Abstract
Established evidence from the last decade has suggested that chronic cytomegalovirus infection has strong impact on the human immune system, resulting in aggravated aging-associated T-cell changes that are associated with poorer vaccination responses, cardiovascular disease and shortened survival. Patients with end-stage renal disease (ESRD), the most severe form of chronic kidney disease, exhibit premature aging phenotypes in almost all organ systems, including the immune system. Longitudinal studies of T-cell aging in healthy humans have been scanty because it requires a large number of study subjects and a study duration for decades. In recent years, it became clear that ESRD patients with cytomegalovirus (CMV) infection exhibit enhanced aging-related immune changes than CMV-seropositive individuals without renal disease, including chronic inflammation, decreased numbers of naïve CD4+ and CD8+ T cells, increased clonality of memory T cells with skewed repertoire and shortened telomeres. These findings lead to the hypothesis that the uremic milieu and treatment for renal failure can lead to premature aging of T cells independent from CMV infection and suggest that ESRD can be an important disease model for studying human aging. Future studies deciphering the underlying mechanisms of accelerated T cell aging in ESRD patients may eventually reveal additional insights into T-cell persistence and function during aging in CMV-seropositive, non-ESRD individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human immune system consistently interacts with microorganisms, and reacts dynamically throughout our entire life time. There are measurable differences in several aspects of the immune system when comparison is made between the younger and the older individuals. These changes were initially termed as “immunosenescence” due to the earliest findings that primarily focused on senescence of cell replications in the elderly. The term was soon challenged by later studies extended to the more diversified immune cell characteristics and to the interaction between immune cells and microorganisms [1]. In humans, T lymphocytes are by far the most studied aspect of immune system in immune aging, and probably one of the aspects that are found to have the most characteristic changes. Some evidence has implied that the most significant life-long T-cell changes are mediated by latent cytomegalovirus (CMV) infection, an infection that had long been thought as largely harmless, albeit highly prevalent in the world (> 50% in some developed countries and > 90% for others) [2, 3].
End-stage renal disease (ESRD), estimated to affect more than 1.5 million people worldwide [4], is a debilitating illness associated with high risks of life-threatening comorbidities. The combination of comorbidities associated with ESRD, such as chronic inflammation, increasing risks of vasculopathy, muscle wasting, infection, and cancer, mimic aging-related physiological changes and risk profiles [5]. Recent studies of ESRD also suggested some resemblance with aging-related subcellular changes, such as increase in DNA damages and shortening of telomere lengths [5]. Emerging studies have also attempted to characterize the T-cell changes in ESRD. In this review, we summarize the collective evidence on T-cell changes in patients with ESRD patients, the resemblance with physiological T-cell aging, and current evidence on the role of CMV in these T-cell changes.
Definition of T-cell aging in humans
Based on surface markers, T cells can be characterized into subsets according to the level of differentiation. From the least to the most differentiated, these subsets are broadly classified into naïve T cells (CD45RA+ CCR7+), central memory T cells (CD45RA− CCR7+), effector memory T cells (CD45RA− CCR7−) and effector memory T cells with RA re-expression (TEMRA cells, CD45RA+ CCR7−). The most differentiated T cells are sometimes referred to as the T cells of the most “aged” phenotype. At least in the peripheral blood, the proportion of naïve T cell decreases markedly in both CD4+ and CD8+ cell populations with increasing age, while other subsets proportionally increased [6]. Some phenotype markers on T cells, such as CD28 and CD27, are found to be lost gradually with increasing age or during repetitive T cell re-stimulation. In contrast, T cell markers such as CD57 and KLRG-1 progressively increase with increasing age. Telomere length of T cells also seems to decrease with increasing age or correlate with more aging T cell profile, such as a greater extent of differentiation or loss of CD28 [7].
