Abstract
Parkinson’s disease (PD), which is caused by degeneration of dopaminergic neurons in the midbrain, results in a heterogeneous clinical picture including cognitive decline. Since the phasic signal of dopamine neurons is proposed to guide learning by signifying mismatches between subjects’ expectations and external events, we here investigated whether akinetic-rigid PD patients without mild cognitive impairment exhibit difficulties in dealing with either relevant (requiring flexibility) or irrelevant (requiring stability) prediction errors. Following our previous study on flexibility and stability in prediction (Trempler et al. J Cogn Neurosci 29(2):298–309, 2017), we then assessed whether deficits would correspond with specific structural alterations in dopaminergic regions as well as in inferior frontal cortex, medial prefrontal cortex, and the hippocampus. Twenty-one healthy controls and twenty-one akinetic-rigid PD patients on and off medication performed a task which required to serially predict upcoming items. Switches between predictable sequences had to be indicated via button press, whereas sequence omissions had to be ignored. Independent of the disease, midbrain volume was related to a general response bias to unexpected events, whereas right putamen volume correlated with the ability to discriminate between relevant and irrelevant prediction errors. However, patients compared with healthy participants showed deficits in stabilisation against irrelevant prediction errors, associated with thickness of right inferior frontal gyrus and left medial prefrontal cortex. Flexible updating due to relevant prediction errors was also affected in patients compared with controls and associated with right hippocampus volume. Dopaminergic medication influenced behavioural performance across, but not within the patients. Our exploratory study warrants further research on deficient prediction error processing and its structural correlates as a core of cognitive symptoms occurring already in early stages of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, which results from depletion of dopamine (DA) production in the substantia nigra (SN). The degeneration of dopaminergic neurons in the SN progressively affects both subcortical and cortical areas along different neuronal pathways (Braak et al. 2003; Goedert et al. 2013). Apart from specific motor symptoms such as bradykinesia, rigidity, and resting tremor, changes in cortico-basal ganglia-cortical projections also result in initially subtle but progressive cognitive impairment (Muslimović et al. 2005; Chaudhuri and Schapira 2009; Kehagia et al. 2010). The question, which structural changes lead to which specific impairment, has moved into the focus of cognitive neuropsychology (see Biundo et al. 2016, for a review), but is thus far not answered conclusively.
Cognitive deficits in PD include impaired prediction of upcoming events implicated by experience-based internal models of the world that guide motor as well as cognitive control by (probabilistic) inference: For example, Schönberger et al. (2013) reported that patients show difficulties in predicting stimulus sequences and, moreover, that these difficulties are intra-individually correlated with the severity of motor dysfunctions. Other studies found compromised learning from prediction errors (PEs), i.e., mismatches between subjects’ expectations and external events (Schott et al. 2007; Schonberg et al. 2010; Galea et al. 2012). PE processing deficiencies are likely to be caused by the disruption of DA production in PD since phasic DA release in the SN appears to be triggered by unpredicted external stimuli (Schultz and Dickinson 2000; Redgrave and Gruney 2006; Schiffer et al. 2015).
Beyond signalling surprise, violations of predictions can be differentiated based on their behavioural implications. They could either signal the need for adapting to lasting changes of the environment (thereby requiring flexible updating), or be caused by temporary distractors (requiring stabilisation of predictions). Irrespective of predictive functioning, Cools and D’Esposito (2011) suggest that optimal dopamine (DA) levels in frontostriatal circuits are responsible for balancing the trade-off between cognitive stability and flexibility. On the one hand, DA receptor activation in the striatum—one of the main target structures of SN projections—is associated with flexible gating of relevant information into working memory (Badre 2012; Chatham and Badre 2015; Frank et al. 2001; Cools et al. 2007; D’Ardenne et al. 2012). In contrast, DA in the lateral prefrontal cortex (PFC) is essential for stabilising working memory representations (Cohen et al. 2002; Miller and Cohen 2001; Bilder et al. 2004; D’Esposito 2007). According to the dual-state theory of working memory, representations in the PFC are regulated by so-called attractor networks, with either high or low energy barriers favouring either maintenance of the current representational state or flexible and fast switching among different states (Durstewitz and Seamans 2008). Regarding PD, there is evidence that in the early stages of PD, when DA depletion primarily affects the striatum, patients perform worse at flexible updating. On the contrary, the ability to maintain working memory content is not affected or can even be improved compared with healthy controls (Cools et al. 2003, 2009).
Within the specific scope of prediction, we recently found slightly different networks for stabilisation and flexible updating of internal models (Trempler et al. 2017). Here, stability was defined as the capacity to shield the internal model against unexpected irrelevant temporary changes, whereas flexible updating was required in response to unexpected, but lasting changes of the environment. Because of this operationalisation approach, the striatum was activated by either relevant or irrelevant sequential PEs highlighting striatal gating to control cognitive and motor representations in the frontal cortex (Chatham and Badre 2015; Cools 2011). Stabilisation was associated with activity of the inferior frontal gyrus (IFG), consistent with its role in active maintenance of working memory representations and inhibition (Baddeley 1986). In contrast, flexible adaptation was accompanied by activation of medial prefrontal cortex (mPFC) and the hippocampus contributing to updating and learning from PEs (Schlichting and Preston 2015; Schiffer et al. 2012). Owing to the evidence for an involvement of DA in prediction on the one hand, and in flexibility and stability on the other, the present study aimed at investigating whether stability and flexibility in the particular case of prediction are both impaired in PD. By reason of the various findings on the relationship between cognitive deficits and grey matter alterations in the striatum as well as in frontal and temporal regions in PD (see Kehagia et al. 2012, for a review on the progression of structural abnormalities in PD), we further explored whether deficient PE processing would relate to structural variations within a priori defined brain regions.
