cardiac changes was found, although its mechanism remains to be determined. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is becoming more prevalent due the growing incidence of childhood obesity [28]. Obese children are predisposed to the development of subclinical cardiovascular alterations early in life [11] and to an increased cardiovascular morbidity and mortality in adulthood [11, 25, 36].
Several studies have shown that obese children present several cardiometabolic risk factors, such as high blood pressure, dyslipidemia, and liver steatosis, already during the prepubertal period [13, 23, 34]. In addition, obese children show early signs of impaired vascular function, such as increased carotid intima–media thickness, arterial stiffness and decreased flow-mediated dilation [16, 23]. Many metabolic and inflammatory factors are involved in the pathogenesis of vascular changes in obese children. In particular, insulin resistance has been proposed as a key link between adiposity and the associated cardiovascular risk, because it mainly participates in the development of vascular endothelial dysfunction [9]. Under physiological conditions, insulin signaling in the endothelial cells results in vasodilation via increased nitric oxide production and bioavailability. In contrast, in conditions of insulin resistance, there is an impaired insulin signaling which leads to vasoconstriction and vascular proliferation, therefore contributing to endothelial dysfunction [33]. During the last years, several studies have focused on the relationship between obesity and potential abnormalities in cardiac structure and function [11]. Adults with severe forms of obesity show a cardiomyopathy attributed to chronic overload, left ventricular (LV) hypertrophy, and ventricular dysfunction [21, 40, 43]. Even milder forms of obesity have been associated with abnormalities in cardiac function and structure [40], which, although often subclinical, represent predisposing factors for increased CVD morbidity and mortality [11].
Some studies performed in obese youth have shown that subclinical cardiac alterations can be detected even at an early age [6, 12, 19, 26, 27, 29, 41]. However, data on the association between childhood obesity and morphological and functional changes in the heart have often reported contrasting findings. In addition, little is known on the potential association between early cardiac alterations and metabolic abnormalities in obese children when compared to healthy normal weight peers.
The aim of this study was to evaluate early cardiac structural and functional abnormalities in obese children and adolescents compared to normal weight controls and the potential association with metabolic alterations, in particular with insulin resistance.
Patients and methods
Study population
The study population included 30 obese and 15 normal weight children and adolescents (Table 1).
Obese children were consecutively recruited from patients attending the Pediatric Endocrinology Clinic of the Department of Pediatrics, University of Chieti, Italy. The inclusion criteria of the case group were age between 6 and 17 years, obesity (body mass index [BMI] > 95th percentile for age and sex), being otherwise in good health, and not affected by any chronic disease. The control group was recruited from children attending the pediatric outpatient clinics for a general check after 1–2 weeks from a previous admission to our pediatric ward for minor diseases (mainly gastroenteritis and trauma). At the time of assessment, these children were in good general health with complete resolution of the original disease. The inclusion criteria of the control group were age between 6 and 17 years, normal weight (BMI between 5th and 85th percentile for age and sex), being otherwise in good health, and not affected by any chronic disease. None of the patients were taking any medication, and none had a history of smoking or alcohol consumption.
At the beginning of the study, a complete physical examination, including anthropometric measurements (height, weight, BMI, and waist circumference [WC]), was performed, and basal blood pressure was measured in all children. Fasting blood samples were collected to measure glucose and insulin levels, lipid profile (total cholesterol, low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C], and triglycerides [TG]), and asymmetric dimethylarginine (ADMA). Homeostasis model assessment of insulin resistance (HOMA-IR) was used as a surrogate index of insulin resistance, and it was calculated as follows: [fasting insulin (mU/l) × fasting glucose (mmol/l) / 22.5] [35].
Doppler two-dimensional echocardiographic assessments were performed in all subjects.
The study was approved by the local ethics committee. Written informed consent was obtained from all parents and oral consent from all children.
Anthropometric measurements
Body weight was determined to the nearest 0.1 kg, and height was measured with Harpenden stadiometer to the nearest 0.1 cm. BMI and WC were used as indexes of adiposity. BMI was calculated as the weight in kilograms divided by the square of the height in meters and was converted in standard deviation scores (SDS) using published reference values for age and sex for the Italian population [8]. WC was measured at its smallest point between iliac crest and rib cage.
