Abstract
Parkinson’s disease (PD) can be considered as the dysfunction in segregation and integration of large-scale structural networks in the late stage of disease progression. However, the altered patterns in the early stage have not been extensively investigated, especially the altered structural rich-club patterns, which is proved powerful to detect the altered patterns of structural networks in Alzheimer’s disease and schizophrenia. To this end, we investigated the rich-club organization of the structural networks derived from diffusion tensor imaging (DTI) data in the early stage of PD, and further investigated the relationship between rich-club organization and clinicopathological measures, including motor and non-motor scales and cerebrospinal fluid (CSF) biomarkers. Two datasets were included for validation in this study. The first one included 41 healthy controls (HC) and 64 PD patients from Parkinson’s Disease Progression Marker Initiative (PPMI) dataset, and the second one included 24 HC and 26 PD patients. Results revealed that PD patients in early stage had disrupted rich-club organization, with abnormal connectivity strength between peripheral regions (two-sample t-test between PD and HC: p < 0.001), whereas connectivity strength between rich-club regions remained relatively stable (two-sample t-test between PD and HC: p = 0.108). The classification accuracies on three types of connections were 59.93%, 73.96% and 77.44% for rich-club, feeder and local connections. Furthermore, abnormal local and feeder connections showed significant correlation with poor clinical scales and CSF biomarkers. In summary, a selective disruption of non-rich-club connections here could be regarded as a potential marker in the early diagnosis of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is classically known as a motor disorder, clinically characterized by resting tremor, muscle rigidity, bradykinesia and other abnormal postures and pace (Jankovic 2008). The most important pathological change that characterizes PD is the degeneration of dopaminergic neurons in the substantia nigra, which causes significantly decreased dopamine content in the striatum and thus results in disease (Dauer and Przedborski 2003; Kalia and Lang 2015). PD has serious effects on patients during the occurrence and development of the disease, aggravating movement disorders, shortening life expectancy, and reducing quality of life. Identification of preclinical biological and imaging markers is pressingly needed for early diagnosis and prevention approaches. The early stage of PD is associated with non-motor symptoms and usually indicates the stages I and II in the Braak model (Braak et al. 2003), which has classified the PD pathological process into six stages. The clinical diagnosis of PD, especially the early diagnosis, is usually based on the Hoehn and Yahr Staging Scale (H&Y scale) (Armstrong and Okun 2020). Recent studies suggest that CSF biomarkers may profile progressive neurodegeneration in PD. It has been demonstrated that intracellular accumulation of \({\alpha }\)-synuclein aggregates may be a marker for PD diagnosis and progression (Klein and Westenberger 2012). In early PD patients (i.e., H&Y stage of I or II) study, CSF \({\alpha }\)-synuclein and total tau are lower than healthy controls (HC) (Kang et al.2016). Longitudinal changes in levels of CSF \({\alpha }\)-synuclein in PD indicates that CSF \({\alpha }\)-synuclein may increase over time or in those with more severe PD (Majbour et al. 2016). Total tau (t-tau) and \({\beta }\)-amyloid 1–42 (A\({\beta }\) 1–42), recognized as pathologic features in Alzheimer’s disease, correlate with PD neurodegeneration as well. The progressive accumulation of misfolded protein aggregates may initially trigger synaptic damage and network dysfunction, suggesting the potential diagnosis power of network measures in PD.
The human brain is a complex network of interconnected regions. An increasing number of studies have demonstrated that brain function is not solely attributable to individual regions and connections but rather emerges from the topology of the network as a whole, the connectome of the brain (Bullmore and Sporns 2009; Sporns et al. 2005). Thus, each functional brain region is connected to various nodes, as can be described by graph theory. Recent studies have called attention to the existence of hub regions that are functionally valuable for their high connectivity and high level of centrality (Hagmann et al. 2008; van den Heuvel and Sporns 2011). Hub regions play important roles in global information integration between different parts of the network. van den Heuvel and Sporns (2011) have reported the existence of a “rich-club” formed by brain hubs, characterized by a tendency for high-degree nodes to be more densely connected among themselves than nodes of a lower degree, providing important information on the higher level topology of the brain network. This topological information has been widely used to uncover abnormal network topology in brain disease. Previous studies (van den Heuvel et al. 2013; Yan et al. 2018) in various brain diseases, including Alzheimer’s disease and schizophrenia, have shown that disturbances of the rich-club within the connectome profile are related to neuropathology. These findings have emphasized the potential importance of the organization and topological properties of the rich-club in understanding brain diseases. Understanding the network dysfunction pattern, especially the rich-club pattern in the early stage of PD patients may advance our understanding of the pathobiology of the disease course.
However, very few studies on PD have adopted the organization of rich-club to reveal topological changes in brain structural networks, and associated the structural network changes with potential biomarkers of PD. Hall, (2018) demonstrated decreased participation coefficients in the right caudate, thalamus, and hippocampus as well as the superior frontal and parietal cortical regions in patients with freezing of gait compared to those without freezing of gait. Some of these nodes belong to the brain’s “rich-club”. Li, (2017) found that the feeder and local connections have lower connectivity strength in PD patients than in HC and detected significantly increased shortest path length and decreased global efficiency in PD patients compared to controls. These findings may provide useful information for an improved understanding of the abnormalities of brain structural networks in PD patients. However, it remains unclear whether these alterations could function as early diagnostic and progression biomarkers in PD patients. In our study, we used two datasets to explore how the patterns of rich-club organization change and investigate the relationship between abnormal brain structural networks and clinical performance in PD patients.