To this end, akinetic-rigid PD patients and healthy controls performed a task previously described in Trempler et al. (2017). The task requires monitoring of a digit sequence for order-violating items. Switches between predictable sequences need to be indicated via button press (requiring prediction flexibility), whereas digit omissions, drifts hereafter, should be ignored (requiring prediction stability). Using correlation analysis on the rate of correctly ignored drifts and detected switches, we first assessed whether (1) cognitive stability and cognitive flexibility of prediction would act in an antagonistic fashion in patients and not in healthy controls (Cools et al. 2003, 2009; Trempler et al. 2017). Moreover, we hypothesised that (2) patients compared with controls would show deficits in discriminating between irrelevant and relevant PEs and, as a result, impaired cognitive stability and flexibility as reflected in lower rates of drift rejection and switch detection, respectively. Since midbrain DA neurons are suggested to respond to unexpected events, (3) midbrain volume variations were expected to be associated with the probability to respond to surprising stimuli per se, i.e., a general response bias irrespective of stimulus identity (Redgrave and Gurney 2006). Striatal volume was (4) predicted to correlate with difficulties in discriminating between drifts and switches (Chatham and Badre 2015; Trempler et al. 2017). Furthermore, we expected (5) deficits in cognitive stability to be accompanied by structural alterations in IFG, which is known to be involved in maintaining working memory content and inhibition (Cohen et al. 2002; Fegen et al. 2015). In contrast, (6) reduced cognitive flexibility would correlate with inter-individual hippocampus volume and mPFC thickness differences due to the role of these regions in updating and learning (Schlichting and Preston 2015). Comparing correlation coefficients between the groups helped to quantify the specificity of the hypothesised brain-behaviour relationships. Finally, in view of the evidence for an involvement of DA in stability and flexibility of prediction, we (7) expected performance at switches and drifts to be partially improved by medication within and across patients and assessed whether daily medication dose would be associated with grey matter changes.
Materials and methods
Participants
23 right-handed patients with akinesia-rigidity dominant idiopathic Parkinson’s disease (abbreviated as PD in the following) (6 females; 58.83 ± 9.24 years old; range 40–72 years) participated in the study. A group of 21 healthy participants (6 females; 60.05 ± 10.05 years old; range 36–74 years) similar to the patients regarding age and gender served as control subjects. Patients were acquired from the neurologic outpatient clinic of the University Hospital of Cologne, Germany. Only those patients that met the United Kingdom Parkinson´s Disease (UKPD) Society Brain Bank Criteria for idiopathic Parkinson’s disease (Hughes et al. 1992) and no patients with atypical parkinsonian syndrome were included in the study, also indicated by a significant response to their individual dopaminergic medication. To select a clinically homogenous group and to minimize potential movement artefacts, only patients of the akinetic-rigid subtype according to a clinical judgment of an experienced movement disorder specialist were selected. No participant had undergone neurosurgical treatment for the disease or had a history of other neurological or psychiatric diseases. Symptoms of nine patients were left-dominant, and symptoms of eight patients were right-dominant (with onset of symptoms as criterion). All patients were tested once on their regular medication and once off medication to investigate whether DA medication could improve not only motor but also cognitive deficits resulting from the disease. Patients in the OFF-state were studied after overnight withdrawal of dopaminergic medication. The levodopa equivalent daily dose (LEDD) was calculated according to Tomlinson et al. (2010) and the severity of clinical symptoms was defined according to Hoehn and Yahr (1967) and to the motor score of the Unified Parkinson’s Disease Rating Scale (UPDRS III) (Fahn and Elton 1987). Hoehn and Yahr ratings ranged between I and III under regular medication. UPDRS III was assessed by a movement disorder specialist and additionally determined on the basis of video tapes by a second specialist blinded for the state of medication (see Supplementary Material). Patients and controls with any evidence of dementia or depression would have been excluded from the study. However, all participants scored between 19 and 30 points in the Parkinson Neuropsychometric Dementia Assessment (PANDA; 18–30 points = “age adequate cognitive performance”) (Kalbe et al. 2008) and lower than 19 points in the Beck depression inventory-II (BDI-II; cut-off for depression: ≥ 20 points) (Hautzinger et al. 2006). Two patients were excluded due to difficulties in completing the main task. Thus, a total of 21 PD patients (6 females, 58.81 ± 9.89-years-old; range 40–72) were included in the analyses. For further assessment, all participants performed the two subtests “Divided Attention” and “Go/NoGo” of the TAP (Testbatterie zur Aufmerksamkeitsprüfung) (Zimmermann and Fimm 1993) and completed the Barratt Impulsiveness Scale (BIS-11; Patton et al. 1995). Table 1 summarises demographic data of patients and controls.
All subjects gave written informed consent prior to participation. The study was performed in accordance with the Declaration of Helsinki and had been approved by the ethics committee of the Medical Faculty of the University Hospital Cologne, Germany. Each participant submitted a signed informed consent notification and received reimbursement for participation afterwards (10 € per hour plus travel expenses).
Procedure and task
Healthy controls and PD patients attended the study on 1 and 3 days, respectively. Controls performed training, experiment, and additional assessments on the same day, whereas patients were first screened for being able to perform the task with their regular dopaminergic medication. The second day, 50% of patients were tested on medication and 50% were tested off medication. The third day was arranged in the same way as day two, except that the other 50% of patients were now tested off medication and vice versa. Healthy controls did not receive any medication.
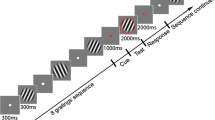
Participants performed a slightly modified version of the paradigm as described in Trempler et al. (2017). They were presented with one of two different digit sequences, which allowed them to predict forthcoming input (ascending model: 1–2–3–4, descending model: 4–3–2–1) (Fig. 1). Digits continuously succeeded one another and were presented one at a time at the centre of the screen for 1 s, separated by an inter-stimulus interval of 100 ms. Sequences repeated constantly to enable the participants to predict the regular sequence. Switches between the sequences, i.e., directional changes, occurred at a random ordinal position within the initial sequence so that participants had to flexibly adapt their prediction according to the new direction of the presented sequence. In addition, single digits were omitted occasionally at variable positions without a temporal gap (drifts, hereafter). Contrary to switches, drifts disturbed the predictive process and required stabilisation of the internal model. Hence, the participants’ task was to signal a switch from one model to the other by button press (switch detection), but to ignore the sequential omissions (drift rejection). Moreover, a motor control task was implemented to assess the individual mean reaction time. Here, one digit of the sequence repeated continuously, but maximally eight times until the participant pressed the response button. Baseline trials with a 6 s presentation of a fixation cross were distributed equally across the experiment.