Blood pressure
Systolic (SBP) and diastolic (DBP) blood pressure were measured twice from the right arm after 10 min of rest in the supine position by using a calibrated sphygmomanometer and averaged. The cuff size, which was selected on the length and circumference of upper arm, was as large as possible without the elbow skin crease obstructing the stethoscope.
Biochemical analyses
Plasma glucose levels were determined by using the glucose oxidase method, and plasmatic insulin levels were measured with two-site immunoenzymetric assay (AIA-PACK IRI; Tosoh, Tokyo, Japan). Total cholesterol, HDL-C, and TG were measured with an enzymatic–calorimetric test. LDL-C was derived according to the Friedewald equation (low-density lipoprotein cholesterol = total cholesterol − high-density lipoprotein cholesterol − triglycerides / 5). The TG/HDL ratio was also calculated as an emerging marker of cardiovascular risk [16].
Levels of human serum ADMA were determined by enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., LTD, Catalog No. CSB-E09298h, People’s Republic of China), according to the manufacturer’s protocols [17]. The minimum detectable dose of human ADMA was less than 2 ng/ml. The intra-assay coefficient of variation was <8 %, and the inter-assay coefficient of variation was <10 %.
Echocardiography
All echocardiographic measurements were performed with Acuson Sequoia, whereas tissue Doppler was performed with Philips Sonos 500. Standard views from the parasternal long and short axis and apical four-chamber views were used. Echocardiographic images were obtained with the subject in the left partial decubitus position. The LV internal dimensions and septal and posterior wall thickness were measured at the end of the diastole and the end of the systole from two-dimensional target M-Mode echocardiographic tracings in the parasternal long axis, according to the criteria of the American Society of Echocardiography. Relative wall thickness (RWT) was calculated as (diastolic posterior wall thickness + diastolic interventricular septum thickness) / LV diastolic diameter (LVDD). LV mass was calculated using Devereux’s method [20].
Left atrial (LA) size assessment was performed by two-dimensional four-chamber view, at end-systole just before the mitral valve opens, and included cranio-caudal diameter, latero-lateral diameter, atrial area, and volume. LA area was measured tracing planimetry of atrial endocardial border and major-axis dimension; atrial volume was measured according to area/length method using planimetered area and minor-axis dimension [volume = 8 × (A1)^2/3p × (L)]. Images were optimized to avoid foreshortening and obtain clear endocardial border delineation.
LA and LV geometric parameters were indexed by dividing height in meters to the allometric power of 2.7 (h2.7), as previously suggested by De Simone et al. [18].
Pulsed Doppler measurements of LV filling were obtained in the apical four-chamber view, with the Doppler beam aligned perpendicularly to the plane of the mitral annulus, and the sample volume was placed between the tips of the mitral leaflets. Three consecutive beats obtained during quiet respiration were acquired for calculating peak early wave (E) and peak late wave (A) velocities and the E wave deceleration time.
The Doppler cursor was placed parallel to mitral inflow, and maximal velocity was measured with the sample volume at the mitral valve leaflet tips. The mitral peak of E (early filling) and A (late filling) waves, E/A ratio, and deceleration time (DcT) of the E wave velocity were measured. Tissue Doppler imaging was performed to acquire mitral annular diastolic myocardial velocities by setting the sample point at the lateral and septal sides of the mitral annulus in the apical four-chamber view (septal and lateral early diastolic mitral annular velocity (Em) and late diastolic mitral annular velocity (Am), in cm/s), and E′/A′ ratio and LV filling index E/Em ratio were calculated in accordance with recent guidelines [31, 37].
All reported measurements represent the average of three consecutive cardiac cycles obtained by a single experienced pediatric cardiologist, with a validated training in cardiac ultrasound. In order to assess the reproducibility of the assessments, a random sample of five obese children and three control children was reassessed by a second cardiologist. The inter-observer correlation was good being between 0.88 and 0.94 for the different cardiac parameters.
