Abstract
Streptococcuspneumoniae, or pneumococcus, is a major respiratory-tract pathogen that causes high levels of mortality and morbidity in infants and elderly individuals. Despite the development of various capsular polysaccharide vaccines to prevent pneumococcal disease, it remains epidemic. Pneumococcal surface protein A (PspA) is a highly immunogenic surface protein existing in all strains of S. pneumoniae, and it can elicit immunizing protection against pneumococcal infection. In our previous studies, a fusion protein (PsaA-PspA23), consisting of PspA and pneumococcal surface antigen A (PsaA), displayed greater immunogenicity and provided better protection in mice against S. pneumoniae strains than either PsaA or PspA. In this study, the fusion protein PsaA-PspA23, together with PspA4, was formulated with four adjuvants Al(OH)3, MF59, AS03, and AS02, and subsequently subjected to dose optimization and immunological evaluation for determination of the antibody titers, bacterial burden, survival rates, and levels of cytokines in mice. All vaccines with high adjuvant doses displayed higher antigen-specific immunoglobulin G (IgG) titers. Bacterial burdens were notably decreased to different extents in the lungs and blood of mice immunized with the antigen and various adjuvants. Among these adjuvants, AS02 provided outstanding protection against challenge with pathogenic bacteria from different families and clades; it also induced high titers of IgG1 and IgG2a. Moreover, only AS02 elicited high levels of cytokines, such as TNF-α, IFN-γ, IL-2, and IL-4. These results suggest that PsaA-PspA23 and PspA4 formulated with AS02 may potentially be used as a subunit vaccine against deadly pneumococcal infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pneumoniae is the major pathogen causing pneumonia, otitis media, ocular infections, bacteremia, and sinusitis [1, 2]. Approximately 1.6 million people worldwide die each year from pneumococcal disease [3]. Moreover, pneumococcal disease results in death in almost 20% of infected children under 5 years of age [4]. The multitude of capsular polysaccharides and their conjugated vaccines have been developed to protect people from the invasion of pneumococcal strains. The most widely used capsular polysaccharide vaccine is Pneumovax 23 (PPV23). However, due to the weak immunogenicity of T-independent antigens, the high morbidity ratio of children and elderly people remains a challenge even after immunization with capsular polysaccharide vaccines [1, 5]. Currently, the main polysaccharide-conjugated vaccines include 7-valent pneumococcal conjugate vaccine (PCV7) and 13-valent pneumococcal conjugate vaccine (PCV13). These vaccines have been proven to protect children from strains of the serotypes covered by those vaccines [6]. However, PCV7 and PCV13 have a limited influence on pneumococcal disease caused by the serotypes not covered by the vaccines [7]. To address these problems, it is crucial to develop an effective and broad-spectrum vaccine to protect against S. pneumoniae.
In recent decades, a number of S. pneumoniae virulence factors have been identified, such as pneumolysin and many cell-surface choline-binding proteins [8,9,10]. Among these proteins, pneumococcal surface protein A (PspA) serves to inhibit complement deposition on the bacterial cell surface; thus, it is one of the major choline-binding virulence factors of pneumococci. Therefore, PspA may potentially be used as a target antigen to restrain S. pneumoniae. PspA is classified into three families based on the sequence variability at its N-terminus: family 1 comprises clades 1 and 2; family 2 comprises clades 3, 4, and 5; and family 3 presently only comprises clade 6 [11,12,13]. More importantly, PspA is a T cell-dependent antigen and has strong immunogenicity [14]; therefore, it can induce a better immunization effect in infants. To achieve complete coverage, it is hypothesized that a PspA-based vaccine should contain at least one PspA from each of the two major families (1 and 2). Our study and previous studies have revealed that parenteral immunization of mice with a recombinant PspA from family 2 (clades 3, 4, and 5) could induce effective protection against lethal pneumococcal strains expressing PspA from families 1 and 2 [15]. Antibodies generated against PspA are highly cross-reactive [16] and cross-protective. The major cross-protective epitopes are located in the N-terminal alpha-helical sequence of PspA, especially in the first and last 100 amino acids [17]. Pneumococcal surface antigen A (PsaA) is another antigen candidate, because it is a conserved lipoprotein present in all pneumococcal strains. PsaA plays several important roles in pneumococcal virulence, such as manganese transport, resistance to oxidative stress, and bacterial adhesion. The efficiency of the PsaA antigen in eliciting an immune response against S. pneumoniae infection has been evaluated in some studies, either as a protein or a recombinant plasmid [18, 19]. In our previous study, we constructed a fusion protein of PsaA-PspA23 containing the adhesion region of PsaA, the N-terminal clade-defining region of PspA3, and full-length PspA2; we found that PsaA-PspA23 plus an Al(OH)3 adjuvant triggered a high antibody titer in mice and conferred effective protection against PspA family-1 and family-2 strains, regardless of serotype [20]. To further improve protective efficiency, we attempted to include the full length of PspA4 into the PsaA-PspA23 formulation in this study. In addition, a series of commercial adjuvants for human use were evaluated to illustrate their potential to enhance antigen immunogenicity and protection efficiency.