Schematic diagram of the task. Stimuli of a simple 4-digit sequence continuously followed each other with a duration of 1 s and an inter-stimulus interval of 100 ms. Subjects had to indicate changes from ascending to descending sequences (and vice versa) (switch), as displayed in the left row, via button press. Moreover, they had to ignore the omission of a single digit (drift), as displayed in the middle row. During a motor control task, depicted on the right, one digit repeated continuously until the participant pressed the response button
The task was binned into 12 blocks that either had a high or low probability of switches, either paired with a high or low probability of drifts. Each block consisted of an average number of 125 trials in a full-factorial 2 (probability: high vs. low) × 2 (event: switch vs. drift) design. Stimulus exposure per block was pseudo-randomised using the stochastic universal sampling method (Baker 1987). Results of this probability manipulation will be reported elsewhere.
The training session contained ten blocks of 80 trials each and a probability of 16% for switch or drift occurrence. To enable participants to get accustomed to the task, presentation speed started at 1400 ms per digit and adapted block-wise with a decrease of 50 ms if the participant reacted correctly to 75% of the events. In addition, patients performed a short training directly prior to the main experiment consisting of three blocks with 80 trials at the main experiment’s digit presentation speed of 1 s. The randomisation was programmed using MATLAB R2012b (The MathWorks Inc., Natick, MA, USA) and stimuli were presented using Presentation 13.1 (Neurobehavioral Systems, San Francisco, CA, USA).
Behavioural data analysis
Unpaired t tests were used to explore group differences in demographic variables. Derived from signal detection theory measures, task performance was assessed by hits (correct detection of switches), correct rejections (CRs) of drifts and, correspondingly, switch misses and false alarms at drifts. Differences in task measures between controls and patients OFF were regarded as effects of the disease, whereas differences between patients ON and OFF were interpreted as effects of DA medication. No comparisons between controls and patients ON were carried out due to lacking hypotheses about potential differences. Individual response time windows per participant were calculated from the mean reaction time plus two standard deviations as determined by the motor control task. If the participants pressed the button within this specific time window after a switch, their response was acknowledged as hit, whereas it counted as CR, if the participants did not press the button after a drift. There were no differences between the groups in the individual response time window length (controls (M = 2432.38, SD = 490.38) vs. patients OFF (M = 2318.50, SD = 649.79): t(40) = 0.644, p = 0.511; patients OFF vs. patients ON (M = 2430.54, SD = 800.24): t(20) = 1.496, p = 0.15).
A discrimination index [Pr; probability of recognition of switches and drifts, i.e., Pr = hit rate - false alarm rate] indicating the participants’ ability to discriminate between drifts and switches and a bias index [Br; response probability in an uncertain state, i.e., Br = false alarm rate/(1 − Pr)] for an assessment of the overall motor threshold were calculated (Snodgrass and Corwin 1988). Data points per participants for each behavioural variable exceeding two standard deviations from the respective group mean were regarded as outliers and excluded from further analyses. This procedure resulted in exclusion of one healthy participant of analyses including the switch measure and the Pr index.
To assess the relationship between drift CRs and switch hits we calculated Pearson’s correlation coefficients for each group separately. Comparisons between control participants and patients OFF regarding behavioural measures were carried out by an analysis of covariance (ANCOVA). Br index served as a covariate as variations in drift CRs and switch hits can partly be explained by the individual probability to respond to surprising stimuli in general. Differences in CRs and hits between PD patients ON and OFF were assessed by paired t tests on the unstandardized residuals of the regression of hits and CRs on Br index. Reaction times were calculated for each group separately and compared by either unpaired t tests to determine differences between controls and patients OFF or by paired t tests for differences between patients ON and OFF. Finally, we calculated the Pearson’s correlation coefficient for an assessment of the relationship between LEDD and task performance in the ON-state. If not stated otherwise, significance tests were performed at α = 0.05, one-sided, based on directional hypotheses with regard to the behavioural data. Statistical analyses were performed using IBM SPSS Statistics 22.
Brain imaging and data processing
Whole-brain imaging data of healthy controls and PD patients were collected at the Research Centre Jülich, Germany, on a 3 T Siemens TIM TRIO MRI scanner using a Tx/Rx CP head coil. Structural data were acquired for each participant using a standard Siemens 3D T1-weighted MPRAGE sequence for detailed reconstruction of anatomy with isotropic voxels (1 × 1 × 1 mm3) in a 256 mm field of view (256 × 256 matrix, 172 slices, TR = 2250, TE = 3.03). In addition, functional images were acquired during the main task using a gradient T2*-weighted single-shot echo-planar imaging (EPI) sequence sensitive to blood oxygenation level dependent (BOLD) contrast. The corresponding results will be reported elsewhere.
Estimates of cortical thickness and volumetric segmentation were obtained using FreeSurfer 5.3.0 (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures have been described in previous publications (Dale et al. 1999; Fischl and Dale 2000). The standardised processing includes motion correction, removal of non-brain tissue, automated Talairach transformation, segmentation of the subcortical white matter (WM) and deep grey matter (GM) volumetric structures, intensity normalization, tessellation of the GM/WM boundary, and automated topology correction. The FreeSurfer data were checked visually after preprocessing for any topological defects in the surface. For each subject, a triangular mesh was used to measure the distance from the pial surface to the GM/WM boundary for each hemisphere. A priori regions of interest (ROIs), i.e., bilateral portions of the inferior frontal gyrus (pars orbitalis, pars triangularis, pars opercularis), bilateral rostral anterior cingulate cortex (rACC) and superior frontal gyrus, based on gyral anatomical landmarks were parcellated on each hemisphere (Desikan et al. 2006). Subcortical GM volume measures for bilateral caudate nucleus, putamen and hippocampus were automatically extracted as part of the standard FreeSurfer pipeline. Midbrain segmentation was automatically performed by the application of a Bayesian algorithm relying on a probabilistic atlas of the brainstem as described in Iglesias et al. (2015).
Statistical analysis of a priori ROIs
Thickness of left and right IFG was computed by averaging the extracted measures of pars orbitalis, pars triangularis, and pars opercularis due to the lack of specific hypotheses on the respective substructures. Thickness of left and right mPFC was computed by averaging measures of rACC and superior frontal gyrus as these regions correspond closest to the activation pattern found in our previous study (Trempler et al. 2017). Thickness per region was proportionally normalised by the mean thickness of the respective hemisphere. Volumes of subcortical ROIs, i.e., midbrain, caudate, putamen, and hippocampus, were normalised by the estimated total intracranial volume. Unpaired t tests were used to assess group differences in volume and thickness of the respective regions.
