Abstract
Palatable foods can stimulate appetite without hunger, and unconstrained overeating underlies obesity and binge eating disorder. Women are more prone to obesity and binge eating than men but the neural causes of individual differences are unknown. In an animal model of hedonic eating, a prior study found that females were more susceptible than males to eat palatable food when sated and that the neuropeptide orexin/hypocretin (ORX) was crucial in both sexes. The current study examined potential extra-hypothalamic forebrain targets of ORX signaling during hedonic eating. We measured Fos induction in the cortical, thalamic, striatal, and amygdalar areas that receive substantial ORX inputs and contain their receptors in hungry and sated male and female rats during palatable (high-sucrose) food consumption. During the test, hungry rats of both sexes ate substantial amounts, and while sated males ate much less than hungry rats, sated females ate as much as hungry rats. The Fos induction analysis identified sex differences in recruitment of specific areas of the medial prefrontal cortex, paraventricular nucleus of the thalamus (PVT), nucleus accumbens (ACB), and central nucleus of the amygdala (CEA), and similar patterns across sexes in the insular cortex. There was a striking activation of the infralimbic cortex in sated males, who consumed the least amount food and unique correlations between the insular cortex, PVT, and CEA, as well as the prelimbic cortex, ACB, and CEA in sated females but not sated males. The study identified key functional circuits that may drive hedonic eating in a sex-specific manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sweet and other palatable tastes can stimulate our appetites and drive eating without hunger. Individuals differ in their propensity to respond to palatable foods when sated (Small 2009; Reppucci and Petrovich 2012; Sun et al. 2015) and understanding the underlying neurobiology is key to resolving hedonic overeating. Women are more susceptible to gain weight and to engage in binge eating than men (Hudson et al. 2007; Mitchell and Shaw 2015), and recently we examined potential behavioral and neural sex differences in an animal model of hedonic eating (Buczek et al. 2020). First, we compared palatable food (high-sucrose) consumption between hungry and sated adult Sprague Dawley rats of both sexes. Then, we examined activation and signaling of the neuropeptide orexin/hypocretin (ORX) (de Lecea et al. 1998; Sakurai et al. 1998), which supports food reward motivated behaviors (Choi et al. 2010; Mahler et al. 2014; Petrovich 2019). We found that females were more prone to hedonic eating than males and that ORX signaling is crucial in both sexes. Across multiple tests, males consumed according to their physiological hunger state prior to testing—sated ate less than food deprived. In contrast, sated females consumed similar amounts of palatable food as hungry rats. Our analysis of Fos induction patterns in hungry and sated male and female rats found that ORX neurons were activated during palatable food consumption independently of fasting. In a follow-up experiment, systemic blockade of ORX receptor 1 signaling decreased palatable food consumption in both sexes (Buczek et al. 2020).
A critical next step is to determine the brain areas where ORX signaling could drive hedonic eating in males and females, and the current study examined potential extra-hypothalamic forebrain targets. Notably, ORX fibers and receptors are widely distributed in the forebrain areas that underlie motivation and reward processing, taste and visceral networks, and associative learning and decision-making functions (Peyron et al. 1998; Trivedi et al. 1998; Marcus et al. 2001; Baldo et al. 2003; Petrovich 2019). Thus, we examined cortical, thalamic, striatal, and amygdalar areas that are involved in these processes and receive substantial inputs from ORX neurons and contain ORX receptors, in the brain tissue from the subjects in our prior study (Buczek et al. 2020). The specific subset of areas across these brain regions were chosen for analysis based on their interconnectivity (Reppucci and Petrovich 2016; Petrovich 2018, 2021). In those areas, we compared the patterns of Fos induction in hungry and sated male and female rats that were either given palatable food or not fed during tests. These groups enabled examination of the effects of consumption separately from food deprivation effects in both sexes. We analyzed forebrain activation patterns and identified the areas where Fos induction correlated with consumption and the network differences that corresponded with sex-specific behavior.
Experimental procedures
Subjects
Sixty-four experimentally naïve, Sprague Dawley rats (32 male, 32 female) were obtained from Envigo at 250–275 g. Rats were individually housed in the colony room that was maintained on a 12 h light/dark cycle (lights on 06:00), and all behavioral tests occurred during the light-phase between 09:00 and 16:00. Upon arrival and prior to any handling or habituation procedures, rats were given 24 h to acclimate to the colony room. Rats had ad libitum access to water and standard laboratory chow (Purina Lab Diet Prolab RMH 3000; calories: 26%, protein, 15% fat, 59% carbohydrates (89% starch)) except when noted otherwise. All housing and testing procedures were in compliance with the National Institute of Health’s Guidelines for the Care and Use of Laboratory Animals and were approved by the Boston College Institution Animal Care and Use Committee. One female rat (sated, no food group) died prior to the start of the experiment.
Experimental design
Behavioral procedures were described previously in detail (Buczek et al. 2020). Prior to any testing, rats were handled daily for 5–6 days and habituated to all experimental conditions, including exposures to ceramic dishes used for testing consumption of palatable food, Test Diet pellets (TD; 5TUL 45 mg Scott Pharma: 21% protein, 13% fat, 67% carbohydrates (all sucrose)). Each rat received 1 g of TD at the bottom of their home cage and on a separate occasion was exposed to an empty ceramic testing dish for 30 min. Then, rats were acclimated to consume TD pellets from the ceramic testing dishes (10 g of TD for 30 min) in their home cages, once under sated state (ad libitum access to chow prior to testing) and once after 20 h of deprivation (testing order was counter-balanced). Rats were also acclimated to transportation on a cart to and from the procedure room, where perfusions to collect brain tissue occurred.
In order to test neural activation patterns due to palatable food consumption under satiety and hunger in both sexes, rats were assigned to sated or deprived condition and to receive food at test or not (four groups per sex). For rats in the deprived condition, food was removed 20 h prior to testing, while rats in the sated condition were allowed ad libitum access to chow (n = 16 per sex). At test, half of the rats in each condition (sated or food-deprived) were given 15 g of palatable food (TD) in ceramic dishes while the other half of the rats were given empty ceramic dishes (no food) for 100 min in home cages (n = 8 per group). The 100 min testing duration was chosen to optimize test-specific Fos induction. Rats were undisturbed during testing and perfused immediately after to collect brain tissue.
Histological procedures
Immediately following the testing sessions, rats were briefly anaesthetized with isoflurane (Henry Schein, Pittsburgh, PA), intraperitoneally injected with tribromoethanol (Sigma Aldrich, St. Louis, MO) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M borate buffer. Brains were then stored for 20–24 h at 4 ℃ in a paraformaldehyde and 12% sucrose mixture, rapidly frozen in hexanes, cooled with dry ice and stored at – 80 ℃. Using a microtome, brains were cut into 30 µm coronal sections and collected in four adjacent series. One tissue series was processed with Fos immunohistochemistry, described in detail below. A second series was mounted from KPBS (0.02 M potassium phosphate-buffered saline; pH 7.4) onto gelatin-coated slides and stained with thionin for identification of cyto-architectonic borders. Another series was processed with double immunohistochemistry for detection of ORX and Fos, as described in Buczek et al.,(2020), and used for analyses of the lateral hypothalamus (Buczek et al. 2020), paraventricular thalamus, central nucleus of the amygdala and posterior agranular insular cortex (see below).