It is well known that adjuvants have been widely used in various vaccines to achieve a high antibody titer, to broaden vaccine response, and/or to overcome immune senescence [21]. Al(OH)3 alum-based adjuvant is the oldest type in use, and it has shown great potential to induce Th2-biased immune responses. Moreover, Al(OH)3 alum adjuvant is comparatively safe, although some rare immune responses, such as cytotoxic T lymphocytes or granulomas at the injection site, have been reported [22]. The mechanisms of Al(OH)3 alum adjuvant are complicated and may vary depending on specific vaccine formulations. The mechanisms involve induction of danger signals, enhancement of antigen presentation, and recruitment of immune cells.
In addition to Al(OH)3 alum adjuvant, squalene-based emulsion adjuvants have attracted wide attention due to their safety and the potential benefits of dose sparing, broadened immune response, and high efficiency in vaccines for the elderly. Squalene-based emulsion adjuvants include MF59, AS03, and AS02. MF59 is a typical, milk-white, oil-in-water emulsion that measures 160 nm and consists of squalene, span85, and tween 80 [23, 24]. Since 1992, MF59-adjuvanted influenza vaccines have been licensed worldwide, and many other MF59-adjuvanted vaccines are in the clinical-study phase [25]. The main target of MF59 is to enhance antigen presentation after intramuscular injection via activation of tissue-resident monocytes, macrophages, and dendritic cells, followed by chemokine-mediated recruitment and differentiation of various immune cells [23, 26]. Similar to MF59, AS03 is an oil-in-water emulsion adjuvant, but it consists of the surfactant tween 80 and two oils: squalene and DL-α-tocopherol [27, 28]. AS03 is reported to induce transient NF-κB, cytokine and chemokine responses and results in an increased recruitment of innate immune cells from the bloodstream. This leads to monocyte activation and improved antigen presentation, which is then followed by the migration of antigen-presenting cells to draining lymph nodes [29]. Another advantage of AS03 is that it enhances the recruitment of innate immune cells in local drainage lymph nodes [30, 31]. Both MF59 and AS03 contribute to Th1 and Th2 immune responses. However, the addition of different immunomodulatory molecules may lead to a Th1- or Th2-biased immune response. For example, AS02, an adjuvant system consisting of a squalene-based oil-in-water emulsion and potent immune stimulants, 3-O-desacyl-4-monophosphory lipid A (MPL) and saponin QS21 (Quillaja saponaria Moline, fraction 21), has been reported to induce a Th1-biased immune response [32]. However, it should be noted that use of different antigens may influence the subsequent immune response [33].
In this study, the fusion protein PsaA-PspA23, together with PspA4, was formulated with four different adjuvants [Al(OH)3, MF59, AS02, and AS03], then assessed to determine the optimal adjuvant dose. The resulting vaccines were further evaluated and compared to illustrate their potential in terms of antibody titers, bacterial burden, and survival rates. Moreover, cytokine levels and antigen-specific antibody titers of IgG subclasses in mice were assessed to reveal possible differences among these vaccines. This study provides valuable information regarding adjuvant selection and development of a pneumococcal subunit vaccine.
Materials and methods
Bacterial strains and culture conditions
The strains used in this study were ATCC 6312 (PspA family 1, clade 1), ATCC 10,813 (PspA family 1, clade 2), ATCC BAA-334 (PspA family 2, clade 3), and ATCC 6303 (PspA family 2, Clade 5). All were provided by the American Type Culture Collection (Manassas, VA, USA). S. pneumoniae bacteria were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) at 37 °C without shaking. Two hours after stocks were maintained at -80 °C in a THY medium containing 10% glycerol, the bacteria were then diluted and cultured on blood agar plates at 37 °C in the presence of 5% CO2 overnight. Resulting colony numbers were then counted to determine colony-forming units (CFU).