For cognitive measures, partial correlations, controlling for age, were performed between midbrain and Br index for each group separately to investigate whether volume variations were associated with the probability to respond to surprising stimuli per se. Correlations between bilateral caudate and putamen volume and Pr index were analysed to determine the contribution of morphology of these regions to differences in the capacity to discriminate between different types of PEs. Furthermore, we examined the relationship between drift CRs and IFG thickness and between switch hits and hippocampus volume by partial correlations, controlling for age, for healthy controls and patients OFF. To investigate the specificity of these effects also the reversed correlations were calculated. Coefficients were compared between the groups to assess whether relationships were specific to the disease or could also apply to healthy controls. For bilateral tests, the alpha level was corrected to p < 0.025. Finally, we performed a regression analysis of GM measures on LEDD, controlling for age and disease duration.
Results
Group differences in task performance
To investigate differences in the relationship between cognitive stability and flexibility in patients and controls, we calculated and compared correlation coefficients between drift CRs and switch hits of each group separately. The correlation was more negative in patients OFF, r = − 0.073, p = 0.753, compared with controls, r = 0.345, p = 0.136, and with patients ON, r = 0.265, p = 0.246, but the differences between these correlation coefficients did not reach significance (Controls vs. Patients OFF: z = 1.28, p = 0.201; Patients ON vs. Patients OFF: z = 1.03, p = 0.303). Thus, (1) there was a weak positive though non-significant relationship in controls and patients ON, but no systematic correlation in patients OFF (Fig. 2a).
a Scatter plot of correlation between switch hits and drift correct rejections (CRs) in healthy controls and akinetic-rigid Parkinson’s Disease (PD) patients in the OFF- and ON-state. As expected, the two measures were not significantly correlated with each other. b Unstandardized residuals of the regression of Br index on switch hits (left) and drift CRs (middle) and Pr index (right) of healthy control participants and patients OFF and ON. In all three measures, PD patients OFF significantly differed from controls, whereas there were no differences between patients OFF and ON. *p < 0.05, one-tailed
Behavioural manifestations of deficits in cognitive stability were determined by comparisons of drift CRs of PD patients OFF and healthy controls, controlling for the general response bias as assessed by the Br index. In contrast, impaired cognitive flexibility was measured by comparisons of switch hits of the two groups, controlling for the Br index. In accordance with our hypothesis, (2) patients OFF compared with healthy controls had lower rates of both drift CRs, F(1,39) = 3.085, p = 0.044, and switch hits, F(1,38) = 3.924, p = 0.028. Accordingly, the Pr index that measures discrimination between drifts and switches significantly differed between the two groups, t(39) = 2.062, p = 0.023 (Fig. 2b). Reaction times of switch hits, [Controls (M = 1394.47, SD = 481.60) vs. Patients OFF (M = 1485.77, SD = 412.92): t(39) = − 0.522, p = 0.604] and drift false alarms [Controls (M = 1393.46, SD = 584.41) vs. Patients OFF (M = 1412.92, SD = 743.73): t(39) = − 0.091, p = 0.928] did not differ between the groups.
Contrary to our hypothesis, (7) patients ON and OFF did not differ in drift rejection, t(20) = 0.413, p = 0.684, and switch detection, t(20) = 0.559, p = 0.582. Likewise, there were no differences in the Pr index, t(20) = 0.867, p = 0.396. Finally, dopaminergic medication did not affect reaction times at switch hits [Patients ON (M = 1494.73, SD = 613.22) vs. Patients OFF: t(19) = − 0.673, p = 0.509] and drift false alarms [Patients ON (M = 1519.05, SD = 557.90: t(19) = − 0.410, p = 0.687]. However, focusing on the effects of individual dopaminergic medication dose on task performance across patients in the ON-state, we found a significant positive correlation between LEDD and the Pr index, r = 0.404, p = 0.035.
Group differences in GM measures
Patients compared with healthy controls exhibited a significant decrease of left caudate volume, t(38) = 3.883, p < 0.001, and differences in right caudate volume at a probability of significance close to the adjusted alpha level, t(39) = 2.33, p = 0.025. Midbrain, bilateral putamen and hippocampus volumes as well as IFG and mPFC thickness did not differ between the two groups (p > 0.13) (Fig. 3).
Volume and thickness of different regions of interest of healthy controls and patients with akinetic-rigid Parkinson’s Disease (PD). Only left caudate nucleus volume significantly differed between the two groups. R, Right; L, Left; Hipp, Hippocampus; IFG, inferior frontal gyrus; mPFC, medial prefrontal cortex. The n-subscript indicates that volume and thickness were normalised by estimated total intracranial volume and mean hemisphere thickness, respectively; *p < 0.025
To explore whether left caudate atrophy contributes to morphological alterations in these apparently non-affected regions, we calculated a post hoc regression analysis of GM measures of midbrain, bilateral putamen, bilateral hippocampus, bilateral IFG and bilateral mPFC on left caudate volume, controlling for age. This analysis revealed a significant positive association with right hippocampus volume, β = 0.647, p = 0.023, but a significant negative relationship with right IFG thickness, β = − 0.411, p = 0.007, and left mPFC thickness, β = − 0.409, p = 0.019, with R2 = 0.558, F(10,28) = 3.535, p = 0.004.
Association of GM measures with PE processing
We hypothesised (3) a relationship between the response bias Br and midbrain volume. Partial correlation controlling for age revealed a non-significant correlation in patients OFF, r = 0.429, p = 0.059, but a significant correlation in controls, r = 0.473, p = 0.042. Correlation became significant when considering the whole group, r = 0.451, p = 0.002 (Fig. 4a). In contrast, our hypothesis (4) of a relationship between the Pr index, which represents the ability to discriminate between different types of PEs, and caudate nucleus volume was not confirmed, neither for patients OFF nor for controls (p > 0.404). However, since there was a trend towards a significant correlation between bilateral putamen and Pr index in both groups [Controls: R (right): r = − 0.471, p = 0.042, L (left): r = − 0.398, p = 0.093; Patients OFF: R: r = − 0.455., p = 0.044, L: r = − 0.401., p = 0.080] we assessed the relationship across the whole sample revealing a significant association of Pr index with right, r = − 0.395, p = 0.012, but not with left putamen, r = − 0.312, p = 0.050 (Fig. 4b). Thus, volume of dopaminergic regions, i.e., midbrain and right putamen, was related to the probability to respond to surprising stimuli per se and to the ability to discriminate between them, respectively, though not specific to the disease.