Statistical analysis
All data were expressed as mean ± SD or median (interquartile range), unless otherwise stated. P values <0.05 were considered statistically significant. All calculations were made with the computer program Statistical Package for the Social Science (SPSS) version 17.0 (SPSS Inc., Chicago, IL, USA). Not normally distributed variables were log transformed before data analysis. Differences between the two study groups in continuous variables were tested by an unpaired t test, whereas differences in categorical variables were assessed by χ 2 test or Fisher’s exact test. Analysis of covariance was used to allow adjustments for potential confounders, such as pubertal stage and sex, followed by Bonferroni test when more than two groups were compared.
Linear regression analysis was performed to assess the potential associations of cardiac geometry and function parameters with demographic and clinical/biochemical data.
Results
Clinical and metabolic characteristics
The study population included 30 obese and 15 normal weight children. The two groups were comparable for age, sex distribution, and height, whereas, as expected, weight, BMI, and WC were higher in obese compared to control children. SBP SDS was also higher in obese than in normal weight children (Table 1).
Obese children showed higher values of LDL-C, TG, and TG/HDL ratio and lower levels of HDL-C, compared to normal weight children. ADMA levels were also higher in obese children than in their normal weight peers (Table 1).
Insulin levels and HOMA-IR values were significantly higher in obese than in normal weight children, whereas there were no differences in fasting glycemia between the two groups (Table 1).
Cardiac geometry and function
Atrial and ventricular size
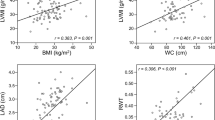
The results of the echocardiographic assessment are reported in Table 2. LA size was significantly increased in obese compared to normal weight children, as indicated by higher LA cranio-caudal and latero-lateral diameters, LA area, and volume (Table 2 and Fig. 1a, b). These differences were maintained after the normalization of atrial measurements for height2.7.
LV mass index was also increased in obese compared to normal weight children, whereas there were no significant differences in other assessed structural LV parameters, such as PWT, IVS, LVDD, and RWT (Table 2).
Diastolic function
Obese children showed subtle abnormalities in the transmitral flow, characterized by an increased peak early filling velocity E and a prolonged DcT of early transmitral blood flow velocity. No significant differences between the two groups were found in the peak late filling velocity A and in the E/A ratio (Table 2).
Regarding myocardial flow velocities, the only different parameter between obese and control children was lateral Am, which was slightly higher in the obese group. The septal and lateral E/Em ratio, an index of LV filling, was not different between the two groups (Table 2).
Associations between cardiac parameters and clinical/biochemical variables
In a univariate model, LA size parameters were all significantly associated with BMI-SDS and WC, as well as with SBP (Table 3). The association between LA size and BMI-SDS and WC persisted after adjusting for age, pubertal stage, and sex, while the association with SBP lost statistical significance (Table 4, model 1). When including BMI-SDS and WC in the same regression model, only BMI-SDS remained significantly associated with all LA size parameters (data not shown).
In the univariate model, LA size was also associated with HOMA-IR, ADMA, and TG/HDL ratio (Table 3). These associations remained statistically significant after adjusting for age, sex, and pubertal stage (Table 4, model A). When BMI-SDS was added into the regression model (Table 4, model B), HOMA-IR remained significantly associated with LA diameter, area, and volume (Fig. 1c, d), whereas ADMA only reached borderline statistical significance (Table 4, model B).
LV mass was significantly associated with adiposity indexes (BMI-SDS and WC) (Table 3). Significant associations were also found between LV mass and HOMA-IR, although this was not maintained after adjusting for BMI (Table 4).
Peak E was only associated with SBP, but this association was lost after adjusting for age, sex, and pubertal stage (r = 0.143, p = 0.459).
To further explore the effect of HOMA-IR on cardiac parameters, the study population was divided into tertiles of HOMA-IR. In an analysis of covariance, after adjusting for BMI-SDS, a significant trend across HOMA-IR tertiles was found in LA area (adjusted mean ± SD = 11.7 ± 2.4 vs 13.0 ± 2.1 vs 14.7 ± 2.3 cm2, p for trend = 0.01), with a significant difference between the first and last tertiles (p = 0.009) and LA volume (24.7 ± 9.7 vs 29.0 ± 8.5 vs 37.7 ± 9.3 ml, p for trend = 0.005), with again a significant difference between the first and last tertiles (p = 0.006).