Mice
BALB/c female mice 4–6 weeks old weighing 16–20 g were procured from the Changchun Institute of Biological Products. Mice were housed in the IVC animal facilities at Jilin University (Changchun, China) and provided with abundant food and water throughout the study. All mouse experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
Vaccine preparation
The antigen consisted of PsaA-PspA23 and PspA4. The fusion protein PsaA-PspA23 included the adhesion area of PsaA, the N-terminal clade-defining region of PspA3 (derived from strain EF3296), and full-length PspA2 (derived from strain Rx1). The construction of PsaA-PspA23 has been described in detail in our previous study [20]. The protein PspA4 (derived from strain EF5668) containing the N-terminal domain and the proline-rich region of PspA (clade 4), was subcloned into the pET-20b expression vector (Novagen, Darmstadt, Germany) which added a histidine tag to the C-terminal of the amino-acid sequence, and expressed in Escherichia coli. Both recombinant antigens were expressed in E. coli and purified using a nickel affinity chromatography column. The purity of the protein was evaluated with an SDS–PAGE gel that was scanned using near-infrared imaging (Odyssey system; LI-COR, Lincoln, NE, USA). Adjuvants used in this study (listed in Table 1) were sterile phosphate-buffered saline (PBS) solution mixed with Al(OH)3, citrate buffer solution (pH 6.5) with MF59, and 50 mM PBS solution (pH 6.8) with AS02 and AS03. Oil-in-water emulsion adjuvants MF59, AS02, and AS03 were prepared under high-pressure homogenization in a Microfluidizer (M-110L; Microfluidics, Newton, MA, USA), in accordance with reported protocols [23]. The particle sizes and polydispersity indexes of MF59, AS03, and AS02 were determined using dynamic light scattering (NanoZS, Malvern Instruments, Malvern, UK). Particle sizes of these three adjuvant formulations range from 150 to 160 nm, and all polydispersity indexes were less than 0.15. Each standard dose of MF59, AS02, and AS03 per mouse used in this study contained 2.15 mg squalene. One standard dose of AS02 per mouse used in this study also contained 20 µg MPL and 15 µg QS21.
Immunization and challenge
Groups of six wild-type BalB/c mice were immunized intramuscularly with a 100 µl vaccine containing 5 µg PsaA-PspA23, 20 µg PspA4, and adjuvants, or 100 µl buffer with Al(OH)3 as a negative control. The PPV23 vaccine was injected as a positive control; mice receiving this vaccine were subcutaneously immunized only once with 100 µl per mouse on day 14. This was done to follow the protocol for human immunization, using one-fifth of the human dose. In the other group, the immunization regimen was followed on days 14 and 28; a bacterial challenge was administered on day 42. The mice used for challenges were anesthetized and intranasally administered 40 µl total of 4 × 106 CFU pneumococcal strains ATCC 6312 (clade 1, family 1), 7 × 105 CFU ATCC 10,813 (clade 2, family 1), 2 × 107 CFU ATCC BAA-334 (clade 3, family 2), and 4 × 105 CFU ATCC 6303 (clade 2, family 2) (shown in Table 2). The mortality rate was monitored for 2 weeks after challenge.
Detection of specific IgG, IgG1, and IgG2a levels in serum
The blood of the mice was sampled by orbital puncture 14 days after the final immunization. Sera were obtained via centrifugation and stored at − 20 °C. Antibody titers were detected using the enzyme-linked immunosorbent assay (ELISA). The 96-well plates were coated with 0.5 µg purified PsaA-PspA23 or PspA4 protein per well, and HRP-anti-mouse IgG, IgG1, and IgG2a were used for the detection of corresponding antibody levels in serum samples. The sample optical density (OD) was observed at 450 nm. The antibody titer was determined by the absorbance of a dilution ratio that was twofold greater than the average OD of the blank group.
Quantification of bacterial burden
Mice were immunized and challenged with strains ATCC 10,813 and ATCC BAA-334. The dose of challenge was 2 × 105 CFU per mouse, which was far lower than the lethal dose. Bacteria were recovered from the blood and lung at 24 h post-challenge. Mice immunized with antigen plus adjuvants, PBS, or PPV23 were sacrificed by cervical dislocation at 24-h post-challenge. The lungs and blood were collected aseptically in PBS, and the lung tissue was homogenized using a burnisher. Samples were diluted tenfold, and 100 µl of every second dilution was cultured on blood agar plates. Plates were incubated at 37 °C in the presence of 5% CO2 for 20 h. Finally, bacterial CFU were counted.
Cytokine measurement
On the 14th day after the final immunization, the mice were euthanized through an approved Institutional Animal Care and Use Committee procedure: they were sprayed with 75% ethanol. The skin was removed and an incision made in the mid-abdomen near the ventral side. The spleen was collected aseptically in RPMI 1640 medium, then placed in a sterile RPMI 1640 1X medium after tissue separation. To split erythrocytes, a lysis buffer (ammonium–chloride–potassium) was added. Five minutes later, the reaction was terminated with RPMI 1640 medium. Collected spleen cells were centrifuged at 300 g for 5 min, then washed with RPMI 1640 three times. Samples containing 1 × 107 spleen cells were mixed with 5 µl PspA (containing 1 µg PsaA-PspA23, 4 µg PspA4), 5 µl ConA (containing 5 µg ConA) as a positive control, or 5 µl PBS as a negative control. The spleen cells were diluted and cultured on RPMI 1640 with 10% fetal bovine serum at 37 °C in the presence of 5% CO2 for 48 h. Then, samples were assessed in accordance with the instructions from the BD Cytometric Bead Array (CBA) Mouse Th1/Th2Cytokine kit (IL-2, IL-4, TNF-α, and IFN-γ) (BD, Franklin Lakes, NJ, USA).