Fos immunohistochemistry
Free-floating tissue sections were rinsed three times in KPBS, incubated for 1 h at room temperature in a blocking solution (KPBS containing 2% normal goat serum (NGS; S-1000, Vector Laboratories, Burlingame, CA), 0.3% Triton X-100 (Sigma Aldrich) and 10% milk (Lab Scientific, Livingstone, NJ)), and then incubated with anti-c-Fos antibody raised in rabbit (1:5000; Synaptic Systems, 226–003) in the blocking solution for 72 h at 4 ℃ with gentle agitation. Sections were then rinsed with KPBS, 2% NGS, and 10% milk, incubated with biotinylated secondary antibody against rabbit (1:500; Vector Laboratories, BA-1000) in the blocking solution, rinsed in KPBS, incubated in avidin biotin complex (ABC; Vector Laboratories, PK-6100), rinsed in KPBS and recycled through the secondary antibody and ABC solutions with KPBS rinses in between. Nuclei for Fos were visualized as gray after a two-minute incubation in nickel-intensified 3,3’-diaminobenzidine (Vector Laboratories, SK-4100). Sections were then rinsed, mounted on SuperFrost slides (Fisher Scientific), dried at 45 °C, dehydrated through graded alcohols, cleared in xylenes, and coverslipped with DPX Mountant (Electron Microscopy Services, Hatfield, PA).
Image acquisition and analysis
Areas within the medial prefrontal cortex (PFC), the agranular insular cortex (AI), the central nucleus of the amygdala (CEA), and the paraventricular nucleus of the thalamus (PVT) were analyzed following parcellation and nomenclature derived from Swanson’s rat brain atlas (Swanson 2018). Sampling areas for the nucleus accumbens (ACB) are described below and shown in Fig. 1 (Fig. 1). The atlas levels mentioned hereafter refer to the levels in the Swanson atlas (Swanson 2018). For each area, images of Fos- and adjacent thionin-stained tissue sections were acquired bilaterally with 10 × objective on an Olympus BX51 light microscope and attached Olympus DP74 camera using DP2-BSW software (Olympus America Inc., Center Valley, PA, USA). Images of Fos-processed tissue were transformed using ImageJ software to 8-bit greyscale, and Fos-positive neurons were identifiable by distinct gray nuclear staining.
Borders for analyses of ACB subregions are shown at anterior (level 11, left) and posterior (level 14, right) levels. Templates adapted from the Swanson brain atlas (Swanson 2018)
All acquisition of images, Fos counting, and data analyses were performed by experimenters blind to the experimental conditions. The areas for analyses were demarcated either by nuclear borders drawn or by a template placed on 10 × images of thionin-stained tissue, following pre-determined criteria outlined for each region and described in detail below. The border or template was then transferred onto images of Fos-stained tissue, and automated counting of Fos-positive cells within the border or template was conducted using ImageJ. The protocol for counting was consistent across different brain areas, and the criteria were pre-determined before the start of analysis. The threshold for counting specific Fos labeling above the background was set manually, based on an area on each section with no specific labeling (background), and accuracy was confirmed by comparing automated with manual counts. Counts from left and right sides were summed for each region within each subject, then the counts from all subjects were averaged for each group to produce a mean of total Fos-positive neurons. For the PVT, left and right sides were acquired together in one image.
Within the medial PFC, the prelimbic (PL) and infralimbic (ILA) areas were analyzed at two mid-rostrocaudal levels (atlas levels 8 and 9; bregma + 3.20 and + 2.80). In order to accurately determine borders for PL and ILA (and the placement of 10 × images), images of thionin- and Fos-stained tissue were first acquired bilaterally under ×4 magnification. Two brains were excluded from analyses due to tissue damage at levels 8 and 9, and one brain (female sated food) was identified in SPSS as an outlier and excluded to correct distribution normality. After exclusions, group sizes for PFC analyses were as follows: males sated no food n = 8, males deprived no food n = 8, males sated food n = 7, males deprived food n = 8, females sated no food n = 6, females deprived no food n = 8, females sated food n = 7, and females deprived food n = 8.
The posterior AI cortex (AIp) was analyzed at atlas level 25 (bregma -1.53) using a rectangular template that conformed to anatomical borders. One brain was excluded from the analyses due to tissue damage, resulting in the following group sizes: males sated no food n = 8, males deprived no food n = 8, males sated food n = 8, males deprived food n = 8, females sated no food n = 7, females deprived no food n = 8, females sated food n = 8, and females deprived food n = 7.
Within the ACB, three subregions were analyzed at representative anterior (aACB; atlas level 11, bregma + 1.7) and posterior (pACB; atlas level 14, bregma + 0.95) levels. The three subregions for analysis, core (ACBc), dorsal shell (ACBshD) and ventral shell (ACBshV), were defined based on clearly identifiable neuroanatomical features and descriptions by Cole et al. (2015a) and in consultation with Zahm and Brog (1992), as shown in Fig. 1. The ACBc was demarcated by a line drawn ventrally from the tip of the lateral ventricle around the anterior commissure at a distance equal to half the width of the anterior commissure (Fig. 1). The ventral border of the anterior ACBc (aACBc) was demarcated at a distance from the external capsule equal to half the anterior commissure width. The ventral border for the ACBshD was a horizontal line that was aligned with the middle of the anterior commissure for the anterior ACBshD (aACBshD) and with the dorsal edge of the anterior commissure for the posterior ACBshD (pACBshD). The ACBshV was demarcated dorsally by the ventral border of the ACBshD and ventrally by Swanson atlas borders, ending at the point aligned vertically with the lateral edge of the anterior commissure. The medial border of the ACBshD and ACBshV was determined based on Swanson atlas’s medial ACB border.
Due to unavailability of tissue or tissue damage, 12 brains were excluded from analyses of the aACB, and nine brains were excluded from analyses of the pACB, resulting in group sizes as follows: anterior (males sated no food n = 6, males deprived no food n = 6, males sated food n = 5, males deprived food n = 6, females sated no food n = 6, females deprived no food n = 7, females sated food n = 8, females deprived food n = 7), posterior (males sated no food n = 7, males deprived no food n = 6, males sated food n = 6, males deprived food n = 8, females sated no food n = 5, females deprived no food n = 8, females sated food n = 7, and females deprived food n = 7). The CEA was analyzed at representative anterior (aCEA; atlas level 26, bregma − 1.78) and posterior (pCEA; atlas level 28, bregma − 2.45) levels for each subregion: capsular (CEAc), lateral (CEAl), and medial (CEAm). Five brains were excluded from aCEA analyses, and eleven brains were excluded from pCEA analyses due to tissue damage, resulting in group sizes as follows: anterior (males sated no food n = 7, males deprived no food n = 7, males sated food n = 8, males deprived food n = 8, females sated no food n = 7, females deprived no food n = 7, females sated food n = 7, females deprived food n = 6), posterior (males sated no food n = 7, males deprived no food n = 7, males sated food n = 7, males deprived food n = 7, females sated no food n = 6, females deprived no food n = 7, females sated food n = 5, and females deprived food n = 5).
Images of PVT were acquired at levels 22–33 (bregma -0.83 to -4.20). Since prior work has demonstrated that anterior (aPVT) and posterior (pPVT) parts of the PVT are distinct (Li and Kirouac 2012; Barson and Leibowitz 2015; Dong et al. 2017; Gao et al. 2020), separate analyses were conducted for the aPVT (atlas level 25, bregma − 1.53) middle PVT (mPVT; atlas level 28, bregma − 2.45), and pPVT (atlas level 31, bregma -3.70). Three brains were excluded from aPVT, pPVT and mPVT analyses due to tissue damage or poor tissue quality. One brain (male deprived no food) was excluded as an outlier from analyses of the aPVT and pPVT to correct distribution normality, and one brain (male sated food) was excluded as an outlier from analyses of the mPVT. Resulting group sizes were as follows: aPVT and pPVT (males sated no food n = 7, males deprived no food n = 6, males sated food n = 8, males deprived food n = 8, females sated no food n = 7, females deprived no food n = 8, females sated food n = 8, and females deprived food n = 7), mPVT (males sated no food n = 7, males deprived no food n = 7, males sated food n = 7, males deprived food n = 8, females sated no food n = 7, females deprived no food n = 8, females sated food n = 8, and females deprived food n = 7).
