Abstract
This report demonstrates a late presenter and long-term survivor (38 months old) of alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV) and with a heterozygous frameshift mutation in FOXF1. The mild phenotype may be due to his residual normal lung tissue as demonstrated in the chest computed tomography (CT) and histopathological findings.
Conclusion: We report the longest survivor of ACD/MPV. The mild phenotype is most likely due to the patient’s residual normal lung tissue.
What is Known: • Patients with ACD/MPV uniformly die early in life. • A previously reported case with the same mutation in FOXF1 died at 15 days after birth. |
What is New: • The presented case is the longest survivor with this disease. • The patient’s mild phenotype can be explained by his residual normal lung tissue. |
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Patients with alveolar capillary dysplasia with misalignment of the pulmonary veins (ACD/MPV; OMIM 265380) usually present with respiratory distress resulting from persistent pulmonary hypertension in the early postnatal period and uniformly die early in life [3]. Recently, Stankiewicz et al. have reported genomic deletions and point mutations in the FOXF1 gene (OMIM 601089) in chromosome 16q24.1 in unrelated patients with histopathologically verified ACD/MPV [9]. So far, 42 FOXF1 variants and 24 genomic and genic deletions identified in the 16q24.1 region have been reported [7, 9, 10]. All of the patients have died in the first 4 months of life. This case report describes a patient as a late presenter and long-term survivor of ACD/MPV with a known frameshift mutation in FOXF1.
Case report
A 5-month-old boy presented with a 2-month history of cyanosis when crying and failure to thrive. He had been asymptomatic and fed well until poor weight gain was noted last month during a routine checkup. He was born at full term at a weight of 3.3 kg via caesarian section for prolonged labor. He was the first child of non-consanguineous parents without significant family history.
On presentation, the patient was crying, pale, tachypneic (60 breaths/min) with mild intercostal muscle retractions, hypoxic (70 % oxygen saturation on 2-L/min nasal cannula), and tachycardic (140 beats/min). His body temperature was 36.7 °C. Good bilateral air entry was noted on lung auscultation while regular S1 and accentuated S2 sounds without murmurs, rubs, or gallops were heard on cardiac auscultation.
Chest radiograph revealed bilateral haziness with a cardiothoracic ratio of 0.49. Chest computed tomography (CT) demonstrated bilaterally widespread ground-glass attenuation and normal density in the less affected areas (Fig. 1). Echocardiogram showed marked pulmonary hypertension estimated at more than 100 mmHg, a small atrial septal defect, ventricular septal defect in the muscular part, and mild tricuspid regurgitation.
The patient was initially treated with inhaled nitric oxide (NO) (20 ppm) and intravenous milrinone (0.3 μg/kg/min) for severe pulmonary hypertension. During the next 2 days, because the number of cyanotic episodes increased with agitation along with more pallor, diaphoresis, and lethargy, he was placed on mechanical ventilation. Methylprednisolone pulse dose therapy and intravenous immunoglobulin with empiric antibiotics were started under the clinical suspicion of interstitial lung disease.
Because the repeated echocardiogram revealed worsening of the pulmonary hypertension on the third hospital day, epoprostenol up to 11 ng/kg/min along with oral pulmonary vasodilators including sildenafil, beraprost, and bosentan was given with symptomatic improvement. Three weeks later, he was extubated although epoprostenol was continued for 2 months.
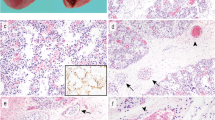
Three weeks after the mechanical ventilation, a surgical lung biopsy from the middle and lower lobes of the right lung was performed. The histopathological findings of the two different lobes of the right lung showed misalignment of the pulmonary veins next to the muscular pulmonary arteries adjacent to the airway in the same bronchiolovascular sheath (Fig. 2a). In the alveolar regions, the alveolar walls were thick with poor development of alveolar capillaries. Only several normal alveolar walls were noted in the diseased lung tissues (Fig. 2b, c). The histopathological diagnosis of ACD/MPV was made.