Statistical analysis
All statistical analyses were performed using GraphPad Prism Software (GraphPad Software, La Jolla, CA, USA) and SPSS 17.0. Statistical data for the antibody titers and cytokine concentration were assessed using a one-way analysis of variance or an unpaired, two-tailed Student’s t-test. Results were expressed as the mean ± standard error of the mean (SEM). Statistical data for the bacterial burden were assessed using a Dunnett’s post-hoc test, and the results were expressed as the dot plots. Statistical data for the survival rate were performed by log-rank Mantel–Cox test. Furthermore, we did not perform a Bonferroni correction or any other procedure that controls the family-wise error rate for the whole paper. All p values are exploratory and none of the results are significant in a strict statistical sense.
Results
Preparation of antigens
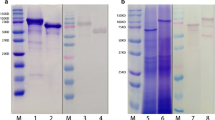
Fusion protein PsaA-PspA23 consists of the adhesion region of PsaA, the N-terminal clade-defining region of PspA3 (derived from strain EF3296), and the full length of PspA2 [20]. Protein PspA4 contained the N-terminal domain and the proline-rich region domain of PspA clade 4. They were expressed in E. coli using the pET-20b expression vector, proteins were purified through Nickel affinity chromatography and analyzed using SDS–PAGE (Fig. 1). The purities of PsaA-Pspa23 and PspA4 were over 85% (measured with Odyssey), and molecular masses were approximately 90 kD and 65 kD, respectively.
SDS–PAGE analysis of PsaA-PspA23 and PspA4 antigens expressed in Escherichia coli. The proteins were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and were detected using direct staining with Coomassie Brilliant Blue. lane 1, Precision Plus Protein Standards Dual Color (BIO-RAD) in kD; lane 2, purified PsaA-PspA23; lane 3, purified PspA4
Effect of adjuvant dose on serum antibody titer
To ensure a suitable dose of adjuvant, mice were inoculated with the antigen at same amount plus adjuvants of different doses. As a control, some mice were immunized with adjuvants only. Sera of the mice were extracted from the vascular plexus of the orbit, 2 weeks after each immunization. The antibody titers of IgG against PspA23 and PspA4 were detected in the serum using ELISA. The results are shown in Fig. 2. No free antigens were detected in the presence of 3% Al(OH)3 adjuvant, suggesting complete adsorption of antigens onto the Al(OH)3 interface. For both PsaA-PspA23- and PspA4-specific antibodies, an increasing dose of Al(OH)3 adjuvant resulted in higher IgG titers after final boost (p < 0.05). Similar to Al(OH)3, AS02, and MF59 displayed higher antibody titers in high-dose adjuvant groups (p < 0.05) after final boost, compared with those of medium- and low-dose adjuvant groups. In the AS03 group, the middle and high doses resulted in comparative IgG titer levels at all sampling points, except that of PsaA-PspA23 at the final boost. Based on these results, we used high-dose Al(OH)3, high-dose MF59, high-dose AS02, and medium-dose AS03 in the subsequent studies to test their potentials for enhancing immunogenicity and immune protection efficiency. In these selected groups, antigens plus adjuvants showed an obvious increase in both anti-PsaA-PspA23 antibody titers (Fig. 2a) and anti-PspA4 antibody titers (Fig. 2b) after the boost (p < 0.05). The increasing antibody titers indicate that memory B-cell response was strengthened via the prime-boost immunization strategy. Furthermore, all adjuvanted vaccines elicited significantly higher IgG titers than negative controls, suggesting an enhanced immune response in the presence of adjuvants and antigen. In addition, all antigen plus adjuvants groups exhibited similar levels of antigen-specific IgG titers after final boost. These results indicate that all four adjuvants used in combination with the antigens PsaA-PspA23 and PspA4 could incite a potent antigen-specific IgG immune response.
PsaA-PspA23 (a) and PspA4 (b)-specific IgG titers in sera of mice immunized with antigen and different doses of Al(OH)3, MF59, AS02, and AS03. Al(OH)3 was used at two different dose levels of 30 µg and 100 µg, respectively. Each standard dose of MF59, AS02 and AS03 per mouse used in this study contained 2.15 mg squalene. One standard dose of AS02 per mouse used in this study also contained 20 µg MPL and 15 µg QS21. The low dose was 50% of the standard dose, the middle dose was standard dose, and the high dose was 150% of the standard dose. The sera of all mice in each group (six mice per group) were mixed into a serum pool and evaluated three times separately by ELISA. Symbols indicate statistically significant differences compared with corresponding controls by one-way ANOVA, followed by Tukey’s multicomparison test. For comparison of the high-dose group with the low-dose group: #p < 0.05. For comparison with the negative-control group: *p < 0.05
Reduction of bacterial burden in the lungs and blood of immunized mice
All groups inoculated with antigen plus adjuvants showed strong systemic immune responses in the serum after immunization. Thus, we evaluated whether the improved IgG titers could protect mice from bacterial infection. It is well known that more than 96% of isolated pneumococcal strains express family 1 or family 2 PspA [34,35,36]. For this reason, we chose ATCC BAA-334 (clade 3; family 2) and ATCC 10,813 (clade 2; family 1) to challenge mice via intranasal administration. Bacteria were recovered from the blood and lungs 24 h after challenge. The numbers of bacteria (CFU/ml) in the blood and lung homogenates of each group are shown in Fig. 3. The numbers of bacteria (CFU/ml) in the blood and lungs of mice immunized with antigen plus adjuvants were less than those of the negative control mice, which suggests the adjuvanted vaccines resulted in effective bacterial clearance (p < 0.05). However, four adjuvanted vaccines, as well as the commercially available PPV23 vaccine, displayed variable efficiency in reducing the bacterial burden in both the blood and lungs of mice. For strain ATCC BAA-334 (clade 3; family 2), use of AS02 resulted in better bacterial clearance in the blood than did use of MF59; this level of clearance is similar to that of PPV23. Use of AS03 and Al(OH)3 resulted in marginal bacterial clearance in the blood; both were less efficient than PPV23. Regarding ATCC BAA-334 burden in the lungs, use of Al(OH)3 and PPV23 resulted in significant bacterial clearance, and both were more efficient than MF59, AS02, and AS03.