Discussion
The present study showed that obese children have an increased LA size and initial alterations in LV geometry and diastolic function when compared to their normal weight peers. Adiposity and insulin resistance were significantly associated with these early cardiac changes.
Increased LA size
Our study showed marked variations in the LA dimension: a clear LA enlargement was observed in obese youth compared to their normal weight peers. In addition, LA size, both in terms of diameters, area, and volume, was independently associated with adiposity indexes, such as BMI and WC. These findings are in line with some previous studies reporting LA enlargement in obese compared to normal weight peers [6, 19, 26, 27, 32, 45]. The present study confirmed that excess body weight is associated with LA enlargement as early as during childhood and adolescence and remarkably in youth without hypertension. This finding is clinically relevant, because in obese adults an increased LA size has been associated with adverse cardiovascular events, including risk for atrial fibrillation, congestive heart failure, and stroke [1, 5, 7, 38].
Increased LV mass and LV diastolic dysfunction
LV mass index was also increased in obese children, and this parameter was independently associated with BMI. This is in line with previous studies where obese and overweight adults and children had increased LV mass even in the absence of arterial hypertension [12, 18, 43]. This finding is clinically relevant because the persistence of the obesity status overtime may lead to the development of hypertension, which in turn may add an additional load for the LV.
Another key finding of the present study was the detection of subtle abnormalities in some parameters related to LV function, suggestive of an initial impairment of LV diastolic function. In particular, the transmitral flow assessment showed that obese children presented higher E values and DcT, whereas in terms of myocardial flow velocities, there was only a small difference between the two groups in the lateral Am. These findings are in line with those from some previous studies [45] but in contrast with some others, mainly performed in adults with a longer duration of obesity, where a reduced peak E was detected [42, 46]. Duration of obesity has been suggested as a main determinant of cardiac remodeling [2], with longer duration being associated with more evident cardiac morphological and functional abnormalities. This aspect, as well as different degrees of obesity [4], may explain different findings between obese adults and children and contrasting results between studies performed within the pediatric population. The findings of the present study might reflect a specific obesity pattern of alterations associated with an early onset and short duration of obesity.
Impact of insulin resistance on cardiac changes
The abnormal cardiac structural findings of the present study are of particular interest mainly because they emerged from a group of apparently healthy obese youth, in the absence of metabolic alterations, such as dyslipidemia, impaired glucose tolerance/type 2 diabetes, or hypertension, a well-known risk factor for cardiac structure modifications [14]. Particularly, in children with hypertension, excess body weight has been associated with LA size, independently of blood pressure [15], suggesting a potential role of other obesity-related mechanisms, including hyperinsulinemia/insulin resistance [32]. Reduced insulin sensitivity is a common finding among obese children, already during the prepubertal period [9]. This metabolic abnormality is not only a risk factor for future development of type 2 diabetes, but it is also associated with the presence of other cardiometabolic risk factors, such as increased blood pressure, dyslipidemia, and liver steatosis [9]. In obese children, insulin resistance represents also a key link between excess body weight and the associated cardiovascular risk, being one of the first mechanisms involved in the development of vascular endothelial dysfunction [9] and early vascular alterations, such as increased carotid intima–media thickness [22]. The independent association between insulin resistance and LA enlargement observed in this study highlights another possible negative effect mediated by insulin resistance in the context of obesity. This is supported by previous data from animal and human studies showing a role of obesity-associated insulin resistance in mediating alterations in myocardial metabolism, by acting on fatty acid uptake and oxidation [39]. Previously, several mechanisms have been proposed to explain the association between insulin resistance and cardiac structure [44]. In particular, the most likely ones in our normoglycemic population might be related to the associated hyperinsulinemia and include a growth effect on the cardiac myocytes, altered vascular compliance, matrix remodeling, and increased sodium reabsorption [44].