Scatter plots of significant correlations between a residuals of midbrain (controlling for age) and Br index indicating a general response bias to unexpected events and b between right putamen residuals (controlling for age) and Pr index measuring the ability to discriminate between different types of events across healthy controls and akinetic-rigid Parkinson’s disease patients in the OFF-state. The n-subscript indicates that volume was normalised by estimated total intracranial volume
We finally predicted disease-specific structural variations in (5) the IFG and (6) the mPFC and hippocampus to be related to deficits in cognitive stability and cognitive flexibility, respectively. Partial correlation analyses controlling for age revealed a significant negative correlation between CRs and right IFG thickness in patients OFF, but not in healthy controls. In contrast, switch hits of patients but not of controls correlated negatively with right hippocampus volume (Fig. 5).
Above, overlay of mean thickness of right inferior frontal gyrus (R IFG) and mean volume of right hippocampus (R Hipp) of healthy controls (in dark colour) and akinetic-rigid Parkinson’s disease (PD) patients (in light colour). Mean thickness and volume did not significantly differ between the groups. Below, scatter plots displaying the relationship between measures of stability (drift correct rejections, CRs) and flexibility (switch hits) and unstandardized residuals of right IFG thickness and hippocampus volume, respectively, for healthy controls and PD patients OFF (controlled for age). There was a significant negative correlation between drift CRs and right IFG thickness and between switch hits and right hippocampus volume in patients, but not in controls
Correlations appeared to be specific as there was no relationship between drift CRs and hippocampus volume or switch hits and IFG thickness as well as no differences in coefficients between the groups. However, contrary to our expectation left mPFC negatively correlated with drift CRs, but not with switch hits in patients only (Table 2).
Association of GM with medication
For an assessment of (7) the relationship between medication and GM, we performed a regression analysis of our GM measures on LEDD, controlling for age and disease duration. This analysis revealed a negative relationship between LEDD and right hippocampus, β = − 2.591, p = 0.049, right caudate, β = − 2.960, p = 0.032, and right putamen, β = − 2.916, p = 0.033, with R2 = 0.947, F(7,31) = 6.807, p = 0.023. Thus, patients with smaller volume of these regions might require a higher dose of dopaminergic medication. Note that left hippocampus, caudate, and putamen negatively relate to LEDD, albeit not reaching significance (p > 0.052) (see Table S3 in the Supplementary Material).
Discussion
In the present study, we investigated whether structural deviations in a priori defined brain regions of akinetic-rigid PD patients are accompanied by deficient processing of unpredicted stimuli that either had to be ignored (indicating cognitive stability) or detected (indicating cognitive flexibility). In sum, (1) there was no significant correlation between our behavioural measures of cognitive stability and flexibility in prediction, neither in patients on and off medication nor in healthy participants. (2) Both functions were affected in patients as reflected in difficulties in ignoring irrelevant and detecting relevant, but unpredicted events. Furthermore, data confirmed our hypothesis that (3) midbrain volume relates to a general response bias to unexpected events, though this was not specific to PD. Likewise, (4) right putamen volume related to the ability to discriminate between relevant and irrelevant events across the whole sample. (5) Morphological differences in right IFG were associated with variations in cognitive stability only in patients, whereas (6) differences in hippocampus volume related to specific deficits in flexible adaptation to relevant input. Contrary to our hypothesis, mPFC thickness correlated with our measure of stability in patients, but not in controls. Finally, (7) although we did not find performance differences between patients on and off medication, individual dose of medication positively correlated with discrimination ability across the patients in the ON-state. Increased daily medication dose was in turn associated with smaller right hippocampus, caudate and putamen volume.
Contribution of morphological variations to general PE detection
We found distinct patterns for the association of different anatomical structures with difficulties in dealing with unpredicted events. Smaller midbrain was related to a lower probability to respond to unpredicted sensory input across the whole sample, i.e., independent of the disease. Phasic DA release in the midbrain projecting to the striatum is involved in signalling mismatches between anticipated and actual events (Redgrave and Gurney 2006), allowing for a fast adaptation of behaviour (D’Esposito and Postle 2015). Our results further suggest that structural variations in the midbrain matter with regard to differences in the ability to identify and respond to PEs as such. Moreover, patients compared with controls exhibited a reduced ability to discriminate different types of PEs which was negatively correlated with volume of the right putamen in the whole sample. This finding corresponds with previous studies on response selection especially within the domain of motor processing (Humphries et al. 2006; Lo and Wang 2006; Howard et al. 2017; Hiebert et al. 2014). It is suggested that the primary role of the basal ganglia entails the selection of behaviours represented in prefrontal and premotor areas to be executed. Cognitive representations within the frontal cortex are selected by striatal actions via caudate-prefrontal loops (Houk and Wise 1995; Jueptner and Weiller 1998) and then executed via the motor loop connecting the putamen to the lateral premotor cortex and the supplementary motor area (Alexander et al. 1986). The motor loop contributes to the prediction of upcoming events (see Schubotz 2007, for a review), and impaired performance in predicting stimulus sequences in PD has been found to be accompanied by motor loop dysfunction (Schönberger et al. 2015). Thus, although we could not replicate our previous finding of a relationship between caudate nucleus and discrimination ability in young healthy subjects (suggesting the involvement of the prefrontal loop) (Trempler et al. 2017), the relationship between thickness of the right putamen and discrimination ability emphasises the importance of overt motor aspects of discrimination ability or cognitive impact on motor control in older age.