Possible role of CMV on T-cell aging in the general population
CMV largely causes life-long latent infection but intermittent breakthrough reactivations are thought to occur. With its ability to present additional antigen burden each time CMV reactivates, the human immunity is under increasing pressure during each reactivation. It has been estimated that in CMV-exposed individuals, as many as 10% of CD8+ T cells and CD4+ T cells are CMV-specific [8]. Although older individuals are consistently found to have smaller proportion of naïve cells in both CD4+ and CD8+ T-cell population compared to younger individuals [9, 10], it is worth noting that this smaller proportion of naïve CD4+ cells associated with aging is not attributed to a decrease in the absolute number of naïve CD4+ cells. Instead, recently it became evident that the age-related reduction in the absolute number of naïve CD4+ cells does not happen during normal aging but only happens in CMV-seropositive individuals. It seems that the absolute number of CD4+ naïve-T cells in CMV-seronegative individuals remains steady throughout life [11], and so the age-related reduction in the proportion of CD4+ naïve-T cells in CMV-seronegative individuals was mainly due to an expansion of other T cells, i.e., memory T cells. For CD8+ cells, however, there was reduction in the absolute number of CD8+ naïve-T cells in older individuals [12] regardless of CMV serostatus, probably due to decreased homeostatic proliferation [13].
The memory cell expansion with increasing age is more obvious in CMV-seropositive individuals than in CMV-seronegative individuals, and this increase is mostly seen in the most differentiated effector memory and terminal effector or TEMRA subsets of memory T cells [14]. As a result, effector memory and TEMRA cell numbers are higher in CMV-seropositive individuals when compared to age-matched seronegative controls [10]. Of note, a large proportion of CMV-specific T cells belongs to the effector memory and TEMRA subsets and comprise a large proportion of cells in these T cell subsets [8, 15]. These cells, either CD45RA+ or CD45RA−, are commonly found to be CD4+ CD28− effector cells and were only found in CMV-seropositive individuals [16]. Memory-cell inflation may lead to shrinkage and clonality of the total T-cell repertoire, although the physiological impact of this change is uncertain [1]. The expansion of memory cells might also be more pronounced in patients with higher level of CMV-IgG, which was speculated to correlate with more frequent CMV reactivations [11].
Current evidence of T-cell aging in ESRD patients and the possible role of CMV
Previous studies based on small numbers of patients with ESRD have shown specific T-cell changes in ESRD at relatively young ages that mimic physiological or CMV-related T-cell aging among older but otherwise healthy individuals. For example, reports from the Netherlands had suggested that patients with ESRD have a lower naïve-to-memory cell ratio [17,18,19], shorter telomere length and higher level of senescent markers [20] compared to age-matched healthy individuals. There was also expansion of CD28− cells and increase in CD57+ cells in patients with ESRD [17]. We recently reported the baseline characteristics from The Immunity in ESRD Study (iESRD) in Taiwan, the largest prospective cohort of patients with ESRD so far, that aimed to characterize both T cell and other immune functions and associated morbidities in patients with ESRD [21]. With more than 400 hemodialysis-dependent patients, we found that patients with ESRD have a smaller absolute number of naïve CD4+ and CD8+ T cells, a greater absolute number of memory T cells, and a more advanced differentiation profile in memory T cells when compared to age-matched healthy individuals (Both ESRD patients and healthy individuals were almost 100% CMV-seropositive). The acceleration of T-cell aging in patients with ESRD was studied in relation to the duration of dialysis. In a recent study it was found that the level of CD28− cells in patients receiving regular peritoneal dialysis was lower than in patients receiving regular hemodialysis, but there was a large difference in the duration of dialysis dependency (63 versus 33 months) [22]. The Taiwan iESRD study have also suggested enhancement of memory-T cell expansion related to increasing duration of hemodialysis [21] and related to p-cresyl sulfate [23, 24], a uremic toxin associated with increased risk for cardiovascular disease.