Histopathological findings of the right lung. a A membranous bronchiole (number sign) is accompanied by muscular pulmonary arteries (arrows) and thin-walled small veins (asterisks). The three ductal structures are situated in the common bronchiolovascular sheath. b Low- and c high-magnification photomicrographs of a secondary lobule show normal and thickened alveolar walls. In normal alveolar walls, the thickness is 1–3 μm with alveolar capillaries. In thickened alveolar walls, the thickness is 5–20 μm with an increase of stromal cells and poor development of alveolar capillaries. Hematoxylin and eosin stain. Magnification: a ×10, bar = 0.2 mm; b ×4, bar = 0.5 mm; and c ×40, bar = 0.1 mm
Genetic analysis using polymerase chain reaction (PCR) and direct DNA sequencing from the patient’s peripheral blood revealed a known heterozygous frameshift mutation c.899Tdel, p.L300RfsX79, in the first exon of FOXF1 [7]. Microarray-based comparative genomic hybridization analysis demonstrated no chromosomal deletions or duplications along the whole genome. The mutation arose de novo as it was not found in his parents.
Two months after admission, cardiac catheterization was performed with results showing a high pulmonary artery pressure (61/28 mmHg) (mean 43 mmHg) compared with femoral artery pressure (86/45 mmHg) (mean 62 mmHg), indicating improvement by the pulmonary vasodilating agents, such as the inhaled NO and epoprostenol. The patient was discharged from the hospital 2 weeks later.
At the time of writing this article, the patient (38 months old) was staying at home and was treated with oral pulmonary vasodilators and supplemental oxygen. Although his current respiratory condition has been stable, he was admitted to the hospital more than ten times for respiratory distress with congestive heart failure. Most of these events were triggered by upper and/or lower respiratory infections.
Discussion
Infants affected with ACD/MPV usually develop respiratory distress and severe pulmonary hypertension, have no sustained response to supportive treatments, and die early in life [3]. Thus far, six infants with ACD/MPV beyond the neonatal period have been reported, and the longest survivor has been an 8-month-old child [1–5, 7–9]. One report describing two sibling cases shows that a patient with less severe symptoms has a patchy pattern of capillary deficiency and abnormal distal air space, which may be correlated with the late onset [4]; however, this finding remains controversial because some reports describing histological appearance of lung tissues taken from early and late presenters have been strikingly similar [1–3, 5, 8]. All of the infants with mutations in the FOXF1 gene, the one responsible for ACD/MPV, have died in the first 4 months of life [7, 9, 10]. The case reported above, however, shows a 38-month-old boy with ACD/MPV carrying a known frameshift mutation in FOXF1. It is interesting to note that a previously reported patient with the same mutation in FOXF1 died just 15 days after birth [7]. To the best of the authors’ knowledge, this case shows the longest survivor with ACD/MPV. Recently, the FOX1 gene has been reported to be imprinted in humans [10]; it is possible that the above patient may have had the mutation in the partially imprinted paternal allele. Further studies will be needed to clarify the phenotypic difference between the above patient and the previously reported case.
The patient in the above case has been asymptomatic from birth to 3 months of age. His chest CT shows bilaterally widespread ground-glass attenuation and normal density in the less affected areas (Fig. 1). To date, only two cases with ACD/MPV have been reported to be asymptomatic during the neonatal period although no lung CT or magnetic resonance imaging is performed [1, 8]. In the above case, the CT shows areas of normal density in the bilateral lungs; the lung biopsy specimens show capillary deficiency and abnormal, patchy development of air spaces. The mild phenotype can be explained by the unique lung CT and histopathological findings.