Materials and methods
Participants
Dataset 1
Diffusion tensor imaging (DTI) and T1-weighted data were collected from Parkinson’s Progression Markers Initiative (PPMI) study to explore changes in early stage of PD patients (defined by clinical baseline assessments) compared to HC, including 41 HC and 64 PD patients aged from 50 to 70 years old in early stage. PPMI study was carried out in accordance with Good Clinical Practice (GCP) regulations and International Conference on Harmonization (ICH) guidelines (Marek et al. 2011). The multicenter PPMI study is aimed to identify and validate PD progression biomarkers and speed therapeutic development for PD. Neuroimaging, cognitive and CSF data from drug-naïve PD patients and HC are available to academia and industry. This study has been approved by the respective institutional review boards of all participating sites including Baylor College of Medicine, The Parkinson’s Institute, John Hopkins University, Emory University, Northwestern University Medical School, Mellen Center Cleavland Clinic, Boca Raton Regional Hospital, University of Tubingen, Paracelsus-Elena Klinik, University of Innsbruck. Each site independently received ethics approval of the protocol and all subjects gave written informed consent in accordance with the Declaration of Helsinki.
Both the PD patients and the HC had been assessed with the Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) (Colosimo et al. 2010) and with additional clinical tests evaluating cognition, such as the Montreal Cognitive Assessment (MoCA). In detail, the MDS-UPDRS contains three main parts: UPDRS-I (part I of the MDS-UPDRS) concerns “non-motor aspects of experiences of daily living”, UPDRS-II (part II of the MDS-UPDRS) concerns “motor experiences of daily living”, and UPDRS-III (part III of the MDS-UPDRS) is retained as the “motor examination” (Goetz et al. 2008). Cerebrospinal fluid (CSF) biomarkers collected from PPMI were analyzed: CSF \({\alpha }\)-synuclein using an ELISA assay available commercially from BioLegend, amyloid-\({\beta }_{1-42}\), total (t)-tau, and phosphorylated tau (P-tau) measured using Elecsys electro-chemi-luminescence immunoassays on the cobase 601 platform (Roche Diagnostics) and ratios between CSF biomarkers (Kang et al. 2016).
In early stage, PD participants were required to be diagnosed with an asymmetric resting tremor or asymmetric bradykinesia or two kinds of bradykinesia, resting tremor and rigidity within two years; to be untreated for PD; to have a dopamine transporter (DAT) deficit on DAT imaging; and to have a Hoehn and Yahr (H&Y) stage of II or lower. Healthy participants were required to have no significant neurological dysfunction, no first-degree family members with PD, and MoCA scores > 26 (Marek et al. 2011). PPMI enabled PD patients to be enrolled in the study at an earlier detectable stage of disease than previous studies, which could enhance the potential to identify progression biomarkers.
Dataset 2
We recruited 24 HC and 26 PD participants through advertising, calling, face-to-face interviewing or from the PD outpatient clinic of Beijing Rehabilitation Hospital, Capital Medical University. PD patients were diagnosed according to the 2015 Movement Disorder Society criteria and Rome III criteria. Subjects who suffered traumatic brain injury and other central nervous system disease, such as epilepsy and brain tumors, were excluded. Moreover, PD patients had been assessed with the H&Y, UPDRS-III and MoCA. In this dataset, patients had a H&Y stage of II or lower and been treated for PD. Healthy participants were required to have no significant neurological dysfunction and no first-degree family members with PD. All subjects gave written informed consent in accordance with the Declaration of Helsinki. And this study has been approved by the Ethics Committee of Beijing Rehabilitation Hospital, Capital Medical University (No. 2018bkky022).
Data acquisition
Dataset 1
All MR scans were acquired on a Siemens 3T TIM Trio scanner using a 12-channel matrix head coil, and structural DTI data and T1-weighted data were collected for all participants. Two-dimensional DTI data were collected using echo-planar imaging with the following parameters: repetition time (TR) = 850 ms, echo time (TE) = 88 ms, flip angle = 90°, voxel size = 2 × 2 × 2 mm3, slices = 72, field of view (FOV) = 232 × 232 mm2, acquisition matrix = 116 × 116, thickness = 2 mm, 64 gradient directions with a b-value of 1000 s/mm2 and one additional image with no diffusion weighting (b = 0 s/mm2).
T1-weighted MR images were obtained with the following parameters: TR = 2300 ms, TE = 2.98 ms, inversion time (TI) = 900 ms, flip angle = 9°, voxel size = 1 × 1 × 1 mm3, slices = 176, FOV = 240 × 256 mm2, acquisition matrix = 240 × 256, and thickness = 1.0 mm. These parameters can be obtained from the online PPMI protocol (http://www.ppmi-info.org/wp-content/uploads/2018/02/PPMI-AM-13-Protocol.pdf).
Dataset 2
In this dataset, MR scans were acquired on a GE SIGNA Pioneer 3.0T scanner, and structural DTI data and T1-weighted data were collected for all participants. Two-dimensional DTI data were collected using echo-planar imaging with the following parameters: TR = 8000 ms, TE = 99.5 ms, flip angle = 90°, voxel size = 2 × 2 × 2 mm3, slices = 50, FOV = 280 × 280 mm2, acquisition matrix = 256 × 256, thickness = 5 mm, 64 gradient directions with a b-value of 1000 s/mm2 and one additional image with no diffusion weighting (b = 0 s/mm2).
T1-weighted MR images were obtained with the following parameters: TR = 8 ms, TE = 3.1 ms, TI = 450 ms, flip angle = 15°, voxel size = 1 × 1 × 1 mm3, slices = 180, FOV = 300 × 300 mm2, acquisition matrix = 512 × 512, and thickness = 1.0 mm.