Statistical analyses
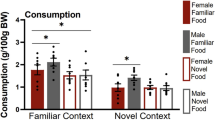
Food consumption analysis was conducted from the data in Buczek et al. (2020). Consumption was analyzed for groups that were given food at test using between-subjects 2-way univariate ANOVAs for sex and physiological state, as previously reported in Buczek et al. (2020). In addition, Bonferroni multiple comparisons were used as a follow-up to between-subjects 1-way univariate ANOVA with group (female sated, female deprived, male sated, male deprived) as the sole factor in order to directly compare consumption between each group. Consumption was analyzed both in grams and as a percentage of body weight (%BW).
Fos induction of males and females was analyzed separately in order to determine sex-specific neuronal activation patterns underlying behavioral differences. For each cortical area examined (ILA, PL, AIp), Fos induction was analyzed using between-subjects 2-way univariate ANOVAs for physiological state (deprived, sated) and presence of food at test (food, no food). For areas with multiple subregions (ACB, CEA, PVT), 2-way multivariate ANOVAs for physiological state and the presence of food at test were used to examine Fos induction either within each subregion at each anatomical level (ACB and CEA) or at each anatomical level (PVT). When appropriate, main effects were followed by Bonferroni multiple comparisons to directly compare groups sharing one condition.
A p value < 0.05 was considered significant for all analyses, except for post hoc analyses in which Bonferroni adjusted alpha level was used. A p value between 0.05 and 0.07 is referred to as trending toward significance. Data were analyzed for normality using the Shapiro–Wilk test and heterogeneity using Levene’s test. If one of these criteria was not met, logarithmic transformations (AIp, aCEAl, aCEAm, pCEAm, mPVT, pPVT), Welch’s F (aACBshD) or non-parametric Kruskal–Wallis tests (pACBshD) were used to verify reported effects. For data not depicted in figures (Table 1), the mean number of Fos-positive neurons and standard error were reported rounded to the nearest whole number. This was an exploratory study and correction for testing multiple brain areas was not done.
For regions with a significant effect of food, bivariate Pearson correlations were conducted in order to examine the relationship between Fos induction and the amount of TD consumed at test. For these analyses, all same-sex rats that were given food at test (sated and food-deprived) were combined. In addition, bivariate Pearson correlations were used to assess the relationship of Fos induction between brain regions for each group. All subregions were included in the analyses and the anterior and poster parts were not collapsed. The dorsal and ventral nucleus accumbens shell parts (ACBshD and ACBshV) were collapsed within anterior and posterior levels (aACBsh, pACBsh). The final Ns for each correlation between two regions included only the subjects that had the data for both regions; if the tissue was not available (e.g., due to damage) for one of the regions, that subject was not included in the correlation analysis (final Ns are provided in the Tables 3, 4, 5 and 6). All statistical analyses were performed using SPSS Version 24.
Results
Food consumption
Sated females ate similar, large amounts of TD as deprived females, while sated males consumed less TD than deprived males (Fig. 2). A 2-way ANOVA for the effects of sex and physiological state on the amount of TD consumed in grams at test yielded significant main effects of sex (F(1,28) = 8.496 p = 0.007) and state (F(1,28) = 25.254 p < 0.001), and a significant interaction of sex by state (F(1,28) = 6.245 p = 0.019). Similar effects were found when TD consumption values were calculated as a percentage of body-weight: sex (F(1,28) = 16.619 p < 0.001), state (F(1,28) = 27.260 p < 0.001), interaction of sex by state (F(1,28) = 5.156 p = 0.031). In addition, 1-way ANOVA for group yielded a significant main effect using either grams (F(1,28) = 13.332 p < 0.001) or %BW (F(1,28) = 16.345 p < 0.001). Post hoc analysis confirmed that sated males consumed significantly less TD at test than any other group, both in grams (p < 0.005 in each case) and as a percentage of body-weight (p ≤ 0.001 in each case), and that there were no other differences between groups (sated and deprived females were similar to deprived males).
Fos induction
Due to pronounced sex differences in consumption patterns, all Fos induction analyses were conducted separately for each sex. Since the sex differences were only in sated condition, a comprehensive comparison of patterns in each sex was deemed to be the most informative in regard to sex-specific circuits, and the most transparent way to show the neural data for each sex, regardless whether they were similar or different.
Infralimbic cortex
For females, there were no overall effects of food or state on Fos induction patterns within the ILA but there was a crossover interaction (Fig. 3). The groups with food versus no food were the opposite for sated and deprived conditions. For sated animals, Fos was slightly greater in those given food compared to those not given food at test, but for deprived animals Fos was slightly greater for those not given food compared to those given food at test (Fig. 3). Indeed, ANOVA revealed a significant interaction between state and food (F(1, 25) = 5.806 p = 0.024) but no significant main effects of food (F(1,25) = 0.010 p = 0.920) or state (F(1,25) = 0.196 p = 0.662).
Fos induction in the ILA. A Total number of Fos-positive neurons (mean ± SEM) in the ILA is shown for female and male rats across experimental conditions. Same lowercase letters indicate post hoc significant difference between groups, # indicates interaction effect. *p < 0.05; **p < 0.01; ***p < 0.001. B Images show Fos induction in the left ILA of representative brains from male sated food and male sated no food groups; scale bar = 200 μm
For males, Fos induction within the ILA was greater for sated animals given food than any other group (Fig. 3). ANOVA yielded no significant main effect of state (F(1,27) = 3.060 p = 0.092) but a significant main effect of food (F(1,27) = 4.864 p = 0.036) and interaction of state by food (F(1,27) = 18.109 p < 0.001). Post hoc analysis confirmed a significant difference between sated males given food at test compared to sated males given no food (p < 0.001) and compared to deprived males given food (p < 0.001). There were no other differences between groups.
Prelimbic cortex
For females, Fos induction within the PL was similar across groups (Table 1). There was no significant main effect of food (F(1,25) = 0.030 p = 0.863), state (F(1,25) = 0.343 p = 0.563), or interaction of state by food (F(1,25) = 3.483) p = 0.074).
For males, there were no overall effects of food or state on Fos induction within the PL but there was a crossover interaction (Table 1). Fos induction was slightly greater for sated animals given food than those not given food at test, while for deprived groups the pattern was the opposite (Table 1). There was a significant interaction between state and food (F(1,27) = 9.387 p = 0.005), but no significant main effects of state (PL, F(1,27) = 0.051 p = 0.824) or food (F(1,27) = 0.402 p = 0.531).
Posterior agranular insular cortex
For females, Fos induction in the AIp was greater for groups given food than groups not given food at test (Fig. 4). There was a significant main effect of food (F(1,26) = 9.385 p = 0.005) but no main effect of state (F(1,26) = 2.098 p = 0.159) or interaction of state by food (F(1,26) = 0.134 p = 0.717). Since AIp Fos induction values were not normally distributed, ANOVA effects were confirmed using transformed values; there was a significant main effect of food (F(1,26) = 10.101 p = 0.004) but no main effect of state (F(1,26) = 2.115 p = 0.158) or state by food interaction (F(1,26) = 0.152 p = 0.700).
Fos induction in the AIp. A Total number of Fos-positive neurons (mean ± SEM) in the AIp is shown for female and male rats across experimental conditions. *p < 0.05; **p < 0.01; ***p < 0.001. B Images show Fos induction in the right AIp of representative brains from female sated food and female sated no food groups; scale bar = 200 μm
For males, Fos induction in the AIp was greater for groups given food than groups not given food at test (Fig. 4). There was a significant main effect of food (F(1,28) = 5.690 p = 0.024) but no main effect of state (F(1,28) = 0.101 p = 0.753) or state by food interaction (F(1,28) = 0.014 p = 0.908).