Bacterial burden in the blood and lungs of mice. Samples after challenge with two bacterial strains for the group of antigen plus adjuvants, negative control, and positive control (PPV23) group. The density of pneumococcal colonization, expressed in log10 of total colony-forming units (CFU) in the lungs and blood, was determined for individual mice at 24 h after challenge. a, b Burden of strain ATCC BAA-334 (family 2, clade 3) in blood and lungs, respectively; c, d burden of strain ATCC 10,813 (family 1, clade 2) in blood and lungs. Six mice per group were examined in each challenge. Statistical difference with negative controls by one-way ANOVA, with Dunnett’s post-hoc test, *P < 0.05, **P < 0.01, ***P < 0.001 versus NC group, and p values shown in this Figure. Results are from two independent experiments
For strain ATCC 10,813 (family 1, clade 2), use of AS02 and MF59 eliminated 100% of the bacteria in the blood, followed by AS03 and Al(OH)3. This clearance trend differed from that observed for ATCC BAA-334, as use of AS03 resulted in higher clearance than Al(OH)3. However, both AS03 and Al(OH)3 were more efficient than PPV23. Obvious superiority of Al(OH)3, MF59, AS02, and AS03 over PPV23 in reducing the bacterial burden was also found in the lung tissue for strain ATCC 10,813. MF59 provided the highest bacterial clearance, followed by Al(OH)3. The different bacterial clearance efficiencies observed for the same adjuvant to different strains may be due to the diversity of pneumococcus [37, 38]. Additionally, different tendencies among pneumococcus strains to colonize the blood and lungs after an intranasal challenge may make bacterial clearance more complicated.
Protection against pneumococci challenge
Although high IgG titers and effective bacterial clearance are important for prevention of pneumococcal infection, the most direct method to evaluate the effectiveness of a pneumococcal vaccine is through challenge with S. pneumoniae. In this study, vaccine effectiveness was determined by increased survival. Two weeks after the last immunization, the immunized mice were challenged intranasally with four pneumococcal strains of PspA family 1 and family 2. Mortality was monitored for 14 days. The survival percentages by group are shown in Fig. 4.
Vaccine-induced protection against lethal intranasal challenge. Mice were immunized subcutaneously three times at 2-week intervals with PspAs in combination with the adjuvant. Two weeks after the last immunization, the immunized mice were challenged intranasally with pneumococcal strains containing PspA family 1 and family 2. Mortality was monitored for 14 days. Six mice per group were examined in each challenge. Statistical difference of test vaccine groups from the negative controls by log-rank Mantel–Cox test: **P < 0.01, ***P < 0.005
The survival rate increased in the groups of mice that received Al(OH)3-, MF59-, AS02-, and AS03-adjuvanted vaccines, compared with those who received corresponding adjuvants and Ppv23. After challenge with ATCC 6312, Al(OH)3 and AS02 showed better protection than MF59 and AS03, compared with non-vaccinated, non-adjuvant, and PPV23 groups. Protection against ATCC 10,813 was 100%, except in the non-vaccinated groups. The protection provided by the antigen with AS02 against ATCC BAA-334 reached only 50%, which remained higher than the general protection against this strain in all other groups. When challenged with ATCC 6303, survival in the Al(OH)3 and AS02 groups was 100%. Furthermore, protection appeared in the negative group, which may be due to relatively low doses of bacteria and relatively weak infectivity of these bacteria.
These results reveal that the antigen with four different adjuvants may provide effective protection against pneumococcal strains from both family 1 and family 2; thus, it shows the potential for broad-spectrum protection. The protection rate of the AS02 group was outstanding against four strains in a different clade.
Measurement of IgG isotypes
To further illustrate the possible differences among vaccines containing the different adjuvants, titers of antigen-specific IgG1 and IgG2a antibodies were determined by ELISA. The results are shown in Fig. 5.