Image preprocessing
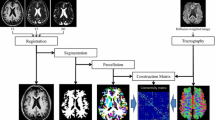
All DTI images were preprocessed using the PANDA toolbox (http://www.nitrc.org/projects/panda) based on FSL 5.0 (www.fmrib.ox.ac.uk/fsl) and Diffusion Toolkit (http://www.trackvis.org/dtk/), with T1-weighted MR images as an anatomical reference. First, eddy current correction was performed on DTI images by realigning the diffusion images to the b0 image to correct for eddy-current-induced distortions and rigid head motion. The quality of the data after eddy current correction was checked. Second, nonbrain tissue was removed to reduce the intensity inhomogeneity of each individual DTI dataset. Third, the fractional anisotropy (FA) of each voxel was computed, which provided information on the level of preferred diffusion direction within a voxel. Finally, the white matter tracts were reconstructed based on the preferred diffusion direction with each voxel in the brain mask. We accomplished this task using deterministic fiber tracking based on the FACT (fiber assignment by continuous tracking) algorithm (Mori et al. 1999; Mori and van Zijl 2002). During the procedure of fiber tracking, a streamline was terminated when it reached a voxel with an FA value < 0.1 or made a sharp turn (< 45°).
T1 images were used for anatomical reference and to select the nodes of the brain network (van den Heuvel and Sporns 2011). We applied an inverse warping transformation by coregistering FA images in native space to corresponding T1-weighted images and registering the T1 images to the International Consortium for Brain Mapping (ICBM) 152 template. The inverse warping transformation from the standard space to the native space was then applied in the Automated Anatomical Labeling (AAL) atlas, which reconstructed the cortical surface into 90 distinct brain regions. Afterwards, the AAL template was warped to the native DTI space for each participant, with each AAL region representing a node of the individual’s brain network.
Network construction
A network was constructed by combining a set of edges with a collection of nodes. For individual brain networks, 90 distinct brain regions in the AAL atlas were defined as nodes, and reconstructed fiber tracts interconnecting brain regions represented edges. To reduce the potential effect of noise, we considered regions \(i\) and \(j\) connected if connections between these two regions existed in at least 50% of participants of each group (Li et al. 2017). Then, based on this assumption, we generated a symmetric binary matrix as a mask for each group, reflecting the underlying patterns of structural connectivity in the cerebral cortex.
FA is a relatively objective and straightforward measure to compare across subjects as a marker for tract integrity (Smith et al. 2006). Higher FA values reportedly indicate a higher level of microstructural organization of white matter connections, and higher FA values for white matter tracts have been linked to faster task performance (Beaulieu 2002; Ewing-Cobbs et al. 2006; Gold et al. 2007), while lower FA values commonly indicate white matter damage in patient studies (Kubicki et al. 2005; Sun et al. 2003; Verstraete et al. 2010). Therefore, FA is believed to be a possible marker of the efficacy of brain connections. In our study, an individual FA-weighted brain structural network was created with the mean FA value along the interregional streamlines as the edge weight, resulting in an FA-weighted connectivity matrix for each participant.
Rich-club organization
In this study, for a given FA-weighted matrix, the degree k of each node i in the network was defined by counting the number of links that node i connected with other nodes in the network. For each group, the group-averaged degree of each node i was calculated by averaging the degrees of node i across participants of this group. After the nodes with degrees \(\le k\) were removed from the network, the remaining nodes were selected as prominent nodes. The rich-club coefficient \({\phi }^{w}\left(k\right)\) for the FA-weighted network was computed with the following equation (Opsahl et al. 2008):
In this equation, the number of links \({E}_{>k}\) was calculated by counting the number of connections between the selected prominent nodes, and their collective weight \({W}_{>k}\) was obtained as the sum of the edge weights of these \({E}_{>k}\) connections. All connections of the examined network were ranked in descending order by weight to generate a vector Wranked. In this study, we generated 5000 comparable random networks of equal size with the same number of nodes and degree distribution as the real brain network (Colizza et al. 2006). \({\phi }_{\mathrm{r}\mathrm{a}\mathrm{n}\mathrm{d}}(k)\) was computed by averaging the rich-club coefficient of these random networks. A normalized rich-club coefficient \({\phi }_{\mathrm{n}\mathrm{o}\mathrm{r}\mathrm{m}}^{w}(k)\) was given by the ratio of \({\phi }^{w}\left(k\right)\) and \({\phi }_{\mathrm{r}\mathrm{a}\mathrm{n}\mathrm{d}}(k)\):
It is believed that the brain network is organized in a rich-club pattern, with a normalized coefficient \({\phi }_{\mathrm{n}\mathrm{o}\mathrm{r}\mathrm{m}}^{w}\) greater than 1 over a range of degrees (k) (van den Heuvel and Sporns 2011). Brain regions with degrees greater than a specified value k were considered rich-club nodes, while the remaining brain regions were defined as peripheral nodes (Yan et al. 2018). Based on the categorization of nodes, the edges of the whole-brain network were classified into three types of connections: rich-club connections, linking rich-club nodes to rich-club nodes; feeder connections, linking rich-club nodes to peripheral nodes; and local connections, linking peripheral nodes to peripheral nodes. The ‘connectivity strength’, a summary measure of connectivity, was calculated as the sum of the edge weights for each connection type.
Statistical analysis
All statistical analyses were conducted using the Statistical Package for the Social Sciences (SPSS) v23.0 (http://www.spss.com/). We used two-sample t-tests to compare age, education, MoCA and UPDRS scores between PD patients and controls and used a chi-squared test for differences in sex.
Two-sample t-tests were performed to evaluate differences between PD patients and HC in rich-club coefficients, normalized rich-club coefficients, three classes of connectivity strength, with age, sex and education as covariates. Significance was set at p < 0.05. Specifically, when we compared the abnormal connections in PD patients and HC, the FA values of connections were extracted according to the mask (created by preserving edges represented in 50% or more of the group of participants) within each group. Two-sample t-tests were then conducted across all these edges within the connection mask, with the false discovery rate (FDR) controlled at < 0.05 to correct for multiple comparisons across all edges.