Anterior nucleus accumbens
For females, Fos induction in aACBshD and aACBshV, but not in the aACBc, was greater for deprived groups than for sated groups (Fig. 5, Table 1).
Fos induction in the aACBshD. A Total number of Fos-positive neurons (mean ± SEM) in the aACBshD is shown for female and male rats across experimental conditions. *p < 0.05; **p < 0.01. B Images show Fos induction in the left aACBshD of representative brains from female deprived no food and female sated no food groups; scale bar = 200 μm
There was a main effect of state for aACBshD (F(1,24) = 9.409 p = 0.005) and aACBshV (F(1,24) = 17.616 p < 0.001), but not for the aACBc (F(1, 24) = 3.516 p = 0.073), and no main effects of food (aACBshD, F(1,24) = 0.521 p = 0.478; aACBshV, F(1,24) = 0.671 p = 0.421; aACBc, F(1, 24) = 0.292 p = 0.594) or state by food interactions (aACBshD, F(1,24) = 0.063 p = 0.804; aACBshV, F(1,24) = 0.254 p = 0.619; aACBc, F(1, 24) = 0.686 p = 0.416).
For males, Fos induction in aACBshD, but not in aACBshV or aACBc, was greater for deprived groups compared to sated groups (Fig. 5, Table 1). There was a significant main effect of state for aACBshD (F(1,23) = 4.843 p = 0.040), but not aACBshV (F(1,23) = 0.069 p = 0.795), a trend for aACBc (F(1,23) = 4.170 p = 0.055), and no significant main effects of food (aACBshD, F(1,23) = 0.076 p = 0.786; aACBshV, F(1,23) = 0.008 p = 0.930; aACBc, F(1,23) = 0.424 p = 0.523) or state by food interactions (aACBshD, F(1,23) = 0.021 p = 0.448; aACBshV, F(1,23) = 0.051 p = 0.823; aACBc, F(1,23) = 0.021 p = 0.887).
Posterior nucleus accumbens
For females, Fos induction in the pACBshD and pACBshV was greater for animals given food than those not given food at test, and in the pACBshV, Fos induction was also greater for groups in a state of deprivation (Fig. 6, Table 1). MANOVA yielded a significant main effect of food in pACBshD (F(1,23) = 7.197 p = 0.013) and pACBshV (F(1,23) = 5.745 p = 0.025) and a trend in pACBc (F(1,23) = 3.605 p = 0.070), and a significant main effect of state in the pACBshV (F(1,23) = 4.614 p = 0.042), but not pACBshD (F(1,23) = 1.168 p = 0.291) or pACBc (F(1,23) = 0.012 p = 0.913), and no significant state by food interactions in any of the regions (pACBshD, F(1,23) = 0.504 p = 0.485; pACBshV, F(1,23) = 2.506 p = 0.127; pACBc, F(1,23) = 0.132 p = 0.720).
Fos induction in the pACBshV. A Total number of Fos-positive neurons (mean ± SEM) in the pACBshV is shown for female and male rats across experimental conditions. *p < 0.05; **p < 0.01. B Images show Fos induction in the left pACBshV of representative brains from female sated food and female sated no food groups; scale bar = 200 μm
For males, Fos induction in the pACBshV, but not in pACBshD or pACBc, was greater for groups that were deprived than sated (Fig. 6, Table 1). There was a significant main effect of state in the pACBshV (F(1,23) = 11.945 p = 0.002), but not in the pACBshD (F(1,23) = 2.027 p = 0.168) or pACBc (F(1,23) = 0.702 p = 0.411), and no significant main effects of food (pACBshD, F(1,23) = 1.796 p = 0.193; pACBshV, F(1,23) = 3.434 p = 0.077; pACBc, F(1,23) = 0.683 p = 0.417) or state by food interactions (pACBshD, F(1,23) = 0.092 p = 0.764; pACBshV, F(1,23) = 0.371 p = 0.548; pACBc, F(1,23) = 0.560 p = 0.462).
Anterior central amygdala
For females, Fos induction in aCEAm and aCEAl was greater for deprived groups than sated groups. In addition, Fos induction was greater in all subregions (aCEAc, aCEAl, aCEAm) in groups that were given food compared to groups not given food at test (Fig. 7, Table 1). MANOVA yielded a significant main effect of state for aCEAm (F(1,23) = 9.196 p = 0.006) and a trend in aCEAl (F(1,23) = 4.028 p = 0.057), but no effect in aCEAc (F(1,23) = 0.191 p = 0.67), a main effect of food for all subregions (aCEAm, F(1,23) = 21.669 p < 0.001; aCEAl, F(1,23) = 54.910 p < 0.001; aCEAc, F(1,23) = 7.41 p = 0.012), and no significant state by food interactions (aCEAm, F(1,23) = 1 p = 0.328; aCEAl, F(1,23) = 1.765 p = 0.197; aCEAc, F(1,23) = 1.757 p = 0.198). Fos induction data for aCEAm and aCEAl were non-normally distributed; therefore, MANOVA results were confirmed with logarithmic transformations. A MANOVA on transformed aCEAm and aCEAl values confirmed the main effects of state and food for aCEAm (state, F(1,23) = 10.357 p = 0.004; food, F(1,23) = 35.956 p < 0.001) and the main effect of food for aCEAl (F(1,23) = 100.853 p < 0.001). The analysis of transformed aCEAl values additionally resulted in a significant main effect of state (F(1,23) = 7.448 p = 0.012).
Fos induction in the aCEAl. A Total number of Fos-positive neurons (mean ± SEM) in the aCEAl is shown for female and male rats across experimental conditions. Same lowercase letters indicate post hoc significant difference between groups. *p < 0.05; **p < 0.01; ***p < 0.001 (#0.05 < p < 0.07). B Images show Fos induction in the left aCEAl of representative brains from female sated food and female sated no food groups; arrows indicate CEAl borders, scale bar = 100 μm
For males, Fos induction in aCEAm, aCEAl, and aCEAc was greater for groups given food than no food at test, and in the aCEAl deprived groups given food had greater Fos induction than sated groups given food (Fig. 7, Table 1). There was a significant main effect of food in each subregion (aCEAm, F(1,26) = 19.983 p < 0.001; aCEAl, F(1,26) = 20.134 p < 0.001; aCEAc, F(1,26) = 12.894 p = 0.001), a trend of state in aCEAl (F(1,26) = 4.153, p = 0.052), but not aCEAm (F(1,26) = 1.151 p = 0.293) or aCEAc (F(1,26) = 0.556 p = 0.463), and a significant state by food interaction in aCEAl (F(1,26) = 4.805 p = 0.038), but not in aCEAm (F(1,26) = 1.116 p = 0.3) or aCEAc (F(1,26) = 1.61 p = 0.216). A post hoc analysis of aCEAl Fos induction revealed a significant difference between sated and deprived rats in groups given food at test (p = 0.005).
Posterior central amygdala
For females, Fos induction in pCEAl and pCEAm was greater for groups given food at test than those not given food (Fig. 8, Table 1). MANOVA yielded a significant main effect of food in pCEAl (F(1,19) = 14.360 p = 0.001) and pCEAm (F(1,19) = 26.988 p < 0.001) but not in pCEAc (F(1,19) = 1.933 p = 0.181). There was no significant main effect of state (pCEAm, F(1,19) = 0.046 p = 0.832; pCEAl, F(1,19) = 0.682 p = 0.419; pCEAc, F(1,19) = 0.147 p = 0.706) or state by food interactions (pCEAm, F(1,19) = 1.179 p = 0.291; pCEAl, F(,19) = 0.042 p = 0.839; pCEAc, F(1,19) = 1.529 p = 0.231).