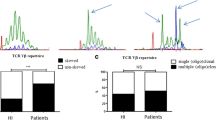
A diverse T-cell receptor (TCR) Vβ repertoire is central to effective T-cell mediated immune responses to antigens. It was found that, age, CMV serostatus and ESRD were independently associated with an increase in shifting of the TCR Vβ repertoire [25]. A higher proportion of ESRD patients (68.9%) had a skewed TCR Vβ repertoire compared to age and CMV serostatus-matched healthy individuals (31.4%). The clonal expansions were predominantly observed within the CD8+ T-cell compartment, and may even be observed in the T cells with naïve phenotype [26].
The sample sizes and designs in published studies so far, including the largest iESRD, are not yet sufficient either for providing concluding evidence that distinguishes CMV-dependent T-cell aging from CMV-independent T-cell aging among patients with ESRD. In the iESRD study, all hemodialysis patients and control individuals were CMV-seropositive; as a result, it is not possible to investigate the CMV-independent effect of T-cell aging in ESRD, which has to be studied simultaneously in CMV-seronegative ESRD individuals. We only found two small reports [17, 18] in which the comparison was made. The acceleration of T cell aging in ESRD can be quantified by comparing T-cell aging characteristics between patients with CMV-seronegative ESRD and healthy controls at different ages. In some reports patients and healthy controls were stratified into younger and older ESRD patients. However, since comparisons were made across dozens of T-cell subtypes (by CD4, CD8, and memory cell differentiations) at a typical stratified sample size between 10 and 50, the risk of false positive and false negative results is high [17, 18], and so interpretation of multiple testing results should be cautious. It is also important to note that the expansion of CD28− was only observed in CMV-seropositive but not in CMV-seronegative patients with ESRD [27], thus ESRD by itself might not promote the expansion of CD4+ CD28− cells.
Impacts of premature T-cell aging on comorbidities in ESRD patients
Cardiovascular events are the main cause of premature death in ESRD [28]. In the general population premature T-cell aging is associated with vasculopathies, and recent studies both in human and in mice have indicated that effector memory-T cells and TEMRA cells can directly damage endothelial cells and promote atherosclerosis [29,30,31]. The association between T-cell aging and adverse cardiovascular profiles in ESRD has also been demonstrated in the larger iESRD study [21] and in other smaller studies [32,33,34], and it is increasingly hypothesized that some cardiovascular events in ESRD are also related to aging-associated immune changes. CMV might play a role in this association between T-cell aging and increased risk of cardiovascular comorbidities among ESRD patients. Hypothetically, patients with ESRD may have immune changes that increase the risk of CMV infection or reactivation, and the increased exposure to CMV antigens may lead to amplification or aggravation of CMV-mediated T-cell aging. It is hoped that by identifying and mitigating these CMV-mediated T-cell aging, some cardiovascular consequences could be prevented or reversed [35].
In studies for the impact of CMV on clinical outcomes in ESRD, CMV-IgG has been the main CMV-specific immune change investigated, although CMV-IgG reflects previous CMV exposure but not reactivation [36, 37]. Consistent with aging in the normal population [38], patients with ESRD were found to have higher level of plasma CMV-IgG compared to healthy individuals in the iESRD study [39], and a higher level of CMV-IgG have been associated with advanced T-cell differentiation and coronary artery disease [39]. Longitudinal studies are required to confirm the causal relationship with more detailed characterization of CMV reactivation and CMV-IgG levels.
Is impaired T-cell effector function also a feature of T-cell aging?