Partial Pearson’s correlations were utilized to assess relationships between abnormal structural connectivity and clinical performance, including MDS-UPDRS scores and CSF biomarkes, controlling age, sex and education.
Validation: classification
Validation by different datasets
It needs to be pointed out that the second dataset was the validation dataset. The first dataset followed all the steps described above, while the validation dataset followed only the image preprocessing and network construction steps. The rich-club connections, feeder connections and local connections in the second dataset were constructed according to the rich-club nodes defined in the first dataset. Thus, we could validate the robustness of our main findings that local edge architecture may be prone to disruption in PD patients and thus could serve as a potential marker to distinguish people with early stage PD from healthy individuals through two datasets.
For each dataset, we used a cross-validation procedure in which we divided the sample dataset into two complementary subsets, one as a training set for training classifiers and the other as a testing set for verifying the validity of the analysis. The rich-club, feeder, local and all the connections were inputed as features. K-fold cross-validation was performed to reduce the variability of the cross-validation results. Each cross-validation included feature selection and a classifier. Specifically, feature selection was applied based on an elastic net model (Witten and Tibshirani 2009), which is a linear regression model using L1 and L2 as feature selection parameters. The classifier was designed using a support vector machine (SVM) algorithm with a linear kernel based on the LIBSVM library toolbox (Chang and Lin 2011). The kernel function was defined as Eq. (3).
The kernel function is K; \({x}_{i}\), \({x}_{j}\) are the input vectors; and \({\Phi }\) is a map to transform the source data from the input space to the feature space. The decision function in the classification with the best parameter set is defined as follows:
where \(\left\{{\alpha }_{m}\right\}\) is a weight series, \({y}_{m}\) is the label of the sample \({x}_{m}\), and \(b\) is a constant coefficient.
In each experiment, we employed inner iterations to determine the feature selection parameters and the model parameters. To further avoid possible biases during partitioning, we repeated the experiments 10 times. We quantified the classification performance by measuring accuracy (percentage of participants classified correctly). In addition, the area under the receiver operating characteristic curve (AUC) was also calculated to evaluate the overall performance of the classifiers. A larger AUC value suggests better classification performance. Here, a higher classification accuracy and a larger AUC value reflect better diagnostic power.
Validation by different parameters
The number of rich-club regions and connections may affect the findings. Thus, we further validated our findings on the two parameters using the first dataset. In this study, the number of rich-club regions varied from 9 to 16 in steps of 1. As for the number of connections, we changed the probability of each connection from 0.1 to 0.9 in steps of 0.1. In detail, the probability of 0.5 indicated that we considered regions \(i\) and \(j\) connected if connections between these two regions existed in at least 50% of participants of each group. Then, we tested the classification power of rich, feeder and local connections with the above parameters. Specifically, we set the number of rich-club regions to 12 when validating the number of connections and set the probability of connections to 0.5 when validating the number of rich-club regions.
Results
Demographic and clinical variables
The demographic characteristics and clinical test results of PD patients and controls are listed in Table 1. In the first dataset, three PD patients and one healthy control were excluded for poor registration quality. No significant group differences were observed in sex, age and education in the two datasets. In the first dataset, the PD patients showed significantly increased UPDRS scores, including UPDRS-I, UPDRS-II and UPDRS-III. However, there appeared to be no significant difference in cognition as measured by MoCA scores. We found that CSF Aβ1–42, p-tau and t-tau levels, but not α-synuclein levels, were significantly lower in PD patients compared with HC.
Rich-club organization
The rich-club organization was constructed based on the first dataset. Normalized rich-club coefficients (\(\phi _{{{\text{norm}}}}^{w}\)) averaged in each group were reported only when the rich-club effects were detected across at least 90% of participants in both controls and PD patients under corresponding degree (k). Rich-club organization was evident in the two groups, with the normalized rich-club coefficient (\(\phi _{{{\text{norm}}}}^{w}\)) greater than 1 (degree range k = 3 to k = 13).
In this study, the top 12 (14%) highest-degree nodes at a rich-club level of k > 13 were identified to represent rich-club regions on the basis of the average nodal degree across the two groups of participants (Fig. 1). The identified rich-club members included the following (in order of degree): the left and right precuneus (PCUN), left calcarine fissure and surrounding cortex (CAL.L), right lenticular nucleus, putamen (PUT.R), right calcarine fissure and surrounding cortex (CAL.R), left caudate nucleus (CAU.L), superior temporal gyrus of the left temporal pole (TPOsup.L), middle temporal gyrus of the right temporal pole (TPOmid.R), left putamen (PUT.L), right caudate nucleus (CAU.R), middle temporal gyrus of the left temporal pole (TPOmid.L) and left middle occipital gyrus (MOG.L).
Rich-club functions of FA-weighted group networks. A Group-averaged normalized rich-club coefficients for controls (blue) and patients (red). The red and blue shade represents standard error of HC and PD, respectively. Group differences are marked as follows: *p < 0.05, **p < 0.01, ***p < 0.001. B Rich-club nodes (red) and peripheral nodes (gray) across both healthy and patient groups. (C) A simplified example of the three classes of connections: rich-club connections, linking two rich-club nodes; feeder connections, linking one rich-club node to one peripheral node; and local connections, linking two peripheral nodes
Rich-club disturbances
The rich-club disturbances were found based on the first dataset. The normalized rich-club coefficient (\({\phi }_{\mathrm{n}\mathrm{o}\mathrm{r}\mathrm{m}}^{w}\)) curve of both PD patients and controls was illustrated in Fig. 1A. For FA weighted brain networks, PD patients showed significantly lower \({\phi }_{\mathrm{n}\mathrm{o}\mathrm{r}\mathrm{m}}^{w}\) than HC at degrees k = 3,4,8,11,12,13. The results indicated significantly reduced rich-club organization in PD patients, reflecting perturbed connectivity between central hubs of the brain.