Fos induction in the pCEAm. A Total number of Fos-positive neurons (mean ± SEM) in the pCEAm is shown for female and male rats across experimental conditions. Same lowercase letters indicate post hoc significant difference between groups. *p < 0.05; **p < 0.01; ***p < 0.001. B Images show Fos induction in the left pCEAm of representative brains from male deprived food and male deprived no food groups; arrows indicate CEAm borders, scale bar = 100 μm
For males, Fos induction was greater in pCEAl and pCEAm for groups given food compared to groups not given food at test, and, in pCEAm, of those given food, deprived groups had greater Fos induction compared to sated groups (Fig. 8, Table 1). There was a significant main effect of food for pCEAl (F(1,24) = 64.289 p < 0.001) and pCEAm (F(1,24) = 15.949 p = 0.001), but not pCEAc (F(1,24) = 2.669 p = 0.115), and a main effect of state for pCEAm (F(1,24) = 4.848 p = 0.038), but not pCEAl (F(1,24) = 0.096 p = 0.76) or pCEAc (F(1,24) = 0.069 p = 0.794). In addition, there was a significant interaction of state by food in pCEAm (F(1,24) = 4.848 p = 0.038) but not pCEAl (F(1,24) = 1.899 p = 0.181) or pCEAc (F(1,24) = 0.336 p = 0.567). Post hoc analysis of pCEAm Fos induction revealed a significant difference between sated and deprived rats given food at test (p = 0.005).
Paraventricular thalamus
For females, Fos induction in the aPVT and mPVT was greater for deprived than sated groups, and Fos induction in the pPVT was greater for groups given food compared to those not given food at test (Fig. 9, Table 1). MANOVA yielded a significant main effect of state for the aPVT (F(1,26) = 10.335 p = 0.003) and the mPVT (F(1,26) = 5.615 p = 0.026) and a trend in the pPVT (F(1,26) = 3.786 p = 0.063). There was no significant effect of food in the aPVT (F(1, 26) = 2.912 p = 0.100) or mPVT (F(1,26) = 0.595 p = 0.447), but the effect of food was significant in the pPVT (F(1, 26) = 5.773 p = 0.024). There was no state by food interaction at any region (aPVT, F(1, 26) = 0.111 p = 0.742; mPVT, (F(1,26) = 0.621 p = 0.438); pPVT, (F(1,26) = 0.031 p = 0.861).
Fos induction in the pPVT. A Total number of Fos-positive neurons (mean ± SEM) in the pPVT is shown for female and male rats across experimental conditions. *p < 0.05 (#0.05 < p < 0.07). B Images show Fos induction in the pPVT of representative brains from female sated food and female sated no food groups (Fos-positive neurons are gray and ORX fibers are brown); scale bar = 200 μm
For males, Fos induction in the aPVT, mPVT and pPVT was not affected by deprivation or food (Fig. 9, Table 1). There were no main effects of state (aPVT, F(1, 25) = 1.126 p = 0.299; mPVT, F(1,25) = 3.422 p = 0.076; pPVT, F(1,25) = 0.358 p = 0.555), food (aPVT, F(1,25) = 0.586 p = 0.451; mPVT, F(1,25) = 0.289 p = 0.596; pPVT, F(1,25) = 0.411 p = 0.527), or interactions of state by food (aPVT, F(1,25) = 0.744 p = 0.397; mPVT, F(1,25) = 0.341 p = 0.564; pPVT, F(1, 25) = 1.243 p = 0.275).
Correlations of Fos induction and consumption
For rats of each sex given food at test, Pearson correlations were completed using the amount of TD consumed in grams and Fos induction data from regions that yielded a significant effect of food. Significant correlations between Fos induction and consumption in grams were confirmed using the amount of TD consumed calculated as a percentage of body-weight (%BW).
For females, the aCEAl was the only region where the counts of Fos-positive neurons were significantly correlated with the amount of palatable food consumed at test (Table 2, Fig. 10). Fos induction in the aCEAl was positively correlated with the amount of TD ingested at test (grams, r = 0.629 p = 0.021; %BW, r = 0.707 p = 0.007).
For males, the counts of Fos-positive neurons in the ILA, aCEAm and aCEAl were correlated with palatable food consumption at test (Table 2, Fig. 10). In the ILA, Fos induction was negatively correlated with the amount of TD ingested at test (grams, r = − 0.727 p = 0.002; %BW, r = − 0.715 p = 0.003), while in the aCEAm and aCEAl, the correlations were positive (aCEAm: grams, r = 0.532 p = 0.034; %BW, r = 0.530 p = 0.035; aCEAl: grams, r = 0.569 r = 0.021; %BW, r = 0.571 p = 0.021).
Correlations of Fos induction between brain regions
Fos induction correlations examined relationships across brain regions and subregions in each group. The correlations for female groups given food at test are shown in Table 3. For sated females given food (left/below diagonal, Table 3), Fos induction values (the number of Fos-positive neurons) in the PL were correlated with the nucleus accumbens core (aACBc) and central nucleus of the amygdala (pCEAc), and the aACBc and pCEAc were also correlated. The AIp was correlated with the central amygdala (aCEAm and cCEAc) and aPVT. The subregions of the CEA and PVT were correlated (pCEAm with mPVT, and aCEAc with aPVT and pPVT). The aACBsh was negatively correlated with pPVT. Fos induction values between subregions were correlated within the CEA (aCEAm, pCEAm, and aCEAl were correlated, and aCEAm with pCEAc) and within the PVT (aPVT and mPVT). The subregions of the nucleus accumbens were not correlated with each other.
For deprived females given food (right/above diagonal, Table 3), Fos induction in the ILA was negatively correlated with pCEAl. The PL was not correlated with any region. The AIp was only correlated with aCEAc. The aACBc was correlated with all other parts of the ACB, and the pACBc was correlated with aACBc and pACBsh. Anterior and posterior CEAc were negatively correlated, and the mPVT and pPVT were correlated.
The correlations for female groups not given food at test are shown in Table 4. For sated females without food (left/below diagonal, Table 4), Fos induction in the PL was negatively correlated with pACBc. The AIp was correlated with the PVT (mPVT and pPVT) but not with the CEA, and the mPVT and aCEAl were correlated. The aACBsh was negatively correlated with aCEAl, while pACBc was correlated with aPVT. There were subregions correlated within the ACB (aACBc with pACBc and pACBsh), within the CEA (pCEAm with pCEAl and pCEAc), and within the PVT (mPVT with pPVT).
For deprived females without food (right/above diagonal, Table 4), Fos induction in the ILA and PL were correlated, but there were no other correlations with these regions. The AIp was correlated with the central amygdala (aCEAm, pCEAm, aCEAl, and pCEAc) and with the PVT (mPVT and pPVT). The pACBsh was correlated with aCEAm and mPVT. The mPVT was also correlated with the aCEAm, as well as with pCEAm, aCEAl, and pCEAc, while the pPVT was correlated with pCEAm and pCEAc. There were subregions correlated within the ACB (aACBc and aACBsh) and the CEA (aCEAm with pCEAm and aCEAl, pCEAm with aCEAl and pCEAc).
The correlations for male groups given food at test are shown in Table 5. For sated males given food at test (left/below diagonal, Table 5), Fos induction in the ILA and PL were correlated, but there were no other correlations with these regions. The AIp was correlated with pACBc and pACBsh. The pACBc was correlated with aCEAm and pPVT, and the pACBsh with pPVT. The aCEAm was correlated with the pPVT, while the pCEAc was negatively correlated with mPVT. There were subregion correlations between the pACBc and pACBsh, between the aCEAm and aCEAl, and between the aPVT and pPVT.