In the present study, we also assessed potential associations between some selected cardiovascular markers, in particular the TG/HDL ratio and ADMA, and left cardiac size. Recently, there has been growing interest in the role of the TG/HDL ratio as a potential new marker of cardiovascular risk. Previous studies have shown that in obese adults and children, the TG/HDL ratio is associated with insulin resistance and early signs of cardiovascular complications, such as increased intima–media thickness [16, 24]. In contrast, in our study, although this index was increased in obese children, it did not emerge to have an independent effect on cardiac structure, but it may be considered as a surrogate marker of obesity in this setting.
Another finding in the present study was the association between LA size and ADMA levels, although this association was mainly mediated by adiposity. ADMA is an endogenous inhibitor of nitric oxide synthase, and it has been associated with an increased cardiovascular morbidity and mortality in adults [3] and with adverse cardiovascular outcomes even in the pediatric population [10]. In line with our finding, a previous study showed an association between cardiac structure parameters and ADMA, which was mainly explained by an effect of BMI on both variables [30], suggesting that increased ADMA and LA size may be the result of common mechanisms acting in the context of obesity. However, further studies are required to better explore the potential causal relationship between LA size and ADMA.
Some limitations of the present study need to be acknowledged, including in particular the small sample size and the cross-sectional design. However, the study population underwent a large number of echocardiographic parameters including not only geometry but also diastolic function parameters, thus allowing a good cardiac evaluation.
In conclusion, this pilot study suggests that the alterations in cardiac geometry and function previously observed in obese adults also occur in the context of obesity in childhood and adolescence. The finding that LA morphological alterations and only few abnormalities in diastolic function parameters occur in obese children compared with their normal weight peers leads to the hypothesis that obesity has an earlier negative impact on the cardiac geometry and only later can affect also LV diastolic function. These morphological cardiac changes were independently associated with insulin resistance, although future longitudinal studies are required to test a pathogenetic role of insulin resistance.
Overall, these findings suggest that assessing cardiac function and structure may represent an important clinical tool for estimating cardiovascular risk in obese youth. At present, the overtime progression of the detected cardiac abnormalities is unknown. Therefore, follow-up of the study population would be of paramount importance to clarify this issue. A further point to clarify is the correlation between the reported abnormalities during childhood and cardiovascular outcomes during adulthood.
Abbreviations
- A :
-
Peak late waves
- Am:
-
Late diastolic mitral annular velocity
- ADMA:
-
Asymmetric dimethylarginine
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- DBP:
-
Diastolic blood pressure
- DcT:
-
Deceleration time
- E :
-
Peak early waves
- Em:
-
Early diastolic mitral annular velocity
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- IR:
-
Insulin resistance
- IVS:
-
Interventricular septum
- LA:
-
Left atrial
- LDL-C:
-
Low-density lipoprotein cholesterol
- LV:
-
Left ventricular
- LVDD:
-
LV diastolic diameter
- PWT:
-
Posterior wall thickness
- RWT:
-
Relative wall thickness
- SBP:
-
Systolic blood pressure
- SDS:
-
Standard deviation score
- TG:
-
Triglycerides
- WC:
-
Waist circumference
References
Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS (2006) Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol 47:2357–2363
Alpert MA, Lambert CR, Panayiotou H, Terry BE, Cohen MV, Massey CV, Hashimi MW, Mukerji V (1995) Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am J Cardiol 76:1194–1197
Alpoim PN, Sousa LP, Mota AP, Rios DR, Dusse LM (2015) Asymmetric dimethylarginine (ADMA) in cardiovascular and renal disease. Clin Chim Acta Int J Clin Chem 440C:36–39