T-cell effector function is a critical aspect of protective immunity. A more effective immune response during chronic infection is thought to be mediated by polyfunctional T cells, which are capable of expressing multiple effector function cytokines such as IL-2, TNFα, and IFNγ [40, 41]. In contrast, accumulation or clonal expansion of non-polyfunctional T cells (or exhausted T cells), often in replicative senescence [42], tends to be seen in suboptimal immune responses, for example in chronic active human immunodeficiency virus [43] and hepatitis C virus [44] infections. In the normal elderly, polyfunctional CMV-specific CD4+ and CD8+ T-cells persist and their functional profiles are surprisingly similar [45]. Although it has been proposed that with increasing age, T-cell function may gradually deteriorate [46] and non-functional CMV-specific T cells may predominate [47], later studies in the general population did not indicate grossly measurable functional impairment of antigen-specific T cells against either acute or latent infections among CMV-seropositive elderly individuals [48,49,50].
The apparent changes in the amount and the proportion of naïve and memory T cells among younger patients with ESRD have been thought to be compatible with T-cell aging in the general population, and so it has also been questioned whether the effector function may deteriorate during T-cell aging in ESRD, and whether this is CMV-related. For example, a possible DUSP6-mediated defect in ERK phosphorylation and an age-associated reduction in CD69 expression were identified in CD4+ T cells during T cell receptor (TCR) activation among a small group of patients with ESRD [51]. In the preliminary findings of Taiwan iESRD study (all CMV-seropositive), there was lower level cytokine secretion of IFNγ and TNFα in T cells among patients with ESRD compared to age-matched healthy individuals (unpublished preliminary results). Therefore, it remains possible that some qualitative difference in T-cell function may exist in ESRD-related T-cell aging and physiological aging, but further mechanistic studies are needed to understand the molecular regulation in T cells in ESRD patients in the face of aging and CMV infection.
Hypothetical mechanistic model of the effects of CMV infection in ESRD
Figure 1 outlines the hypothetical mechanism underlying CMV-induced immunosenescence and its role in ESRD patients. When compared with non-ESRD individuals, patients with ESRD have presumably more frequent viral reactivations (or higher antigen expression levels), which lead to higher levels of plasma CMV-IgG, aggravated immunosenescence and effector memory-T cell expansion. Subsequently, reactivations of CMV viruses in the vascular endothelium combined with effector memory-T cell expansion may directly cause vascular injury. In addition, the uremic milieu, composed of high levels of inflammatory cytokines and uremic toxins, collaboratively promote more advanced T-cell differentiation and atherosclerosis. These changes, acting simultaneously or interactively, contribute to the high prevalence of infectious complications, cardiovascular diseases and premature mortality in ESRD.
Hypothetical mechanistic model of how chronic CMV infection may lead to premature mortality in ESRD patients. Compared to individuals without renal disease, chronic CMV infection in ESRD patients leads to a higher level of anti-CMV-IgG and higher degree of T-cell differentiation (represented as “immunosenescence” in the figure) and increased risk for cardiovascular and infectious complications. Uremic toxins and inflammatory cytokines collaboratively promote the processes. Certain potential pathways were suggested based on the findings in CMV-seropositive individuals without renal disease because parallel evidence in ESRD patients is still lacking
Conclusion and future direction
In all, there is accumulating evidence suggesting parallel CMV-dependent findings between the T-cell changes in ESRD and the T-cell changes commonly seen in healthy individuals at older ages. The underlying drive of this apparent accelerated T-cell aging is not fully understood, and the extent of this parallelism requires future investigations. Future research directions in ESRD-related immune changes include other important aspects of aging-related biological changes such as tissue-resident memory cells, regulatory T cells and epigenetic control of T-cell aging. Given the sustained chronic inflammation and compatible aging-related T-cell phenotypic changes in ESRD patients, ESRD might also be an ideal disease model to study human T-cell aging and associated morbidities.
Change history
09 June 2019
Unfortunately in the original article the first author name incorrectly published as TienYu Yang. The correct name is TienYu Owen Yang.