Impaired cognitive stability and flexibility in PD
Compared with controls patients rejected and detected significantly lower amounts of drifts and switches, respectively, suggesting deficits in both cognitive stability and flexibility of prediction. It has been suggested that cognitive inflexibility in PD, i.e., difficulties in updating in response to relevant input, appears to be beneficial for stability by becoming less prone to distraction (Cool et al. 2003, 2009). It is noteworthy that unlike previous studies on the interplay of stability and flexibility, we here measured the two functions in a predictive setting. We define stability as the ability to shield the initial internal model that allows prediction of visual input in the face of distraction, thereby also inhibiting motor reactions. In contrast, flexibility refers to the ability to update the internal model in response to a lasting change, along with an appropriate motor reaction. To avoid confounds with working memory capacity influencing performance in both, stability and flexibility, we used an overlearned predictable digit sequence serving as the internal model. Thus, our operationalisation of stability and flexibility refers to the ability to deal with different types of PEs and is, thus, only partly comparable to previous studies. Usually, active maintenance of working memory content (without external input) plays a substantial role for stability (Durstewitz 2000), whereas flexibility is measured by switching between different tasks or task sets (Stelzel et al. 2010; Fröber and Dreisbach 2017). However, as Cools and D’Esposito (2011) point out it is conceivable that stability and flexibility are accomplished by two separate mechanisms that nevertheless influence and partly oppose each other.
Hence, in the present study we did not find a significant negative relationship between the rate of drift CRs and the rate of switch hits. Measures of cognitive stability and flexibility showed a non-significant trend towards a weak positive relationship in healthy controls and patients in the ON-state, whereas there was no relationship in the OFF-state. Thus, in case of prediction flexible updating might diminish with disease progression, but independent of the ability to reject irrelevant distractors (or vice versa). The weak positive relationship in controls and patients ON might point to some superior function modulated by DA, from which both stability and flexibility can profit, so that withdrawal results in a more incidental dealing with unpredicted events.
Structural correlates of cognitive stability and flexibility
We found that deficits in cognitive stability relate to higher thickness of right IFG (note that we will discuss possible mechanisms regarding the direction of this effect in the following section). The lateral PFC is responsible for stabilisation of working memory representations, i.e., active maintenance (Baddeley 1986) and cognitive inhibition in the face of distractors (Lavie 2005). The involvement of IFG in drift rejections in our previous and in the present study might point to heightened verbal working memory load in response to sequential interruptions because the common task strategy was subvocalization of the digit sequence (Shergill et al. 2002; Fegen et al. 2015). However, it has been suggested that particularly the right IFG is relevant for motor inhibition (see Aron 2007, for a review) and attentional control when facing salient stimuli (Hampshire et al. 2010). Structural abnormalities in the right IFG have been reported for neuropsychiatric disorders that are linked to impaired inhibition, such as attention-deficit hyperactivity disorder (Depue et al. 2010) and obsessive–compulsive spectrum disorders (Menzies et al. 2007). The present results further indicate that higher thickness in the right, but not in the left IFG of PD patients is related to a decline in inhibitory control and maintenance of the internal model in face of unpredicted distractors. However, thickness of the left mPFC, activated in response to switches in our previous fMRI study (Trempler et al. 2017), was also associated with our measure of cognitive stability in the patient sample. As right IFG and left mPFC thickness negatively correlated with left caudate volume, changes within these two regions might contribute to the same dysfunctional profile in patients, namely overreacting to unexpected but irrelevant input. We suggest that frontal contribution to instable responses reflects impaired inhibition on the one hand (due to the involvement of right IFG) but also concurrent reliance on cognitive control over behavioural responses by recruitment of left mPFC (di Pellegrino et al. 2007; Alexander and Brown 2011). Thus, compensatory but mal-adaptive attentional resources might be recruited to maintain motor control to some degree in the early stage of the disease, whereby this results in rather unspecific and even unstable responses to unexpected stimuli (see Seidler et al. 2010, for a review).
Considering cognitive flexibility, right hippocampus volume negatively correlated with the rate of correctly detected switches in patients, but not in controls. In line with the hippocampus’ role in updating and learning (Ross et al. 2009; Chen et al. 2011), morphological changes within this region might result in specific impairment in flexible dealing with behavioural relevant PEs. Structural changes of the hippocampus of PD patients have been found in several studies, commonly associated with a progression towards dementia, independent from frontal dysfunction (Shimada et al. 2009). It has been suggested that posterior cortical changes in PD are caused by cholinergic loss and not by dopaminergic (dys-)function (Hall et al. 2014). Although it is not provable by the present results whether deficits in flexible updating of prediction in PD result from changes in cholinergic transmission in temporal regions, they suggest subtle differences in morphology to be accompanied by cognitive deficits associated with learning and memory already in early stages of the disease.
Cerebral reorganisation in Parkinson’s disease
It is not a trivial question why increased GM was related to cognitive performance deficits in the present study. Both GM increases and decreases in PD have been described in previous studies, and heterogeneous findings mirror the complexity of the underlying pathophysiology including multiple neurotransmitter deficiencies as well as genetic risk factors (see Biundo et al. 2016, for a review). Additionally, WM fractional anisotropy could be the underlying cause of the reported effects since it has been shown to contribute to variations in GM estimates (Villain et al. 2010; Freund et al. 2011; Henry et al. 2009). For instance, Price et al. (2016) found that a reduced prefrontal fractional anisotropy in addition to reduced GM caudate volume predicted processing speed of PD patients, which highlights the importance of GM-WM interactions for cognitive symptoms in PD.