The connectivity strength of each of the three distinct types of connections was computed by averaging the FA values of all existing connections of each type. Figure 2 shows the results of three classes of connectivity strength compared between PD patients and controls. PD patients showed significantly increased local connections compared to controls, which might be accounted for as a function of compensation. Although no significant difference was found in either feeder connections or rich-club connections, increased feeder connections and rich-club connections in PD patients indicated a tendency toward damaged communication from peripheral nodes to rich-club nodes. The disruption seemed to appear first in local connections between peripheral and peripheral nodes in PD patients in early stage. By testing the distribution of aberrant rich-club, feeder, and local edges in PD patients compared to HC, we found that these three types of connections were disproportionately affected. 2 out of 25, 34 out of 207 and 72 out of 366 connections were affected in rich-club, feeder and local connections, respectively (Table 2).
Comparison of rich-club organization between the PD patients and the controls (HC). A Distributions of the rich-club, feeder, and local connections. B The aberrant connections in PD patients relative to HC. C Comparison of mean connectivity strength (marked with standard error) of rich-club, feeder and local connections corrected by age, sex and education (*** represents significance level p < 0.001)
Disrupted edge architecture
To investigate the relationship between the disrupted edge architecture and clinical behavioral performance, we correlated the strength of aberrant connections with four clinical scales (MoCA, UPDRS-I, UPDRS-II and UPDRS-III) and four CSF biomarkers (Aβ1-42, α-synuclein, t-tau and p-tau). As shown in Table 3 and Fig. 3, a total of five abnormal connections were related to clinical performance, including three local connections and two feeder connections. Specially, the local connections between the right cuneus (CUN.R) and right superior parietal gyrus (SPG.R), inferior temporal gyrus (ITG.R) were significantly negatively correlated with Aβ1-42 levels. The higher local connection between the left olfactory cortex (OLF.L) and the right gyrus rectus (REC.R) showed significant relationships with higher UPDRS-I scores. As for feeder connections, higher connections between the lef inferior frontal gyrus, orbital part (ORBinf.L) and TPOmid.L showed significant relationships with higher α-synuclein levels, while higher connections between the right supramarginal gyrus (SMG.R) and PUT.R showed significant relationships with lower MoCA scores.
The relationships between abnormal connections and clinical performance or CSF biomarkers controlled for age, sex and education; specially, A indicated the abnormal connections which correlated with clinical performance or CSF biomarkers; B and C indicated the relationships for feeder connections; D–F for local connections
Validation: classification
Validation by different datasets
In this study, we estimated the robustness of detected changes in the edge architecture of PD patients by taking the rich-club, feeder, local and all connections as features inputted into the classifier and observing their classification effect under different datasets. As shown in Table 4 and Fig. 4, local connections showed the highest accuracy and AUC. In detail, we obtained an accuracy of 59.93% for rich-club, 73.96% for feeder, 77.44% for local and 76.32% for all connections when using the first dataset. As for the second dataset, we obtained an accuracy of 68.85% for rich-club, 61.55% for feeder, 72.15% for local and 69.3% for all connections. All these results indicated a pattern in which local edge architecture was prone to disruption in PD patients, which is consistent with the main results of this study.
Receiver operating characteristic (ROC) curves. A Classification results of rich-club, feeder, local and all connections from PPMI data (Dataset 1). B Classification results of rich-club, feeder, local and all connections from validation data (Dataset 2). Each cure showed the relationship between true positive rate (TPR) and false positive rate (FPR)
Validation by different parameters
Classification results on different parameters were shown in Fig. 5, local connections showed the highest accuracy. The validation of rich-club regions showed the highest accuracy of 78.84% for local connections when the number of rich-club regions was 10. Compared to local connections, the accuracy for rich-club connections was 59.19% and for feeder connections was 70.94%. As for the validation of connections, we obtained the highest accuracy of 79.61% for local connections when the probability of connections was 0.8. Compared to local connections, the accuracy for rich-club connections was 60.26% and for feeder connections was 67.55%. All these results indicated a pattern in which local edge architecture was prone to disruption in PD patients, which is consistent with the main results of this study.
Classification performances (accuracy) on different parameters. A Performances varied with the probability of connections. The probability of 0.5 indicated that we considered regions \(i\) and \(j\) connected if connections between these two regions existed in at least 50% of participants. (B) Performances varied with the given number of rich-club regions. Rich-club, feeder and local connections were used to classify PD patients and HC. And the outer circle indicated the higher accuracy
Discussion
The main finding of our study is characterized an increased level of interconnectivity of peripheral nodes in the early stage of PD, suggesting that disruption starts in peripheral regions and tends to gradually extend to rich-club regions. In the early stage of PD, connections between peripheral brain regions seem to have more severe damage than other connections, while rich-club connections remain relatively stable. Moreover, we used two datasets to validate the findings. The fact that local connections showed higher classification power than feeder or rich-club connections demonstrated this point. These findings reveal a disrupted pattern of how the abnormality of structural connectivity would affect the brain dysfunctions of PD patients in early stage.