PsaA-PspA23- (a) and PspA4- (b) specific IgG isotype antibody titers in sera of mice immunized with PBS, antigen, and antigen with Al(OH)3, MF59, AS02, and AS03. Sera were obtained after immunization with adjuvant plus antigen at 14 days after final boost, then determined by ELISA. Six mice per group were examined in each group. Statistical difference of IgG1 titer from the IgG2a titer: *P < 0.05; **P < 0.01 and p values shown in this figure
For PsaA-PspA23, groups immunized with adjuvant-containing preparations exhibited higher IgG1 and IgG2a titers than those immunized with antigen alone, suggesting an improved immunogenicity of antigen in the presence of adjuvants. Among these, Al(OH)3, MF59, and AS03 showed comparable efficiency in inducing IgG1; AS03 showed lower efficiency. High IgG1 titer usually indicates a strong Th2 immune response. In contrast, AS02 was more effective in inducing higher titers of IgG2a antibodies than other adjuvants. The above tendency was also observed in the case of PspA4 (Fig. 5b). The high antibody titers of IgG1 and IgG2a elicited by use of AS02 indicated mixed Th1 and Th2 immune responses; this phenomenon was considerably different from the responses elicited by use of Al(OH)3 and MF59.
Production of cytokines in splenocytes
To further illustrate the possible differences resulting from various adjuvants, cytokines produced by isolated splenocytes in the mice were assessed 14 days after the final immunization. The results are shown in Fig. 6. All groups immunized with the antigen and adjuvants showed higher cytokine levels than the negative control. Among these, use of AS02 elicited higher levels of TNF-α, IFN-γ, IL-2, and IL-4 than Al(OH)3, MF59, or AS03 (p < 0.05). For TNF-α, Al(OH)3 was the second-best adjuvant, as it elicited higher cytokine levels than MF59 or AS03.
Cytokine production in murine splenocytes. Mice were immunized with PsaA-PspA23 and PspA4 in combination with various adjuvants three times at 2-week intervals. Fourteen days after the third immunization, splenocytes were collected and stimulated in vitro with PBS, PspA4, and PsaA-PspA23. Cytokine TNF-α (a), IFN-γ (b), IL-2 (c), and IL-4 (d) expression at 48 h after stimulation was detected via CBA assay. Six mice were examined in each group. P values shown in this figure
Discussion
Pneumococcus is an invasive bacterium that can cause the death of infants and the elderly. Many pneumococcal proteins have been evaluated as protein-based vaccine candidates against S. pneumoniae infection. PspA is highly immunogenic and has the potential to induce cross-reactive immunization and broad-spectrum protection [39]. However, most investigations use Al(OH)3 as an adjuvant for the PspA protein [40,41,42,43]. The obvious advantage of this adjuvant is that it rarely causes a cytotoxic T lymphocyte response or granulomas at the injection site. However, the efficiency of Al(OH)3 in stimulating antibodies and enhancing the immune response is unsatisfactory. Therefore, it is important to seek an alternative adjuvant.
In this study, dose optimization of the four adjuvants was first performed by evaluating antigen-specific IgG titers. Then, the optimized doses of high-dose Al(OH)3, high-dose MF59, high-dose AS02, and medium-dose AS03 were chosen to formulate with antigen candidates PsaA-PspA23 and PspA4. Evaluations of immunogenicity and immune protection, in terms of IgG titer, bacterial burden, and survival rate, were then carried out. Cytokines and IgG subclass were also measured to illustrate possible differences in the types of immune response.
The results showed that antigens with four different adjuvants induced comparable antigen-specific IgG titers in sera. High IgG titers usually indicate increased immune protection against infection by pneumococcal strains. Furthermore, the presence of PspA-specific IgG titers may elicit protection by complement-dependent phagocytosis, thereby reducing the multiplication of bacteria in the blood. However, due to the diversity of pneumococcal strains and the incomplete coverage of the target antigens, efficiency of immune protection may vary. For example, in a previous report, high antibody titers against pneumococcal histidine triad protein D did not provide optimal passive protection against several pneumococcal strains [44]. Therefore, it is necessary to determine the immune protection efficiency of high PspA-specific IgG titers against different pneumococcal strains.
Further investigation confirmed that all use of formulations of antigen with adjuvants resulted in varying levels of inhibition of bacteria in lung tissue and blood and yielded higher survival rates after challenge with different pneumococcal strains, compared with the negative control and PPV23. The AS02 group showed superb protection against strains compared with other groups; it even reached 100% protection against strains ATCC 6312, 10,813, and 6303. Moreover, it showed an outstanding ability to achieve bacterial clearance in the blood during challenge with strains ATCC BAA-334 and 10,813. In the negative control group, protection against some strains (ATCC 6312, 10,813, and 6303) was also observed. This may be due to relatively low doses of bacteria and relatively weak infectivity of these bacteria. Similar phenomena have also been reported by other research groups [44, 45]. In general, the results showed a similar trend between bacterial burden and survival rate. All groups of the antigen plus adjuvant showed obvious clearance of bacterial burden when compared with the negative control. The AS02 and MF59 groups showed excellent clearance, especially in the blood; they even reached 100% clearance of ATCC 10,813, which indicated that the antigen provided 100% protection against this strain. Thus, the effectiveness of the vaccine may be evaluated by bacterial burden.