Antonini-Canterin F, Mateescu AD, Vriz O, La Carrubba S, Di Bello V, Carerj S, Zito C, Sparacino L, Marzano B, Usurelu C et al (2014) Cardiac structure and function and insulin resistance in morbidly obese patients: does superobesity play an additional role? Cardiology 127:144–151
Ayer JG, Almafragy HS, Patel AA, Hellyer RL, Celermajer DS (2008) Body mass index is an independent determinant of left atrial size. Heart Lung Circ 17:19–24
Ayer JG, Sholler GF, Celermajer DS (2010) Left atrial size increases with body mass index in children. Int J Cardiol 141:61–67
Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS (2004) Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc 79:1008–1014
Cacciari E, Milani S, Balsamo A, Spada E, Bona G, Cavallo L, Cerutti F, Gargantini L, Greggio N, Tonini G et al (2006) Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Investig 29:581–593
Chiarelli F, Marcovecchio ML (2008) Insulin resistance and obesity in childhood. Eur J Endocrinol 159(Suppl 1):S67–S74
Chiavaroli V, Diesse L, de Giorgis T, Giannini C, Marcovecchio ML, Chiarelli F, Mohn A (2014) Is asymmetric dimethylarginine associated with being born small and large for gestational age? Antioxid Redox Signal 20:2317–2322
Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM (2013) Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol 62:1309–1319
Crowley DI, Khoury PR, Urbina EM, Ippisch HM, Kimball TR (2011) Cardiovascular impact of the pediatric obesity epidemic: higher left ventricular mass is related to higher body mass index. J Pediatr 158:709–714, e701
D’Adamo E, Impicciatore M, Capanna R, Loredana Marcovecchio M, Masuccio FG, Chiarelli F, Mohn AA (2008) Liver steatosis in obese prepubertal children: a possible role of insulin resistance. Obesity (Silver Spring) 16:677–683
Daniels SR, Loggie JM, Khoury P, Kimball TR (1998) Left ventricular geometry and severe left ventricular hypertrophy in children and adolescents with essential hypertension. Circulation 97:1907–1911
Daniels SR, Witt SA, Glascock B, Khoury PR, Kimball TR (2002) Left atrial size in children with hypertension: the influence of obesity, blood pressure, and left ventricular mass. J Pediatr 141:186–190
de Giorgis T, Marcovecchio ML, Di Giovanni I, Giannini C, Chiavaroli V, Chiarelli F, Mohn A (2014) Triglycerides-to-HDL ratio as a new marker of endothelial dysfunction in obese prepubertal children. Eur J Endocrinol 170:173–180
De Marco S, Marcovecchio ML, Caniglia D, De Leonibus C, Chiarelli F, Mohn A (2014) Circulating asymmetric dimethylarginine and lipid profile in pre-pubertal children with growth hormone deficiency: effect of 12-month growth hormone replacement therapy. Growth Hormon IGF Res 24:216–220
de Simone G, Daniels SR, Devereux RB, Meyer RA, Roman MJ, de Divitiis O, Alderman MH (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20:1251–1260
Dencker M, Thorsson O, Karlsson MK, Linden C, Andersen LB, Wollmer P (2012) Body fat, abdominal fat, and body fat distribution is related to left atrial diameter in young children. Obesity (Silver Spring) 20:1104–1108
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458
Di Bello V, Santini F, Di Cori A, Pucci A, Palagi C, Delle Donne MG, Fierabracci P, Marsili A, Talini E, Giannetti M et al (2006) Obesity cardiomyopathy: is it a reality? An ultrasonic tissue characterization study. J Am Soc Echocardiogr 19:1063–1071
Giannini C, de Giorgis T, Scarinci A, Cataldo I, Marcovecchio ML, Chiarelli F, Mohn A (2009) Increased carotid intima-media thickness in pre-pubertal children with constitutional leanness and severe obesity: the speculative role of insulin sensitivity, oxidant status, and chronic inflammation. Eur J Endocrinol 161:73–80
Giannini C, de Giorgis T, Scarinci A, Ciampani M, Marcovecchio ML, Chiarelli F, Mohn A (2008) Obese related effects of inflammatory markers and insulin resistance on increased carotid intima media thickness in pre-pubertal children. Atherosclerosis 197:448–456
Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, Pierpont B, Weiss R (2011) The triglyceride-to-HDL cholesterol ratio: association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 34:1869–1874
Gunnell DJ, Frankel SJ, Nanchahal K, Peters TJ, Davey Smith G (1998) Childhood obesity and adult cardiovascular mortality: a 57-y follow-up study based on the Boyd Orr cohort. Am J Clin Nutr 67:1111–1118
Hirschler V, Acebo HL, Fernandez GB, de Lujan Calcagno M, Gonzalez C, Jadzinsky M (2006) Influence of obesity and insulin resistance on left atrial size in children. Pediatr Diabetes 7:39–44