Rich-club organization
We found obvious rich-club organization in the brains of PD patients, as observed in previous studies (Li et al. 2017). The rich-club phenomenon has been found in humans across a broad age range, from the newborn human brain to the aged adult brain, not only in healthy populations but also in various populations with brain disease, including schizophrenia and Alzheimer’s disease (Cao et al. 2014; Crossley et al. 2014; Onoda and Yamaguchi 2015). Specific brain hubs are efficiently interconnected to form a rich-club, which plays important roles in enabling efficient neuronal signaling and communication and is critically important for the information integration underpinning various behavioral and cognitive tasks (van den Heuvel and Sporns 2013). In this study, a rich-club network of densely connected brain hubs was constructed in both groups, involving the parietal, occipital, temporal cortices and basal ganglia, partly overlapping with reports of parietal and basal ganglia hubs in PD patients (Li et al. 2017). The structural core contains brain regions that form the posterior components of the human default-mode network, such as the bilateral precuneus (Hagmann et al. 2008). In addition, the bilateral precuneus was found to play an important role in between-module connectivity as midline cortical rich-club nodes, and the bilateral putamen is a valuable part of module structure (van den Heuvel and Sporns 2011).
Disrupted edge architecture in PD patients
Our main finding of reduced local connectivity agrees with previous findings that non-rich-club connections were prone to disruption in PD patients (Li et al. 2017). The classification results underscore this point. The local connections showed the highest classification power in distinguishing PD patients and HC compared to feeder and rich-club connections. A similar pattern was found in patients with Alzheimer’s disease (Yan et al. 2018), while the opposite phenomenon was demonstrated in schizophrenia (Crossley et al. 2014). Rich-club architectures exhibit a high cost but high value feature (Collin et al. 2014), and they are beneficial to functional repertoire in the brain network (Senden et al. 2014). The architectures may help with keeping functional diversity, so not behave disrupted in the early stage of PD. In addition, we observed disproportions of aberrant connections in rich-club (8%), feeder (16.43%) and local connections (19.67%). All these findings suggest that in the early stage of PD, impaired connectivity may be concentrated in connections linked to peripheral regions rather than equally affecting all white matter connections. According to Gollo et al. (2015), hub regions are closely synchronized with affective experiences, while peripheral regions are related to fast perceptual processes. In the present study, perturbations of peripheral regions, characterized by faster activity than perturbations of hub regions, have a greater impact on functional disorder in PD patients. Our results also support the notion that brain hubs are more resilient than peripheral regions to focal perturbations and critically contribute to maintaining stability in global brain dynamics.
Relationship with clinical features
We detected abnormal structural connections and further explored their relationship with clinical features (MoCA, UPDRS-I, II, III scores and Aβ1-42, α-synuclein, t-tau, p-tau levels). Our findings revealed that different abnormal structural connections correlated to different clinical features. That may indicate that different connections contribute to different clinical performance. Note that, we failed to find significant relationships between connections and UPDRS-II, UPDRS-III after Bonferroni corrected. The lack of significant relationships may because UPDRS II and III are less sensitive to early stage of PD, especially in H&Y Stage 1 and 2 (Skorvanek et al. 2017). Higher connections between OLF.L and REC.R, belonging to within prefrontal structural connectivity, related to higher UPDRS-I scores, indicating that increased connectivity strength may lead to poor non-motor aspects of experiences of daily living of PD patients. The findings are consistent with a previous DTI study, which also found the abnormal FA values of OLF and REC in the early stages of PD (H&Y stages I and II) (Ibarretxe-Bilbao et al. 2010). Additionally, higher connectivity strength between SMG.R and PUT.R correlated with lower MoCA scores, indicating that higher connectivity between parietal and basal ganglia may lead to poor cognitive performance. Previous study has found the higher mean diffusivity of SMG and PUT in PD patients (Kim et al. 2013). As for the connectivity strength between SMG.R and PUT.RA, a fMRI study has shown that it is related to vocalization in PD patients (Rektorova et al. 2012). Abnormal structural interconnections of the occipital (CUN.R), frontal (REC.R), parietal (SPG.R) and temporal (OLF.L and ITG.R) cortex suggested widespread microstructural damage to frontal, parietal and occipital white matter in the early stages of PD.
Previous study based on PPMI dataset has found the levels of Aβ1-42 and α-synuclein have the potential to predict the cognitive and motor function in PD patients (Irwin et al. 2020), while the relationship with brain structural network is not clear. In this study, we found that lower levels of Aβ1-42 were related to higher connectivity strength between CUN.R and SPG.R, higher connectivity strength between CUN.R and ITG.R. Besides, lower connectivity strength between ORBinf.L and TPOmid.L was significantly associated with lower α-synuclein, supporting the impression that protein α-synuclein is a central component to the pathogenesis of the disease. The early stages of PD patients have showed the cortical atrophy of ITG (Wolters et al. 2020), CUN, SPG (Uribe et al. 2018) and left temporal pole (Jubault et al. 2011), which may cause the low levels of CSF biomarkers. Previous studies have also found the higher brain iron accumulation (Uchida et al. 2019), lower regional cerebral blood flow (Imamura et al. 2011), and decreased perfusion (Melzer et al. 2011) of CUN in PD patients. A positron emission tomography (PET) study found that hypometabolism in ORBinf.L involved in fatigue in PD patients (Zhang et al. 2018). Our findings added more evidences to the point that lower Aβ1-42 and α-synuclein levels were associated with PD diagnosis.
Conclusions
In summary, this study found that disturbance of PD patients in early stage focused on abnormal connections linked to peripheral regions rather than connections linked to rich-club regions. Some of these abnormal local and feeder connections were significantly related to clinical features or CSF biomarkers. Knowledge of this disruption pattern in the early stage of PD may support investigations into prevention and treatment strategies for early PD patients.
Availability of data and material
In this study, the first dataset is available in the PPMI data repository (http://www.ppmi-info.org/access-dataspecimens/download-data/) PPMI is funded by the Michael J. Fox Foundation for Parkinson’s Research and other funding partners, including a consortium of industry players, non-profit organizations and private individuals. Please visit PPMI website (http://www.ppmi-info.org/) for details regarding the PPMI sponsors. And the second dataset is available from the corresponding author on request.