The above differences in bacterial clearance and protection efficiency may be closely related to IgG isotypes and cytokine simulation. Further investigation revealed that use of AS02 induced high titers of IgG1 and IgG2a antibodies, indicating mixed Th1 and Th2 immune responses. However, use of other adjuvants, such as Al(OH)3 and MF59, only induced high titers of IgG1 antibodies, suggesting a Th2-biased immune response. The balanced Th1 and Th2 immune responses of AS02 were further confirmed by the results of cytokine assays, which revealed that immunization with the antigen and AS02 induced higher levels of IL-2, IL-4, TNF-α, and IFN-γ, characteristic factors in Th1 and Th2 pathways. The high IgG1 and IgG2a titers, which are present in the balanced Th1 and Th2 immune response, may contribute to an efficient immune response and enhanced protection against respiratory pathogens. Further investigations, such as those involving opsonophagocytosis and complement deposition, are needed to illustrate the possible mechanism.
In conclusion, the PsaA-PspA23 and PspA4 antigens, combined with four different adjuvants, enhanced systemic immune responses and provided protection and effective elimination in the blood and lungs against pneumococcal strains from different families. The AS02 group displayed the best performance, compared with the other groups in which MF59, AS03, and Al(OH)3 were used as adjuvants. PsaA-PspA23 and PspA4 antigens plus AS02 also provided more efficient immune protection than the commercially available PPV23 vaccine. These results suggest that PsaA-PspA23 and PspA4 antigens plus AS02 may be used as a potential subunit vaccine against pneumococcal invasion. Further investigation is needed to illustrate mechanisms involved in the enhanced immune protection and the possible dose-sparing effect of various adjuvants on antigens. These insights, together with the findings in this study, may pave the way for successful development of an effective pneumococcal vaccine.
References
Pletz MW, Maus U, Krug N, Welte T, Lode H (2008) Pneumococcal vaccines: mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents 32:199–206
Pichichero ME (2017) Pneumococcal whole-cell and protein-based vaccines: changing the paradigm. Expert Rev Vac 16:1181–1190
Zhanel GG, James KD, Karlowsky WA (2015) Clinical cure rates in subjects treated with azithromycin for community-acquired respiratory tract infections caused by azithromycin-susceptible or azithromycin-resistant Streptococcus pneumoniae. J Antimicrob Chemother 3170
Bryce J, Boschi-Pinto C, Shibuya K, Black RE (2005) WHO estimates of the causes of death in children. Lancet 365:1147–1152
Lin H, Peng Y, Lin Z, Zhang S, Guo Y (2015) Development of a conjugate vaccine against invasive pneumococcal disease based on capsular polysaccharides coupled with PspA/family 1 protein of Streptococcus pneumoniae. Microb Pathogen 83–84:35–40
Shinefild RH, Steve Black M (2000) Efficacy of pneumococcal conjugate vaccines in largescale field trials. Pediatr Infect Dis J 19:394–397
Hsu HE et al (2009) Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. The New Engl J Med 360:244–256
Janulczyk R, Iannelli F, Sjoholm AG, Pozzi G, Bjorck L (2000) Hic, a novel surface protein of Streptococcus pneumoniae that interferes with complement function. J Biol Chem 275:37257–37263
Dave S, Brooks-Walter A, Pangburn MK, McDaniel LS (2001) PspC, a pneumococcal surface protein, binds human factor H. Infect Immun 69:3435–3437
McKay JT et al (2015) PD-1 suppresses protective immunity to Streptococcus pneumoniae through a B cell-intrinsic mechanism. J Immunol 194:2289–2299
Crain MJ et al. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. 58, 3293–3299 (1990)
Tu AH, Fulgham RL, McCrory MA, Briles DE, Szalai AJ. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immunol 67:4720–4724 (1999)
Croney CM, Coats MT, Nahm MH, Briles DE, Crain MJ (2012) PspA family distribution, unlike capsular serotype, remains unaltered following introduction of the heptavalent pneumococcal conjugate vaccine. Clin Vac Immunol: CVI 19:891–896
Ren B, Szalai AJ, Hollingshead SK, Briles DE (2003) Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface. Infect Immun 72:114–122
Moreno AT et al (2010) Immunization of mice with single PspA fragments induces antibodies capable of mediating complement deposition on different pneumococcal strains and cross-protection. Clin Vac Immunol 17:439–446
Briles DE et al (2000) Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis 182:1694–1701
Goulart C et al (2011) Selection of family 1 PspA molecules capable of inducing broad-ranging cross-reactivity by complement deposition and opsonophagocytosis by murine peritoneal cells. Vaccine 29:1634
Whaley MJ et al (2010) Concomitant administration of recombinant PsaA and PCV7 reduces Streptococcus pneumoniae serotype 19A colonization in a murine model. Vaccine 28:3071
Oliveira MLS et al (2006) Induction of systemic and mucosal immune response and decrease in Streptococcus pneumoniae colonization by nasal inoculation of mice with recombinant lactic acid bacteria expressing pneumococcal surface antigen A. Microbes Infection 8:1016–1024