For deprived males given food at test (right/above diagonal, Table 5), Fos induction in the ILA and PL were correlated but there were no other correlations with these regions. The AIp was correlated with pCEAm and pCEAc. The pACBsh was correlated with pPVT, the pCEAm with mPVT, and the pCEAc with mPVT and pPVT. There were subregions correlated within the ACB (aACBc with aACBsh) and the CEA (pCEAm with aCEAc, pCEAc, and aCEAc with pCEAc).
The correlations for male groups not fed at test are shown in Table 6. For sated males not given food at test (left/below diagonal, Table 6), The ILA and PL were correlated and the PL was negatively correlated with pCEAc. The AIp was not correlated with any region. The aACBsh was negatively correlated with pCEAc, while the pACBc was correlated with aPVT. The pPVT was correlated with the aCEAm, aCEAl and aCEAc. There were correlations between subregions within the ACB (aACBc with pACBc and pACBsh, and pACBc with pACBsh), and the CEA (aCEAm with aCEAl and aCEAc; pCEAm with pCEAl; and aCEAl with aCEAc).
For deprived males not given food at test (right/above diagonal, Table 6), the ILA and PL were correlated but there were no other correlations with these regions. The AIp was correlated with pCEAm and pCEAc, as well as the mPVT and pPVT. The aACBc was correlated with the mPVT and pPVT, and the aACBsh with the pCEAl, and mPVT with the pPVT. The aCEAm was negatively correlated with aPVT, while the pCEAm was correlated with mPVT, and the pCEAl and pCEAc with pPVT. There were subregion correlations within the ACB (aACBc with aACBsh; aACBsh negatively with pACBsh) and within the CEA (pCEAm with aCEAc and pCEAc; aCEAl with aCEAc), and between the mPVT and pPVT.
Discussion
In this study, we determined forebrain neural activation patterns in male and female rats during hedonic eating. We analyzed Fos induction within the cortical, thalamic, striatal, and amygdalar areas that receive substantial inputs from ORX neurons (see Introduction). During the test, hungry and sated males and females were given palatable, high-sugar food. Control groups of hungry and sated rats of both sexes were tested under the same conditions but were not fed during the test, in order to dissociate potential effects of consumption from food deprivation. During the test, all hungry rats ate substantial amounts, however, sated males and females differed. Sated males ate much less than hungry males, while sated females ate as much as hungry females. Because of these differences in consumption, we analyzed neural activation patterns separately in each sex in order to determine if different networks drive hedonic eating in males and females. Our analysis identified areas that correlated with consumption, and network differences between males and females that corresponded to behavioral differences.
There were sex differences in recruitment of specific areas of the prefrontal cortex (ILA, PL), paraventricular thalamus (pPVT), nucleus accumbens (pACBshV), and central nucleus of the amygdala (pCEAm). In females, in each of these regions, except the prefrontal cortex, there was more Fos induction in the groups given palatable food, irrespective of hunger state. That pattern in females corresponded with their behavior during the test. Interestingly, the medial prefrontal cortex (ILA) was robustly activated in sated males, which is the group that consumed the least amount of food during the test. There were also similar activation patterns in males and females. In both sexes, the insular cortex (AIp) was selectively activated in the food condition irrespective of the amounts eaten or hunger state. The ACB shell was activated by hunger, while Fos induction in the ACB core was similar across groups. The CEA was activated by food and hunger, although more robustly by food, and Fos induction in the CEA correlated with the amounts of food consumed during the test (Table 2).
Medial and lateral prefrontal cortex
Within the medial PFC, Fos induction patterns were complex, possibly because neuronal ensembles that drive and inhibit consumption were differently recruited under hunger and satiety. In the ILA of both sexes, and in the PL of males, the patterns of Fos induction in the groups given food compared to the groups not given food during the test were opposite under sated versus deprived conditions, with more recruitment in sated but less in hungry food groups. Furthermore, in sated males, there was a unique, robust Fos induction in the ILA, which was negatively correlated with consumption (Table 2, Fig. 10). Sated males consumed less palatable food than any other group at test. These results suggest an intriguing possibility that the ILA recruitment is related to suppression of eating during satiety and that such control may be reduced in females. In agreement with this premise, a recent study found that ILA inactivation potentiated sucrose consumption in sated rats that was induced by mu-opioid agonist infusions into the insular cortex (Giacomini et al. 2022). In the same study, inactivation of ILA alone, without insular stimulations, had no effect on feeding, suggesting that the ILA controls ongoing behavior triggered by other sites. The ILA is also critical when suppression of behavioral action (lever pressing) is required to obtain a sucrose pellet reward (Capuzzo and Floresco 2020). Similar function was highlighted in a recent integrative review of brain network differences in binge eating disorder (BED), where decreased activity in the medial PFC was linked to decreased cognitive control over food-related decisions in BED (Hartogsveld et al. 2022). In this regard, ORX signaling within the ventromedial PFC is required in an animal model of cognitive control of feeding. Systemic blockade of ORX-Receptor1 abolished learned food cue-potentiated feeding in sated rats and induced robust Fos in the ventromedial PFC (ILA and PL) (Cole et al. 2015b), and a follow-up study confirmed the necessity of ORX signaling within the ventromedial PFC in this task (Cole et al. 2020).
Within the lateral PFC, the Fos induction patterns in the AIp indicate that it was robustly and selectively activated by the taste of palatable food. Fos induction was high in the AIp in the food groups, irrespective of hunger state, and the numbers of Fos-positive neurons were not correlated with the amounts of food consumed during the test. The role of insular cortex within the taste processing network is well known, and its broader function in the context of learning and memory and feeding decisions was recently reviewed (Boughter Jr. and Fletcher 2021). More directly related to the current results, stimulation of mu-opioid receptors in the insular cortex can drive sucrose consumption (Giacomini et al. 2022). Thus, AIp network differences, particularly connections with the PVT (Li and Kirouac 2012), may underlie sex differences in hedonic feeding. In that regard, while the AIp was recruited similarly in the food groups of both sexes, it was differently correlated with the PVT, CEA and ACB in males and females (see below).
Paraventricular thalamus
In the current study, there were sex differences in PVT recruitment—food and hunger induced Fos in females but not in males (Fig. 9, Table 1). In females, all three rostrocaudal PVT parts analyzed were recruited in the hunger condition, while the pPVT was selectively recruited in the food groups (Fig. 9, Table 1). In males, neither food nor hunger groups differed in Fos induction in the PVT. These results suggest that the PVT is a component of a sex-specific network that drives hedonic eating differently in sated males and females. The PVT is a complex structure that integrates interoceptive signals in the context of energy balance, arousal and stress (Petrovich 2021). The PVT has been shown to exert behavioral control via the ACB, and optogenetic stimulation of the aPVT-ACB pathway increased palatable (high-fat) food consumption but not chow in sated (male) mice (Christoffel et al. 2021). Notably, ORX fibers and receptors within the PVT are dense, and ORX signaling within the PVT, and together with the vmPFC, has been implicated in hedonic and cognitive control of eating (Choi et al. 2012; Cole et al. 2015b). Orexin-Receptor1 knockdown in the posterior PVT decreased palatable food (high-fat) consumption (Choi et al. 2012), and disruption of cue-potentiated eating with systemic blockade of ORX-Receptor1 was accompanied by a robust Fos induction in the anterior PVT (Cole et al. 2015b).