One hypothesis is that cortical thickening may occur due to temporary compensatory, but inefficient cytoarchitectural reorganisation that cannot counterbalance cognitive decline (Rektorova et al. 2014). Evidence for this assumption is provided by a negative correlation between left caudate volume and thickness of right IFG and left mPFC in the present study suggesting that striatal volume loss is accompanied by compensatory thickening of target frontal regions. Biundo et al. (2013) proposed a non-linear progression of structural changes with patients initially exhibiting hyperactivation or thickening in task-relevant brain structures reflecting compensatory mechanisms, and subsequently developing hypoactivation or cortical thinning with disease progress. However, regarding the hippocampus its positive relationship with left caudate indicates hippocampus atrophy rather than thickening. Because smaller volume of the right hippocampus was associated with higher LEDD, which in term influenced PE discrimination ability, it may be possible that patients with decreased volume of these regions might benefit more from medication than those with higher volume and, as a result, perform better at switches. This would correspond with the suggestion of the inverted-U-shaped action of DA on cognitive functions according to which either too little or too much DA action can reduce performance on cognitive tasks (Cools and D’Esposito 2011). In line with this, some studies relate aberrant cerebral alterations to a hyperdopaminergic state induced by DA medication. DA is involved in modulating synaptogenesis, dendritic arborisation, and can induce cytotoxic long-term effects (Tessitore et al. 2016). Thickening of the anterior cingulate and orbitofrontal cortex in PD patients with impulse control disorder (Tessitore et al. 2016) as well as of the right IFG in levodopa induced dyskinesia has been reported (Cerasa et al. 2013). These findings indicate that DA medication might initially preserve cognitive and motor functions, at least to some degree, whereas other areas become hyperactive due to DA overdose, also accompanied by structural alterations. In this regard, future investigations of cognitive dysfunctions in PD should take possible paradoxical effects of DA medication into account (see Cools 2006, and; Vaillancourt et al. 2013, for a review).
Finally, the detected associations could also result from GM variations from birth, with subjects with thinner cortex and smaller subcortical volume being resistant to cognitive deficits, whereas subjects with thicker and larger structures are more prone to cognitive decline (Cerasa et al. 2013). This might also explain why we did not find differences between the two groups in thickness and volume of the corresponding regions.
Medication effects
Contrary to our expectation, there was no difference between patients on and off medication in behavioural performance. Since most of the patients were in the early stage of the disease (Hoehn and Yahr ratings between I and III) the remaining neurons in the SN were possibly still capable of storing DA medication also in the off-state (Chase et al. 2013). Moreover, some of the patients took slow release dopaminergic medication and, due to ethical concerns, we decided not to affect these patients too much by longer-term withdrawal. Thus, although withdrawal affected motor performance as seen in significant differences in UPDRS-score between patients ON and OFF, the patients’ cognitive functioning was possibly retained. Alternatively, loss of other neurotransmitters might contribute to deficits in stability and flexibility as operationalised in our study. As already mentioned, deficits in flexible updating of prediction may result from changes in cholinergic transmission in temporal regions. Moreover, there is growing evidence for noradrenaline depletion in PD (Delaville et al. 2011) that might cause cognitive inflexibility (Vazey and Aston-Jones 2012).
However, we found a positive relationship between the dose of individual dopaminergic medication and performance under medication across patients. This suggests that in the long run DA can indeed enhance the ability to identify and deal with different types of unpredicted events either requiring cognitive stability or flexibility, though short-term withdrawal does not provoke a significant drop in performance. In this connection, it is also interesting that—albeit not reaching the level of significance—there was a weak positive relationship between stability and flexibility in patients on medication (as observed in healthy controls), whereas the correlation was absent or rather negative in the OFF-state. Thus, stable and flexible responses to PEs appear to be influenced equally by dopaminergic medication.
Limitations
We acknowledge the relatively small sample size of 21 patients and its heterogeneity in terms of disease duration, PD subtypes, and stages as limitations of the current study. Future studies should elaborate on deficits in cognitive stability and flexibility of prediction, in particular because the reported effects could be more pronounced when examining a larger sample. To assess a non-linear progression of specific cerebral morphological patterns, longitudinal studies with a larger PD cohort including patients with mild cognitive impairment should be conducted. Regarding the small effects of medication, we acknowledge that we did not assess the presence or absence of medication fluctuations per patient. This could have been beneficial to take into account potential interactions with present task performance.
Furthermore, we are unable to rule out that participants missed, that is, did not detect drifts instead of actively rejecting them. Although there is no reason to assume that subjects would only signal switches (given the fact that there was no systematic relationship between switch misses and drift CRs), functional imaging could ensure that drifts elicited activity in areas associated with stability of prediction.
Finally, to understand the specific pathophysiological mechanisms causing grey matter changes multimodal studies and joint research between disciplines from subcellular or cellular to functional level are required. Therefore, we highlight our considerations on causes of cerebral reorganisation to be still speculative. However, deriving hypotheses on the DA-mediated interactions between cognitive PE processing and cerebral morphology, our exploratory study holds great potential for future investigations.
Conclusion
Taken together, our study is the first to show that inter-individual differences in cerebral morphology of akinetic-rigid PD patients are linked to deficits in cognitive processing of either relevant or irrelevant unpredicted sensory information. Deficient stabilisation is accompanied by disruption of dopaminergic frontostriatal circuits, which is reflected in cerebral alterations in prefrontal areas, whereas impaired updating of current predictions is associated with structural hippocampal alterations.