Lu J et al (2015) Protective immune responses elicited by fusion protein containing PsaA and PspA fragments. Immunological investigations 44:482
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608
Shi L, Zhang YJ, Xue rui YI, Liu SR Cytotoxic T cell responses to hepatitis B virus (HBV) small surface antigen in mice. Chinese Journal of Nosoconmiology (2003)
Fang JH, Hora M (2000) The adjuvant MF59: a 10-year perspective gary ott. Ramachandran Radhakrishnan 42:211–228
Traquina P, Morandi M, Contorni M, Nest GV (1996) MF59 adjuvant enhances the antibody response to recombinant hepatitis B surface antigen vaccine in primates. J Infect Dis 174:1168–1175
Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A (2008) The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol 180:5402–5412
Calabro S et al (2011) Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29:1812–1823
Morel S et al (2011) Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 29:2461–2473
McElhaney JE et al (2013) AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis 13:485–496
Yam KK et al (2016) Comparison of AS03 and Alum on immune responses elicited by A/H3N2 split influenza vaccine in young, mature and aged BALB/c mice. Vaccine 34:1444–1451
Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P (2010) AS03(A)-adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 51:668–677
Moris P et al (2011) H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol 31:443–454
Rénia L et al (2009) A Randomized Trial Assessing the Safety and Immunogenicity of AS01 and AS02 Adjuvanted RTS,S Malaria Vaccine Candidates in Children in Gabon. PloS one 4:e7611
Casimiro DR et al (2010) Efficacy of multivalent adenovirus-based vaccine against simian immunodeficiency virus challenge. J Virol 84:2996–3003
Beall B, Gherardi G, Facklam RR, Hollingshead SK (2000) Pneumococcal pspA sequence types of prevalent multiresistant pneumococcal strains in the United States and of internationally disseminated clones. J Clin Microbiol 38:3663–3669
Brandileone MC et al (2004) Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine 22:3890–3896
Hollingshead SK et al (2006) Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol 55:215–221
Echenique J, Kadioglu A, Romao S, Andrew PW, Trombe MC (2004) Protein serine/threonine kinase StkP positively controls virulence and competence in Streptococcus pneumoniae. Infect Immun 72:2434–2437
Miyaji EN et al (2015) Evaluation of a vaccine formulation against Streptococcus pneumoniae based on choline-binding proteins. Clin Vac Immunol: CVI 22:213–220
María Claudia Vela Coral et al (2001) Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from colombian children. Emerg Infect Dis 7:5
Ogunniyi AD et al (2007) Contributions of pneumolysin, pneumococcal surface protein A (PspA), and PspC to pathogenicity of Streptococcus pneumoniae D39 in a mouse model. Infect Immun 75:1843–1851
Haughney SL et al (2013) Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles. Acta Biomaterialia 9:8262–8271
Nabors GS et al (2000) Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 18:1743
Oliveira MLS et al (2010) Combination of pneumococcal surface protein A (PspA) with whole cell pertussis vaccine increases protection against pneumococcal challenge in mice. PloS one 5:e10863
Leroux-Roels I et al (2015) Adjuvant system AS02V enhances humoral and cellular immune responses to pneumococcal protein PhtD vaccine in healthy young and older adults: randomised, controlled trials. Vaccine 33:577–584
Piao Z et al (2014) Protective properties of a fusion pneumococcal surface protein A (PspA) vaccine against pneumococcal challenge by five different PspA clades in mice. Vaccine 32:5607–5613
Gordon CL et al (2012) Comparison of immunoglobulin G subclass concentrations in severe community-acquired pneumonia and severe pandemic 2009 influenza A (H1N1) infection. Clin Vac Immunol 19:446–448
Ekdahl K, Braconier JH, Svanborg C (1997) Immunoglobulin deficiencies and impaired immune response to polysaccharide antigens in adult patients with recurrent community-acquired pneumonia. Scand J Infect Dis 29:401–407
Acknowledgements
We are grateful for support from Jilin Provincial Industrial Innovation Special Project for the New Vaccine Adjuvant Innovation Technology Platform (Grant No. 2018C004).
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical standards
All mouse experiments in this paper were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council).
Additional information
Edited by: B. Opitz.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, X., Li, B., Yu, J. et al. Comparison of four adjuvants revealed the strongest protection against lethal pneumococcal challenge following immunization with PsaA-PspA fusion protein and AS02 as adjuvant. Med Microbiol Immunol 208, 215–226 (2019). https://doi.org/10.1007/s00430-019-00579-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-019-00579-9