References
Sansoni P, Vescovini R, Fagnoni FF, Akbar A, Arens R, Chiu YL, Cicin-Sain L, Dechanet-Merville J, Derhovanessian E, Ferrando-Martinez S, Franceschi C, Frasca D, Fulop T, Furman D, Gkrania-Klotsas E, Goodrum F, Grubeck-Loebenstein B, Hurme M, Kern F, Lilleri D, Lopez-Botet M, Maier AB, Marandu T, Marchant A, Mathei C, Moss P, Muntasell A, Remmerswaal EB, Riddell NE, Rothe K, Sauce D, Shin EC, Simanek AM, Smithey MJ, Soderberg-Naucler C, Solana R, Thomas PG, van Lier R, Pawelec G, Nikolich-Zugich J (2014) New advances in CMV and immunosenescence. Exp Gerontol. https://doi.org/10.1016/j.exger.2014.03.020
Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE (2011) Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 6(2):e16103. https://doi.org/10.1371/journal.pone.0016103
Lachmann R, Loenenbach A, Waterboer T, Brenner N, Pawlita M, Michel A, Thamm M, Poethko-Muller C, Wichmann O, Wiese-Posselt M (2018) Cytomegalovirus (CMV) seroprevalence in the adult population of Germany. PLoS One 13(7):e0200267. https://doi.org/10.1371/journal.pone.0200267
Thomas B, Wulf S, Bikbov B, Perico N, Cortinovis M, Courville de Vaccaro K, Flaxman A, Peterson H, Delossantos A, Haring D, Mehrotra R, Himmelfarb J, Remuzzi G, Murray C, Naghavi M (2015) Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol 26(11):2621–2633. https://doi.org/10.1681/ASN.2014101017
Kooman JP, Kotanko P, Schols AM, Shiels PG, Stenvinkel P (2014) Chronic kidney disease and premature ageing. Nat Rev Nephrol 10(12):732–742. https://doi.org/10.1038/nrneph.2014.185
Fulop T, Larbi A, Pawelec G (2013) Human T cell aging and the impact of persistent viral infections. Front Immunol 4:271. https://doi.org/10.3389/fimmu.2013.00271
Akbar AN, Vukmanovic-Stejic M (2007) Telomerase in T lymphocytes: use it and lose it? J Immunol 178(11):6689–6694
Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ (2005) Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 202(5):673–685. https://doi.org/10.1084/jem.20050882
Czesnikiewicz-Guzik M, Lee WW, Cui D, Hiruma Y, Lamar DL, Yang ZZ, Ouslander JG, Weyand CM, Goronzy JJ (2008) T cell subset-specific susceptibility to aging. Clin Immunol 127(1):107–118. https://doi.org/10.1016/j.clim.2007.12.002
Weinberger B, Lazuardi L, Weiskirchner I, Keller M, Neuner C, Fischer KH, Neuman B, Wurzner R, Grubeck-Loebenstein B (2007) Healthy aging and latent infection with CMV lead to distinct changes in CD8+ and CD4+ T-cell subsets in the elderly. Hum Immunol 68(2):86–90. https://doi.org/10.1016/j.humimm.2006.10.019
Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Zugich D, Kaye J, Nikolich-Zugich J (2014) Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol 192(5):2143–2155. https://doi.org/10.4049/jimmunol.1301721
Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, Casti A, Franceschi C, Passeri M, Sansoni P (2000) Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood 95(9):2860–2868
Goronzy JJ, Weyand CM (2017) Successful and maladaptive T cell aging. Immunity 46(3):364–378. https://doi.org/10.1016/j.immuni.2017.03.010
Le Saux S, Weyand CM, Goronzy JJ (2012) Mechanisms of immunosenescence: lessons from models of accelerated immune aging. Ann N Y Acad Sci 1247:69–82. https://doi.org/10.1111/j.1749-6632.2011.06297.x
Pita-Lopez ML, Gayoso I, DelaRosa O, Casado JG, Alonso C, Munoz-Gomariz E, Tarazona R, Solana R (2009) Effect of ageing on CMV-specific CD8 T cells from CMV seropositive healthy donors. Immun Ageing 6:11. https://doi.org/10.1186/1742-4933-6-11