Respiratory distress and cyanosis are the most common clinical presentation of ACD/MPV [3]. Thus far, no patient has shown any response to the available pulmonary vasodilators although transient improvement has been observed in some cases. This case’s patient, however, recovers from his pulmonary hypertension crisis by a combination therapy of inhaled NO, epoprostenol, milrinone, and oral pulmonary vasodilators. Although his current respiratory condition remains stable, he may need to undergo lung transplantation when his cardiorespiratory symptoms deteriorate. In Japan, the waiting time for a cadaveric lung transplantation is about two and a half years. No child under 5 years old, however, has received a cadaveric lung transplantation, and only two children have received a living-donor lung transplantation by the end of 2013 [6]. If the patient’s symptoms worsen, he will be treated with epoprostenol or ECMO while waiting for a living-donor lung transplantation.
Abbreviations
- ACD/MPV:
-
Alveolar capillary dysplasia with misalignment of the pulmonary veins
- CT:
-
Computed tomography
- NO:
-
Nitric oxide
- PCR:
-
Polymerase chain reaction
References
Abdallah HI, Karmazin N, Marks LA (1993) Late presentation of misalignment of lung vessels with alveolar capillary dysplasia. Crit Care Med 21:628–630
Ahmed S, Ackerman V, Faught P, Langston C (2008) Profound hypoxemia and pulmonary hypertension in a 7-month-old infant: late presentation of alveolar capillary dysplasia. Pediatr Crit Care Med 9:e43–e46
Bishop NB, Stankiewicz P, Steinhorn RH (2011) Alveolar capillary dysplasia. Am J Respir Crit Care Med 184:172–179
Boggs S, Harris MC, Hoffman DJ, Goel R, McDonald-McGinn D, Langston C, Zackai E, Ruchelli E (1994) Misalignment of pulmonary veins with alveolar capillary dysplasia: affected siblings and variable phenotypic expression. J Pediatr 124:125–128
Kodama Y, Tao K, Ishida F, Kawakami T, Tsuchiya K, Ishida K, Takemura T, Nakazawa A, Matsuoka K, Yoda H (2012) Long survival of congenital alveolar capillary dysplasia patient with NO inhalation and epoprostenol: effect of sildenafil, beraprost and bosentan. Pediatr Int 54:923–926
Sato M, Okada Y, Oto T, Minami M, Shiraishi T, Nagayasu T, Yoshino I, Chida M, Okumura M, Date H, Miyoshi S, Kondo T (2014) Registry of the Japanese Society of Lung and Heart-Lung Transplantation: official Japanese lung transplantation report, 2014. Gen Thorac Cardiovasc Surg 62:594–601
Sen P, Yang Y, Navarro C, Silva I et al (2013) Novel FOXF1 mutations in sporadic and familial cases of alveolar capillary dysplasia with misaligned pulmonary veins imply a role for its DNA binding domain. Hum Mutat 34:801–811
Shankar V, Haque A, Johnson J, Pietsch J (2006) Late presentation of alveolar capillary dysplasia in an infant. Pediatr Crit Care Med 7:177–179
Stankiewicz P, Sen P, Bhatt SS et al (2009) Genomic and genic deletions of the FOX gene cluster on 16q24.1 and inactivating mutations of FOXF1 cause alveolar capillary dysplasia and other malformations. Am J Hum Genet 84:780–791
Szafranski P, Dharmadhikari AV, Brosens E et al (2013) Small noncoding differentially methylated copy-number variants, including lncRNA genes, cause a lethal lung developmental disorder. Genome Res 23:23–33
Acknowledgments
We thank Dr. Christine Kwan for editing and revising this manuscript.
Conflict of interest
The authors declare no conflict of interest.
Authors’ contribution
YI, TD, SK, and YK treated the patient (YI wrote the manuscript under SK’s supervision, and YK took all responsibility of the therapy). MT and MK made the diagnosis using biopsy samples, and TA identified the FOXF1 mutation. KC and MY were consulted about the patient.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Beat Steinmann
Rights and permissions
About this article
Cite this article
Ito, Y., Akimoto, T., Cho, K. et al. A late presenter and long-term survivor of alveolar capillary dysplasia with misalignment of the pulmonary veins. Eur J Pediatr 174, 1123–1126 (2015). https://doi.org/10.1007/s00431-015-2543-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-015-2543-3