Hirschler V, Acebo HL, Fernandez GB, Ferradas S, Oestreicher K (2012) Association between left atrial size and measures of adiposity among normal adolescent boys. Pediatr Cardiol 33:245–251
Imai CM, Gunnarsdottir I, Gudnason V, Aspelund T, Birgisdottir BE, Thorsdottir I, Halldorsson TI (2014) Faster increase in body mass index between ages 8 and 13 is associated with risk factors for cardiovascular morbidity and mortality. Nutr Metab Cardiovasc Dis 24:730–736
Ippisch HM, Inge TH, Daniels SR, Wang B, Khoury PR, Witt SA, Glascock BJ, Garcia VF, Kimball TR (2008) Reversibility of cardiac abnormalities in morbidly obese adolescents. J Am Coll Cardiol 51:1342–1348
Lieb W, Benndorf RA, Benjamin EJ, Sullivan LM, Maas R, Xanthakis V, Schwedhelm E, Aragam J, Schulze F, Boger RH et al (2009) Plasma asymmetric dimethylarginine, L-arginine and left ventricular structure and function in a community-based sample. Atherosclerosis 204:282–287
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T (2010) Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr 23:465–495, quiz 576–467
Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, Zimmer M, Mende M, Linke A, Kiess W et al (2014) Childhood obesity: impact on cardiac geometry and function. J Am Coll Cardiol Img 7:1198–1205
Manrique C, Lastra G, Sowers JR (2014) New insights into insulin action and resistance in the vasculature. Ann N Y Acad Sci 1311:138–150
Marcovecchio ML, Patricelli L, Zito M, Capanna R, Ciampani M, Chiarelli F, Mohn A (2006) Ambulatory blood pressure monitoring in obese children: role of insulin resistance. J Hypertens 24:2431–2436
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH (1992) Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med 327:1350–1355
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193
Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, Brodsky M, Barrell P, Greene HL (2005) Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. J Am Coll Cardiol 45:2026–2033
Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, Dence C, Klein S, Marsala J, Meyer T et al (2004) Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation 109:2191–2196
Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR (2011) Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol 57:1368–1374
Sanchez AA, Singh GK (2014) Early ventricular remodeling and dysfunction in obese children and adolescents. Curr Treat Options Cardiovasc Med 16:340
Stoddard MF, Tseuda K, Thomas M, Dillon S, Kupersmith J (1992) The influence of obesity on left ventricular filling and systolic function. Am Heart J 124:694–699
Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH (2004) Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 110:3081–3087
Young ME, McNulty P, Taegtmeyer H (2002) Adaptation and maladaptation of the heart in diabetes: part II: potential mechanisms. Circulation 105:1861–1870
Yu JJ, Yeom HH, Chung S, Park Y, Lee DH (2006) Left atrial diameters in overweight children with normal blood pressure. J Pediatr 148:321–325
Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW (1991) Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol 68:377–381
Acknowledgments
MLM is the recipient of the 2014 “L’Oréal Italia per le Donne e la Scienza” (For Women in Science) fellowship.
Authors’ contribution
MLM wrote the first draft of the manuscript, contributed to the acquisition of data, and performed statistical analysis. MG contributed to the acquisition of data and data analysis. SG supervised cardiac assessments, contributed to discussion, and reviewed the manuscript. EDA contributed to data acquisition and discussion. RDC contributed to the conception and design of the study and reviewed the manuscript. FC contributed to discussion and reviewed the manuscript. AM collected clinical data, contributed to data analysis, and reviewed the manuscript. GR contributed to the conception and design of the study, supervised the study, and reviewed the manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Beat Steinmann
Revisions received: 23 July 2015/27 July 2015
Rights and permissions
About this article
Cite this article
Marcovecchio, M.L., Gravina, M., Gallina, S. et al. Increased left atrial size in obese children and its association with insulin resistance: a pilot study. Eur J Pediatr 175, 121–130 (2016). https://doi.org/10.1007/s00431-015-2608-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2608-3