Libri V, Azevedo RI, Jackson SE, Di Mitri D, Lachmann R, Fuhrmann S, Vukmanovic-Stejic M, Yong K, Battistini L, Kern F, Soares MV, Akbar AN (2011) Cytomegalovirus infection induces the accumulation of short-lived, multifunctional CD4+ CD45RA + CD27 + T cells: the potential involvement of interleukin-7 in this process. Immunology 132(3):326–339. https://doi.org/10.1111/j.1365-2567.2010.03386.x
Meijers RW, Litjens NH, de Wit EA, Langerak AW, van der Spek A, Baan CC, Weimar W, Betjes MG (2013) Cytomegalovirus contributes partly to uraemia-associated premature immunological ageing of the T cell compartment. Clin Exp Immunol 174(3):424–432. https://doi.org/10.1111/cei.12188
Litjens NH, de Wit EA, Betjes MG (2011) Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing 8(1):2. https://doi.org/10.1186/1742-4933-8-2
Huang L, Langerak AW, Baan CC, Litjens NH, Betjes MG (2016) Latency for cytomegalovirus impacts T cell ageing significantly in elderly end-stage renal disease patients. Clin Exp Immunol 186(2):239–248. https://doi.org/10.1111/cei.12846
Betjes MG, Langerak AW, van der Spek A, de Wit EA, Litjens NH (2011) Premature aging of circulating T cells in patients with end-stage renal disease. Kidney Int 80(2):208–217. https://doi.org/10.1038/ki.2011.110
Chiu YL, Shu KH, Yang FJ, Chou TY, Chen PM, Lay FY, Pan SY, Lin CJ, Litjens NHR, Betjes MGH, Bermudez S, Kao KC, Chia JS, Wang G, Peng YS, Chuang YF (2018) A comprehensive characterization of aggravated aging-related changes in T lymphocytes and monocytes in end-stage renal disease: the iESRD study. Immun Ageing 15:27. https://doi.org/10.1186/s12979-018-0131-x
Ducloux D, Legendre M, Bamoulid J, Rebibou JM, Saas P, Courivaud C, Crepin T (2018) ESRD-associated immune phenotype depends on dialysis modality and iron status: clinical implications. Immun Ageing 15:16. https://doi.org/10.1186/s12979-018-0121-z
Wu IW, Hsu KH, Hsu HJ, Lee CC, Sun CY, Tsai CJ, Wu MS (2012) Serum free p-cresyl sulfate levels predict cardiovascular and all-cause mortality in elderly hemodialysis patients—a prospective cohort study. Nephrol Dial Transplant 27(3):1169–1175. https://doi.org/10.1093/ndt/gfr453
Duranton F, Cohen G, De Smet R, Rodriguez M, Jankowski J, Vanholder R, Argiles A, European Uremic Toxin Work G (2012) Normal and pathologic concentrations of uremic toxins. J Am Soc Nephrol 23(7):1258–1270. https://doi.org/10.1681/ASN.2011121175
Huang L, Langerak AW, Wolvers-Tettero IL, Meijers RW, Baan CC, Litjens NH, Betjes MG (2015) End stage renal disease patients have a skewed T cell receptor Vbeta repertoire. Immun Ageing 12:28. https://doi.org/10.1186/s12979-015-0055-7
Huang L, Betjes MGH, Klepper M, Langerak AW, Baan CC, Litjens NHR (2017) End-stage renal disease causes skewing in the TCR Vbeta-repertoire primarily within CD8(+) T cell subsets. Front Immunol 8:1826. https://doi.org/10.3389/fimmu.2017.01826
Betjes MG, Huisman M, Weimar W, Litjens NH (2008) Expansion of cytolytic CD4+ CD28− T cells in end-stage renal disease. Kidney Int 74(6):760–767. https://doi.org/10.1038/ki.2008.301
Foley RN, Parfrey PS, Sarnak MJ (1998) Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 9(12 Suppl):S16–S23