The nucleus accumbens
Within the ACB, there was regional specificity in Fos induction patterns (Figs. 5 and 6, Table 1). Hunger recruited the medial shell in both sexes (anterior dorsal and posterior ventral in both sexes, and anterior ventral in females), while food selectively recruited the posterior (dorsal and ventral) medial shell in females. There were no differences in the ACB core across any groups. These findings are in agreement with important prior work that established the medial ACB shell in the control of food consumption (Kelley 2004). The ACB neurons respond to food deprivation (Carr 2011) and receive inputs from the glucose-responsive PVT neurons that are activated by hypoglycemia and when optogenetically stimulated drive sucrose-seeking (Labouèbe et al. 2016). Classic studies have shown that pharmacologically stimulating GABA-A receptors with muscimol within the anterior medial ACB shell, or antagonizing glutamate receptors with DNQX, can induce robust feeding in sated animals (Maldonado-Irizarry et al. 1995; Stratford and Kelley 1997). Furthermore, activating mu-opioid receptors with agonist, DAMGO infusions into the ACB shell, including the region that overlaps with the pACBsh in the current study, increased high-fat intake in sated (male) rats (Zhang and Kelley 2000).
Interestingly, ACB stimulation can also elicit aversive behaviors, and distinct rostrocaudal zones for appetitive and aversive behaviors can dynamically change in a stressful environment such that the aversive zone expands (Raynolds and Berridge 2008). In the current study, Fos activation in the hungry rats was broad and overlapped with the appetitive and aversive zones (Raynolds and Berridge 2008). In addition, there was unique activation of the posterior shell in the food condition in females but not in males. That area overlaps with the region where microinjections of ORX enhanced consumption (Thorpe and Kotz 2005; Castro et al. 2016) and microinjections of corticotropin releasing hormone/factor enhanced incentive motivation for sucrose reward (Peciña et al. 2006). The unique activation of the posterior medial shell in females in the food condition is intriguing, and suggests that it may be a component of a sex-specific network that motivates sated females (however, that Fos induction was not correlated with consumption). The ACBsh receives inputs from the PVT, the region where the current study found robust Fos induction in females, but not in males. There were also sex differences in ACB-PVT Fos induction correlations (see below). The same population of ORX neurons projects to the ACBsh and PVT (Lee and Lee 2016). In terms of sex-specific behavioral networks, the ACB shell is where the lateral hypothalamic hunger peptide MCH (melanin-concentrating hormone) neurons were shown to promote a male-specific increase in consumption of chow and palatable foods (Terrill et al. 2020).
The central amygdala
Within the CEA, food and hunger induced Fos patterns in males and females, although the patterns differed by region and sex (Figs. 7, 8 and 10, Tables 1 and 2). Food robustly activated the CEA in both sexes throughout the anterior and posterior medial and lateral, as well as anterior capsular regions. The number of Fos-positive neurons in the anterior lateral part (aCEAl) in both sexes and the anterior medial (aCEAm) in males were positively correlated with the amount food consumed at test (Table 2, Fig. 10). Hunger induced Fos in the anterior lateral (aCEAl) in both sexes and in the medial (aCEAm) in females. Interestingly, the posterior medial (pCEAm) was recruited in a sex-specific way that corresponded to the consumption patterns at test. In males, Fos induction was grater in hungry rats compared to sated rats, while in females, there was similar Fos induction in the food groups regardless of hunger. Indeed, the CEA was the only brain region analyzed in the current study where Fos induction was positively correlated with food consumption. The CEA has been known to control food consumption and it has been suggested, based on functional neuroanatomy, that dysregulation of CEA circuitry could cause excessive hedonic eating or unnecessary food avoidance (Petrovich 2018, 2021). Cell-specific manipulations have shown that different CEA neurons drive or inhibit feeding behavior (protein kinase C-delta (Cai et al. 2014), serotonin receptor 2a (Douglass et al. 2017), prepronociceptin (Hardaway et al. 2019), and corticotropin releasing hormone/factor (Baumgartner et al. 2021). All these studies, except for one (Baumgartner et al. 2021), were conducted in males only (behavioral manipulations in (Douglass et al. 2017) were in males only). The results of the current study highlight the need to evaluate the CEA circuitries in both sexes.
Activation of sex-specific networks during hedonic eating
One aim of this study was to determine if different networks were recruited in males and females during hedonic eating. To accomplish this, we analyzed correlations of Fos inductions between regions in each group (Tables 3, 4, 5 and 6). Interestingly, for both sexes, the highest number of correlations were in the deprived rats not fed (20 for each sex), while the lowest were in the deprived rats given food (8 for females and 11 for males) during test. The PL and ILA were correlated in all male groups but only in the deprived females not given food. In males, the PL and ILA were not correlated with any other regions, except for sated males not given food where PL and CEA were negatively correlated. In females, the ILA was negatively correlated with the CEA in deprived rats given food at test. The PL was positively correlated with the ACB and CEA in sated females fed, and negatively with the ACB in sated females not fed at test.
In sated females given food at test, there were three unique sets of correlations. The AIp, and multiple subregions of the CEA and PVT were correlated with each other. The PL was correlated with both the nucleus accumbens core (aACBc) and the central amygdala (pCEAc), which were also correlated. The accumbens shell (aACBsh) was negatively correlated with pPVT and there were no subregion correlations within the ACB. In contrast, in sated females not fed during the test, there were negative correlations between the PL and pACBc and between the pACBsh and pCEAl, and the AIp was only correlated with the PVT.
In deprived females given food, there were unique negative correlations between the ILA and pCEAl, and between the anterior and posterior CEAc. The AIp was only correlated with aCEAc. In contrast, in deprived females not given food at test, the ILA and PL were correlated, and the AIp and pACBsh were correlated with the CEA and PVT, which were correlated.
In males, in all conditions the PL and ILA were correlated. In sated males given food, different subregions of the CEA and PVT were positively and negatively correlated. The AIp was correlated with the ACB but not with the CEA or PVT. The posterior accumbens core (pACBc) and shell (pACBsh) were correlated with pPVT and pACBc also with aCEAm. In sated males not given food, there were negative correlations between the PL and pCEAc, and between the aACBsh and pCEAc. The pACBc was correlated with aPVT, while the AIp was not correlated with any regions.
In deprived males given food, the AIp was correlated with the CEA, while the pACBsh was correlated with pPVT, and the CEA and PVT were correlated. In deprived males not given food, the AIp was correlated with CEA and PVT, and the accumbens core (aACBc) and shell (aACBsh) were correlated with the PVT, and the aACBsh with the CEA. Different subregions of the CEA and PVT had positive and negative correlations.
Based on these results, the most striking differences between the patterns in sated females and sated males given food—the groups of interest because of their consumption differences—were AIp correlations with the CEA and PVT in females but not in males. In addition, the PL was correlated with the ACB and CEA in females, while the AIp was correlated with the ACB in males. Subregions of the ACB and PVT were negatively correlated in females but positively in males. This is intriguing, because the CEA was the only area analyzed in the current study where Fos induction was positively correlated with consumption at test, and because of the connectional network between the PVT, ACB and CEA (Dong et al. 2017). Thus, specific PVT-CEA and PVT-ACB systems may have been selectively recruited in females, under the control of AIp and PL to drive hedonic eating independent of hunger. In accordance with this hypothesis, stimulation of PVT-ACB increased, while stimulation of PFC pathways to the ACB decreased palatable (high fat) consumption in sated male mice (Christoffel et al. 2021).