Code availability
The code that support the findings of this study are openly available from the corresponding author on request.
Abbreviations
- AAL:
-
Automated anatomical labeling
- AUC:
-
Area under the receiver operating characteristic curve
- CSF:
-
Cerebrospinal fluid
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- HC:
-
Healthy controls
- PD:
-
Parkinson’s disease
- PPMI:
-
Parkinson’s Disease Progression Marker Initiative
- SVM:
-
Support vector machine
References
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of parkinson disease a review. JAMA J Am Med Assoc 323:548–560. https://doi.org/10.1001/jama.2019.22360
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455. https://doi.org/10.1002/nbm.782
Braak H, Del Tredici K, Rub U, de Vos RAI, Steur E, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211. https://doi.org/10.1016/s0197-4580(02)00065-9
Bullmore ET, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198. https://doi.org/10.1038/nrn2575
Cao M et al (2014) Topological organization of the human brain functional connectome across the lifespan. Dev Cogn Neurosci 7:76–93. https://doi.org/10.1016/j.dcn.2013.11.004
Chang CC, Lin CJ (2011) LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2:27. https://doi.org/10.1145/1961189.1961199
Colizza V, Flammini A, Serrano MA, Vespignani A (2006) Detecting rich-club ordering in complex networks. Nat Phys 2:110–115. https://doi.org/10.1038/nphys209
Collin G, Sporns O, Mandl RCW, van den Heuvel MP (2014) Structural and functional aspects relating to cost and benefit of rich club organization in the human cerebral cortex. Cereb Cortex 24:2258–2267. https://doi.org/10.1093/cercor/bht064
Colosimo C et al (2010) Task force report on scales to assess dyskinesia in parkinson’s disease: critique and recommendations. Mov Disord 25:1131–1142. https://doi.org/10.1002/mds.23072
Crossley NA, Mechelli A, Scott J, Carletti F, Fox PT, McGuire P, Bullmore ET (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137:2382–2395. https://doi.org/10.1093/brain/awu132
Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909. https://doi.org/10.1016/s0896-6273(03)00568-3
Ewing-Cobbs L, Hasan KM, Prasad MR, Kramer L, Bachevalier J (2006) Corpus callosum diffusion anisotropy correlates with neuropsychological outcomes in twins disconcordant for traumatic brain injury. Am J Neuroradiol 27:879–881
Goetz CG et al (2008) Movement disorder society-sponsored revision of the unified parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170. https://doi.org/10.1002/mds.22340
Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA (2007) Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia 45:2439–2446. https://doi.org/10.1016/j.neuropsychologia.2007.04.011
Gollo LL, Zalesky A, Hutchison RM, van den Heuvel M, Breakspear M (2015) Dwelling quietly in the rich club: brain network determinants of slow cortical fluctuations. Philos Trans R Soc B Biol Sci 370:54–68. https://doi.org/10.1098/rstb.2014.0165
Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen V, Sporns O (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:1479–1493. https://doi.org/10.1371/journal.pbio.0060159
Hall JM et al (2018) Alterations in white matter network topology contribute to freezing of gait in Parkinson’s disease. J Neurol 265:1353–1364. https://doi.org/10.1007/s00415-018-8846-3
Ibarretxe-Bilbao N et al (2010) Olfactory impairment in Parkinson’s disease and white matter abnormalities in central olfactory areas: a voxel-based diffusion tensor imaging study. Mov Disord 25:1888–1894. https://doi.org/10.1002/mds.23208
Imamura K, Okayasu N, Nagatsu T (2011) The relationship between depression and regional cerebral blood flow in Parkinson’s disease and the effect of selegiline treatment. Acta Neurol Scand 124:28–39. https://doi.org/10.1111/j.1600-0404.2010.01443.x
Irwin DJ et al (2020) Evolution of Alzheimer’s disease cerebrospinal fluid biomarkers in early Parkinson’s disease. Ann Neurol 88(3):574–587. https://doi.org/10.1002/ana.25811
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376. https://doi.org/10.1136/jnnp.2007.131045
Jubault T, Gagnon JF, Karama S, Ptito A, Lafontaine AL, Evans AC, Monchi O (2011) Patterns of cortical thickness and surface area in early Parkinson’s disease. Neuroimage 55:462–467. https://doi.org/10.1016/j.neuroimage.2010.12.043
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Kang JH et al (2016) CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s progression markers initiative study. Acta Neuropathol 131:935–949. https://doi.org/10.1007/s00401-016-1552-2
Kim HJ et al (2013) Alterations of mean diffusivity in brain white matter and deep gray matter in Parkinson’s disease. Neurosci Lett 550:64–68. https://doi.org/10.1016/j.neulet.2013.06.050
Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2:15. https://doi.org/10.1101/cshperspect.a008888
Kubicki M et al (2005) DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage 26:1109–1118. https://doi.org/10.1016/j.neuroimage.2005.03.026
Li CH et al (2017) Impaired topological architecture of brain structural networks in idiopathic Parkinson’s disease: a DTI study. Brain Imaging Behav 11:113–128. https://doi.org/10.1007/s11682-015-9501-6
Majbour NK et al (2016) Longitudinal changes in CSF alpha-synuclein species reflect Parkinson’s disease progression. Mov Disord 31:1535–1542. https://doi.org/10.1002/mds.26754
Marek K et al (2011) The Parkinson progression marker initiative (PPMI). Prog Neurobiol 95:629–635. https://doi.org/10.1016/j.pneurobio.2011.09.005
Melzer TR et al (2011) Arterial spin labelling reveals an abnormal cerebral perfusion pattern in Parkinson’s disease. Brain 134:845–855. https://doi.org/10.1093/brain/awq377