Olson NC, Doyle MF, Jenny NS, Huber SA, Psaty BM, Kronmal RA, Tracy RP (2013) Decreased naive and increased memory CD4(+) T cells are associated with subclinical atherosclerosis: the multi-ethnic study of atherosclerosis. PLoS One 8(8):e71498. https://doi.org/10.1371/journal.pone.0071498
Ammirati E, Cianflone D, Vecchio V, Banfi M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A, Garlaschelli K, Manfredi AA, Catapano AL, Maseri A, Palini AG, Norata GD (2012) Effector memory T cells are associated with atherosclerosis in humans and animal models. J Am Heart Assoc 1(1):27–41. https://doi.org/10.1161/JAHA.111.000125
van de Berg PJ, Yong SL, Remmerswaal EB, van Lier RA, ten Berge IJ (2012) Cytomegalovirus-induced effector T cells cause endothelial cell damage. Clin Vaccine Immunol CVI 19(5):772–779. https://doi.org/10.1128/CVI.00011-12
Betjes MG, Litjens NH, Zietse R (2007) Seropositivity for cytomegalovirus in patients with end-stage renal disease is strongly associated with atherosclerotic disease. Nephrol Dial Transplant 22(11):3298–3303. https://doi.org/10.1093/ndt/gfm348
Buyukhatipoglu H, Tiryaki O, Tahta K, Usalan C (2007) Inflammation as a risk factor for carotid intimal-medial thickening, a measure of subclinical atherosclerosis in haemodialysis patients: the role of chlamydia and cytomegalovirus infection. Nephrology (Carlton) 12(1):25–32. https://doi.org/10.1111/j.1440-1797.2006.00742.x
Betjes MG, de Wit EE, Weimar W, Litjens NH (2010) Circulating pro-inflammatory CD4posCD28null T cells are independently associated with cardiovascular disease in ESRD patients. Nephrol Dial Transplant 25(11):3640–3646. https://doi.org/10.1093/ndt/gfq203
Aiello AE, Chiu YL, Frasca D (2017) How does cytomegalovirus factor into diseases of aging and vaccine responses, and by what mechanisms? GeroScience 39(3):261–271. https://doi.org/10.1007/s11357-017-9983-9
Gomez-Mora E, Massanella M, Garcia E, Giles D, Bernado M, Urrea V, Carrillo J, Ouchi D, Puig J, Negredo E, Clotet B, Blanco J, Cabrera C (2017) Elevated humoral response to cytomegalovirus in HIV-infected individuals with poor CD4+ T-cell immune recovery. PLoS One 12(9):e0184433. https://doi.org/10.1371/journal.pone.0184433
Mansfield SA, Dwivedi V, Elgharably H, Griessl M, Zimmerman PD, Limaye AP, Cook CH (2019) Cytomegalovirus immunoglobulin G titers do not predict reactivation risk in immunocompetent hosts. J Med Virol. https://doi.org/10.1002/jmv.25389
Parry HM, Zuo J, Frumento G, Mirajkar N, Inman C, Edwards E, Griffiths M, Pratt G, Moss P (2016) Cytomegalovirus viral load within blood increases markedly in healthy people over the age of 70 years. Immun Ageing 13:1. https://doi.org/10.1186/s12979-015-0056-6
Yang FJ, Shu KH, Chen HY, Chen IY, Lay FY, Chuang YF, Wu CS, Tsai WC, Peng YS, Hsu SP, Chiang CK, Wang G, Chiu YL (2018) Anti-cytomegalovirus IgG antibody titer is positively associated with advanced T cell differentiation and coronary artery disease in end-stage renal disease. Immun Ageing 15:15. https://doi.org/10.1186/s12979-018-0120-0
Seder RA, Darrah PA, Roederer M (2008) T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol 8(4):247–258. https://doi.org/10.1038/nri2274
Larsen M, Sauce D, Arnaud L, Fastenackels S, Appay V, Gorochov G (2012) Evaluating cellular polyfunctionality with a novel polyfunctionality index. PLoS One 7(7):e42403. https://doi.org/10.1371/journal.pone.0042403
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12(6):492–499
Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, Kwan R, Mican JM, Davey RT Jr, Connors M (2009) Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol 83(22):11876–11889. https://doi.org/10.1128/JVI.01153-09
Rodrigue-Gervais IG, Rigsby H, Jouan L, Sauve D, Sekaly RP, Willems B, Lamarre D (2010) Dendritic cell inhibition is connected to exhaustion of CD8+ T cell polyfunctionality during chronic hepatitis C virus infection. J Immunol 184(6):3134–3144. https://doi.org/10.4049/jimmunol.0902522
Chiu YL, Lin CH, Sung BY, Chuang YF, Schneck JP, Kern F, Pawelec G, Wang GC (2016) Cytotoxic polyfunctionality maturation of cytomegalovirus-pp65-specific CD4+ and CD8+ T-cell responses in older adults positively correlates with response size. Sci Rep 6:19227. https://doi.org/10.1038/srep19227
Liu B, Carle KW, Whisler RL (1997) Reductions in the activation of ERK and JNK are associated with decreased IL-2 production in T cells from elderly humans stimulated by the TCR/CD3 complex and costimulatory signals. Cell Immunol 182(2):79–88
Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G (2004) Dysfunctional CMV-specific CD8(+) T cells accumulate in the elderly. Exp Gerontol 39(4):607–613. https://doi.org/10.1016/j.exger.2003.11.016
Lelic A, Verschoor CP, Ventresca M, Parsons R, Evelegh C, Bowdish D, Betts MR, Loeb MB, Bramson JL (2012) The polyfunctionality of human memory CD8+ T cells elicited by acute and chronic virus infections is not influenced by age. PLoS Pathog 8(12):e1003076. https://doi.org/10.1371/journal.ppat.1003076
Van Epps P, Banks R, Aung H, Betts MR, Canaday DH (2014) Age-related differences in polyfunctional T cell responses. Immun Ageing 11:14. https://doi.org/10.1186/1742-4933-11-14
Bajwa M, Vita S, Vescovini R, Larsen M, Sansoni P, Terrazzini N, Caserta S, Thomas D, Davies KA, Smith H, Kern F (2016) Functional diversity of cytomegalovirus-specific T cells is maintained in older people and significantly associated with protein specificity and response size. J Infect Dis 214(9):1430–1437. https://doi.org/10.1093/infdis/jiw371
Huang L, Litjens NHR, Kannegieter NM, Klepper M, Baan CC, Betjes MGH (2017) pERK-dependent defective TCR-mediated activation of CD4(+) T cells in end-stage renal disease patients. Immun Ageing 14:14. https://doi.org/10.1186/s12979-017-0096-1
Acknowledgements
The authors thank Mr. Ethan Chiu for his assistance with the graphic abstract.
Funding
This work was supported by Far Eastern Memorial Hospital Grant FEMH-2015-C-007, Ministry of Science and Technology Grant 104-2314-B-418-017, 105-2314-B-418-002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest related to this manuscript or the iESRD study.
Ethical approval
All procedures performed in the iESRD study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration.
Informed consent
All authors consent to the publication of this final version of manuscript.
Additional information
Edited by: Matthias J. Reddehase.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Special Issue on Immunological Imprinting during Chronic Viral Infection.
Rights and permissions
About this article
Cite this article
Yang, T.O., Chuang, YF. & Chiu, YL. T-cell aging in end-stage renal disease: an evolving story with CMV. Med Microbiol Immunol 208, 281–287 (2019). https://doi.org/10.1007/s00430-019-00596-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00596-8