In conclusion, the current study conducted a comprehensive analyses of extra-hypothalamic forebrain activation in males and females during palatable food consumption under sated and hungry conditions. Four areas were distinctively activated in a sex-specific manner. The ILA was robustly recruited in sated males given food and was negatively correlated with consumption. The CEA was the only region positively correlated with consumption and the pCEAm recruitment corresponded to sex-specific patterns of consumption at test. The posterior ACB shell and pPVT were uniquely activated in sated females. These patterns suggest sex-specific medial prefrontal cortex (ILA) control of the CEA, ACB, and PVT networks during palatable food consumption. The correlation analyses additionally indicated that the AIp and PL were differently correlated within these networks in males and females and that the ILA and PL were correlated in males under all conditions but only in hungry females without food. Understanding how the medial prefrontal cortex areas, ILA and PL, converge with the AIp within the CEA, ACB, and PVT networks to control hedonic eating in hungry and sated males and females is an important avenue of future research.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Baldo BA, Daniel RA, Berridge CW, Kelley AE (2003) Overlaping distribution of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol 464:220–237
Barson JR, Leibowitz SF (2015) GABA-induced activation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci Lett 609:92–96
Baumgartner HM, Schulkin J, Berridge KC (2021) Activating corticotropin-releasing factor systems in the nucleus accumbens, amygdala, and bed nucleus of stria terminalis: Incentive motivation or aversive motivation? Biol Psychiatry 89:1162–1175
Boughter JD Jr, Fletcher M (2021) Rethinking the role of taste processing in insular cortex and forebrain circuits. Curr Opin Physio 20:52–56
Buczek L, Migliaccio J, Petrovich GD (2020) Hedonic eating: sex differences and characterization of orexin activation and signaling. Neuroscience 436:34–45
Cai H, Haubensak W, Anthony TE, Anderson DJ (2014) Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 17(17):1240–1248
Capuzzo G, Floresco SB (2020) Prelimbic and infralimbic prefrontal regulation of active and inhibitory avoidance and reward-seeking. J Neurosci 40:4773–4787
Carr KD (2011) Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav 104:162–167
Castro DC, Terry RA, Berridge KC (2016) Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal shell shifts ‘liking’ toward ‘disgust’ and ‘fear.’ Neuropsychopharmacology 41:2101–2111
Choi DL, Davis JF, Fitzerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167:11–20
Choi DL, Davis JF, Magrisso IJ, Fitzerald ME, Lipton JW, Benoit SC (2012) Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience 210:243–248
Christoffel DJ, Walsh JJ, Heifets BD, Hoerbelt P, Neuner S, Sun G, Ravikumar VK, Wu H, Halpern CH, Malenka RC (2021) Input-specific modulation of murine nucleus accumbens differentially regulates hedonic feeding. Nature Comunications 12:2135
Cole S, Hobin MP, Petrovich GD (2015a) Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience 286:187–202
Cole S, Mayer HS, Petrovich GD (2015b) Orexin/hypocretin-1 receptor antagonism selectively reduces cue-induced feeding in sated rats and recruits medial prefrontal cortex and thalamus. Sci Rep 5:16143
Cole S, Keefer SE, Anderson LC, Petrovich GD (2020) Medial prefrontal cortex neural plasticity, orexin receptor 1 signaling, and connectivity with the lateral hypothalamus are necessary in cue-potentiated feeding. J Neurosci 40:1744–1755
de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327
Dong X, Li S, Kirouac GJ (2017) Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct 222:3927–3943
Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Morales PLA, Conzelmann KK, Lüthi A, Klein R (2017) Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci 20:1384–1394
Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, Ramakrishnan C, Deisseroth K, Penzo MA (2020) Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23:217–228
Giacomini JL, Sadeghian K, Baldo BA (2022) Eating driven by the gustatory insula: contrasting regulation by infralimbic vs. prelimbic cortices. Neuropsychopharmacology 47:1358–1366
Hardaway JA, Halladay LR, Mazzone CM, Pati D, Bloodgood DW, Kim M, Jensen J, DiBerto J, Boyt KM, Shiddapur A, Erfani A, Hon OJ, Neira S, Stanhope CM, Sugam JA, Saddoris MP, Tipton G, McElligott Z, Jhou TC, Stuber GD, Bruchas MR, Bulik CM, Holmes A, Kash TL (2019) Central amygdala prepronociceptin-expressing neurons mediate palatable food consumption and reward. Neuron 102:1037–1052
Hartogsveld B, Quaedflieg CWEM, van Ruitenbeek P, Smeets T (2022) Volume and connectivity differences in brain networks associated with cognitive constructs of binge eating. eNeuro 9:1–31
Hudson JI, Hiripi E, Harrison GP, Kessler RC (2007) The prevalence and correlates of eating disorders in the national comorbidity survey replication. Biol Psychiatry 61:348–358
Kelley AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765–776
Labouèbe G, Boutrel B, Tarussio D, Thorens B (2016) Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci 8:999–1002
Lee EY, Lee HS (2016) Dual projections of single orexin-or CART-immunoreactive, lateral hypothalamic neurons to the paraventricular thalamic nucleus and nucleus accumbens shell in the rat: Light microscopic study. Brain Res 1634:104–118
Li S, Kirouac GJ (2012) Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 217:257–273
Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G (2014) Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci 17:1298–1303
Maldonado-Irizarry CS, Swanson CJ, Kelley AE (1995) Glutamate receptors in the nucleus accumbens shell control feeding behavior via the lateral hypothalamus. J Neurosci 15:6779–6788
Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435:6–25
Mitchell S, Shaw D (2015) The worldwide epidemic of female obesity. Best Pract Res Clin Obstet Gynaecol 29:289–299
Peciña S, Schulkin J, Berridge KC (2006) Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol 4:8
Petrovich GD (2018) Feeding behavior survival circuit: anticipation & competition. Curr Opin Behav Sci 24:137–142
Petrovich GD (2019) Orexins and control of feeding by learned cues. In: Fadel JR, Burk JA (eds) The orexins/hypocretins system: functional roles and therapeutic potential, 1st edn. Academic Press, pp 85–98
Petrovich GD (2021) The function of paraventricular thalamic circuitry in the adaptive control of feeding behavior. Front Behav Neurosci 15:671096
Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015
Raynolds SM, Berridge KC (2008) Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci 11:423–425
Reppucci CJ, Petrovich GD (2012) Learned food-cue stimulates persistent feeding in sated rats. Appetite 59:437–447
Reppucci CJ, Petrovich GD (2016) Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 221:2937–2962
Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585
Small DM (2009) Individual differences in the neurophysiology of reward and the obesity epidemic. Int J Obes 33:S44–S48
Stratford TR, Kelley AE (1997) GABA in the nucleus accumbens shell participates in the central regulation of feeding behavior. J Neurosci 17:4434–4440
Sun X, Kroemer NB, Veldhuizen M, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, Sinha R, Small DM (2015) Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 35:7964–7976
Swanson LW (2018) Brain maps 4.0—Structure of the rat brain: an open access atlas with global nervous system nomenclature ontology and flatmaps. J Comp Neurol 526:935–943
Terrill SJ, Subramanian KS, Lan R, Liu CM, Cortella AM, Noble E, Kanoski SE (2020) Nucleus accumbens melanin-concentrating hormone signaling promotes feeding in a sex-specific manner. Neuropharmacology 178:108270
Thorpe AJ, Kotz CM (2005) Orexin A in the nucleus accumbens stimulates feeding and locomotor activity. Brain Res 1050:156–162
Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438:71–75
Zahm DS, Brog JS (1992) On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50:751–767
Zhang M, Kelley AE (2000) Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and Fos expression. Neuroscience 99:267–277
Acknowledgements
This work was supported by the National Institutes of Health, NIDDK grant R01DK085721 to GDP. We thank Dr. Ehri Ryu for helpful advice regarding statistical analyses.
Author information
Authors and Affiliations
Contributions
GDP conceptualized the study and supervised the experiments and directed the data analysis. GDP, LB and JM designed the experiments. LB and JM carried out the experiment. WP, EG, LB, JM, EC, AMKM conducted the histological preparation and analyses. WP and EG prepared the figures. WP and GDP wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parsons, W., Greiner, E., Buczek, L. et al. Sex differences in activation of extra-hypothalamic forebrain areas during hedonic eating. Brain Struct Funct 227, 2857–2878 (2022). https://doi.org/10.1007/s00429-022-02580-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-022-02580-0