Mori S, van Zijl PCM (2002) Fiber tracking: principles and strategies—a technical review. NMR Biomed 15:468–480. https://doi.org/10.1002/nbm.781
Mori S, Crain BJ, Chacko VP, van Zijl PCM (1999) Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 45:265–269. https://doi.org/10.1002/1531-8249(199902)45:2%3c265::aid-ana21%3e3.0.co;2-3
Onoda K, Yamaguchi S (2015) Dissociative contributions of the anterior cingulate cortex to apathy and depression: topological evidence from resting-state functional MRI. Neuropsychologia 77:10–18. https://doi.org/10.1016/j.neuropsychologia.2015.07.030
Opsahl T, Colizza V, Panzarasa P, Ramasco JJ (2008) Prominence and control: the weighted rich-club effect. Phys Rev Lett 101:4. https://doi.org/10.1103/PhysRevLett.101.168702
Rektorova I, Mikl M, Barrett J, Marecek R, Rektor I, Paus T (2012) Functional neuroanatomy of vocalization in patients with Parkinson’s disease. J Neurol Sci 313:7–12. https://doi.org/10.1016/j.jns.2011.10.020
Senden M, Deco G, de Reus MA, Goebel R, van den Heuvel MP (2014) Rich club organization supports a diverse set of functional network configurations. Neuroimage 96:174–182. https://doi.org/10.1016/j.neuroimage.2014.03.066
Skorvanek M et al (2017) Differences in MDS-UPDRS scores based on Hoehn and Yahr stage and disease duration. Mov Disord Clin Practice 4:536–544. https://doi.org/10.1002/mdc3.12476
Smith SM et al (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Sporns O, Tononi G, Kotter R (2005) The human connectome: a structural description of the human brain. PLoS Comput Biol 1:245–251. https://doi.org/10.1371/journal.pcbi.0010042
Sun ZG et al (2003) Abnormal anterior cingulum in patients with schizophrenia: a diffusion tensor imaging study. NeuroReport 14:1833–1836. https://doi.org/10.1097/00001756-200310060-00015
Uchida Y et al (2019) Voxel-based quantitative susceptibility mapping in Parkinson’s disease with mild cognitive impairment. Mov Disord 34:1164–1173. https://doi.org/10.1002/mds.27717
Uribe C et al (2018) Cortical atrophy patterns in early Parkinson’s disease patients using hierarchical cluster analysis Parkinsonism. Relat Disord 50:3–9. https://doi.org/10.1016/j.parkreldis.2018.02.006
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. https://doi.org/10.1523/jneurosci.3539-11.2011
van den Heuvel MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17:683–696. https://doi.org/10.1016/j.tics.2013.09.012
van den Heuvel MP et al (2013) Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiat 70:783–792. https://doi.org/10.1001/jamapsychiatry.2013.1328
Verstraete E, van den Heuvel MP, Veldink JH, Blanken N, Mandl RC, Pol HEH, van den Berg LH (2010) Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS ONE 5:9. https://doi.org/10.1371/journal.pone.0013664
Witten DM, Tibshirani R (2009) Covariance-regularized regression and classification for high dimensional problems. J R Stat Soc Ser B Stat Methodol 71:615–636. https://doi.org/10.1111/j.1467-9868.2009.00699.x
Wolters AF et al (2020) Grey matter abnormalities are associated only with severe cognitive decline in early stages of Parkinson’s disease. Cortex 123:1–11. https://doi.org/10.1016/j.cortex.2019.09.015
Yan TY, Wang WH, Yang L, Chen KW, Chen R, Han Y (2018) Rich club disturbances of the human connectome from subjective cognitive decline to Alzheimer’s disease. Theranostics 8:3237–3255. https://doi.org/10.7150/thno.23772
Zhang L et al (2018) Brain metabolic correlates of fatigue in Parkinson’s disease: a PET study. Int J Neurosci 128:330–336. https://doi.org/10.1080/00207454.2017.1381093
Acknowledgements
This work was supported by the National Key R&D Program of China (grant number 2018YFC0115400), the National Natural Science Foundation of China (grant numbers U20A20191, 61727807, 82071912), the Beijing Municipal Science and Technology Commission (grant numbers Z191100010618004, Z201100007720009). We gratefully acknowledge the Michael J. Fox Foundation for Parkinson’s Research and other funding partners, who establish the PPMI database (www.ppmi-info.org/data). In addition, we acknowledge the participants and clinical doctors at the Parkinson’s disease outpatient clinic of Beijing Rehabilitation Hospital, Capital Medical University.
Funding
This work was supported by the National Key R&D Program of China (grant number 2018YFC0115400), the National Natural Science Foundation of China (grant numbers U20A20191, 61727807, 82071912), the Beijing Municipal Science and Technology Commission (grant numbers Z191100010618004, Z201100007720009).
Author information
Authors and Affiliations
Contributions
Tiantian Liu participated in data analysis and wrote the paper. Yan Yan performed the data analysis. Jing Ai collected the data. Duanduan Chen modified the paper. Jinglong Wu verified the analytical methods. Tianyi Yan and Boyan Fang conducted literature research and conceived of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
This study was approved by the Ethics Committee of Beijing Rehabilitation Hospital, Capital Medical University (No. 2018bkky022).
Consent to participate
All participants were provided with written informed consent, which they signed prior to any experimental procedures.
Consent to publication
All authors discussed the study, read the manuscript, and approved its submission to your journal. The manuscript has not been published previously, and it is not under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, T., Yan, Y., Ai, J. et al. Disrupted rich-club organization of brain structural networks in Parkinson’s disease. Brain Struct Funct 226, 2205–2217 (2021). https://doi.org/10.1007/s00429-021-02319-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-021-02319-3