References
Alexander WH, Brown JW (2011) Medial prefrontal cortex as an action-outcome predictor. Nat Neurosci 14(10):1338–1344
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 9(1):357–381
Aron AR (2007) The neural basis of inhibition in cognitive control. Neurosci 13(3):214–228
Baddeley AD (1986) Working memory. Oxford University Press, Oxford
Badre D (2012) Opening the gate to working memory. Proceedings of the National Academy of Sciences, USA, 109, 19878-79
Baker JE (1987) Reducing Bias and Inefficiency in the Selection Algorithm. Proceedings of the Second International Conference on Genetic Algorithms and their Application, 14–21
Bilder RM, Volavka J, Lachman HM, Grace AA (2004) The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29:1943–1961
Biundo R, Calabrese M, Weis L, Facchini S, Ricchieri G, Gallo P, Antonini A (2013). Anatomical correlates of cognitive functions in early Parkinson’s disease patients. PLoS One, 8(5):e64222
Biundo R, Weis L, Antonini A (2016) Cognitive decline in Parkinson’s disease: the complex picture. NPJ Parkinson’s Dis 2:16018
Braak H, Del Tredici K, Rüb U, De Vos RA, Steur ENJ, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, Arabia G, Quattrone A (2013) Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 19(1):123–125
Chase TN (1990) Motor response complications with chronic levodopa therapy. Adv Neurol 53:377–381
Chatham CH, Badre D (2015) Multiple gates on working memory. Curr Opin Behav Sci 1:23–31
Chaudhuri KR, Schapira AH (2009). Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol, 8(5), 464 – 74
Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD (2011) Associative retrieval processes in the human medial temporal lobe: hippocampal retrieval success and CA1 mismatch detection. Learn Memory 18(8):523–528
Cohen JD, Braver TS, Brown JW (2002) Computational perspectives on dopamine function in prefrontal cortex. Curr Opin Neurobiol 12(2):223–229
Cools R (2006) Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev 30(1):1–23
Cools R (2011) Dopaminergic control of the striatum for high-level cognition. Curr Opin Neurobiol 21(3):402–407
Cools R, D’Esposito M (2011) Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiat 69:e113-25
Cools R, Barker RA, Sahakian BJ, Robbins TW (2003) L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia 41(11):1431–1441
Cools R, Sheridan M, Jacobs E, D’Esposito M (2007) Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci 27:5506–5514
Cools R, Miyakawa A, Sheridan M, D’Esposito M (2009). Enhanced frontal function in Parkinson’s disease. Brain awp301
D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD (2012). Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences, USA, 109, 19900-9
D’Esposito M, Postle BR (2015) The cognitive neuroscience of working memory. Annu Rev Psychol 66:115–142
Dale AM, Fischl B, Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9(2), 179 – 94
Delaville C, De Deurwaerdère P, Benazzouz A (2011). Noradrenaline and Parkinson’s disease. Front Syst Neurosci, 5
Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT (2010) Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry Res Neuroimaging 182(3):231–237
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31(3):968–980
di Pellegrino G, Ciaramelli E, Làdavas E (2007) The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci 19(2):275–286
Durstewitz D, Seamans JK (2008) The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiat 64:739–749
Durstewitz D, Seamans JK, Sejnowski TJ (2000) Neurocomputational models of working memory. Nat Neurosci 3:1184–1191
Fahn S, Elton RL (1987). Unified rating scale for Parkinson’s disease. Recent developments in Parkinson’s Disease, 153 – 63
Fegen D, Buchsbaum BR, D’Esposito M (2015) The effect of rehearsal rate and memory load on verbal working memory. NeuroImage 105:120–131
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050-5
Frank MJ, Loughry B, O’Reilly RC (2001) Interactions between the frontal cortex and basal ganglia in working memory: a computational model. Cognit Affective Behav Neurosci 1:137–160
Freund P, Weiskopf N, Ward NS, Hutton C, Gall A, Ciccarelli O, Craggs M, Friston K, Thompson AJ (2011) Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134:1610–1622
Fröber K, Dreisbach G (2017) Keep flexible–Keep switching! The influence of forced task switching on voluntary task switching. Cognition 162:48–53
Galea JM, Bestmann S, Beigi M, Jahanshahi M, Rothwell JC (2012). Action reprogramming in Parkinson’s disease: response to prediction error is modulated by levels of dopamine. J Neurosci 32(2):542–550
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9(1):13–24
Hall H, Reyes S, Landeck N, Bye C, Leanza G, Double K, Thompson L, Halliday G, Kirik D (2014). Hippocampal Lewy pathology and cholinergic dysfunction are associated with dementia in Parkinson’s disease. Brain, 137(9):2493–2508
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010) The role of the right inferior frontal gyrus: inhibition and attentional control. NeuroImage 50(3):1313–1319
Hautzinger M, Keller F, Kühner C (2006) Beck depressions-inventar (BDI-II). Harcourt Test Services, Frankfurt
Henry RG, Shieh M, Amirbekian B, Chung S, Okuda DT, Pelletier D (2009) Connecting white matter injury and thalamic atrophy in clinically isolated syndromes. J Neurol Sci 282:61–66
Hiebert NM, Vo A, Hampshire A, Owen AM, Seergobin KN, MacDonald PA (2014) Striatum in stimulus–response learning via feedback and in decision making. NeuroImage 101:448–457
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Houk JC, Wise SP (1995) Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cerebral Cortex 5(2):95–110
Howard CD, Li H, Geddes CE, Jin X (2017) Dynamic nigrostriatal dopamine biases action selection. Neuron 93(6):1436–1450
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Humphries MD, Stewart RD, Gurney KN (2006) A physiologically plausible model of action selection and oscillatory activity in the basal ganglia. J Neurosci 26(50):12921–12942
Iglesias JE, Van Leemput K, Bhatt P, Casillas C, Dutt S, Schuff N, Truran-Sacrey D, Boxer A, Fischl B (2015) Bayesian segmentation of brainstem structures in MRI. NeuroImage 113:184–195
Jueptner M, Weiller C (1998) A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain J Neurol 121(8):1437–1449
Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, Wittchen HU, Dodel R, Otto J, Ebersbach G, Kessler J (2008) Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Rel Disord 14(2):93–101
Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson’s disease. Lancet Neurol 9(12):1200–1213.
Kehagia AA, Barker RA, Robbins TW (2012) Cognitive impairment in Parkinson’s disease: the dual syndrome hypothesis. Neurodegenerative Dis 11(2):79–92
Lavie N (2005) Distracted and confused?: Selective attention under load. Trends Cognit Sci 9:75–82
Lo CC, Xiao-Jing W (2006) Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat Neurosci 9(7):956
Menzies L, Achard S, Chamberlain SR, Fineberg N, Chen CH, Del Campo N, … Bullmore E (2007) Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain 130(12):3223–3236
Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202
Muslimović D, Post B, Speelman JD, Schmand B (2005) Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology 65(8):1239–1245
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–74
Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, Brumback B, Okun MS, Mareci TH, Bowers D (2016). Gray and white matter contributions to cognitive frontostriatal deficits in non-demented Parkinson’s disease. PloS One, 11(1):e0147332
Redgrave P, Gurney K (2006). The short-latency dopamine signal: a role in discovering novel actions?. Nat Rev Neurosci 7(12):967–75
Rektorova I, Biundo R, Marecek R, Weis L, Aarsland D, Antonini A (2014). Grey matter changes in cognitively impaired Parkinson’s disease patients. PloS One, 9(1):e85595
Ross RS, Brown TI, Stern CE (2009) The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus 19(9):790–799
Schiffer AM, Ahlheim C, Wurm MF, Schubotz RI (2012). Surprised at all the entropy: hippocampal, caudate and midbrain contributions to learning from prediction errors. PloS One, 7(5), e36445
Schiffer AM, Waszak F, Yeung N (2015) The role of prediction and outcomes in adaptive cognitive control. J Physiol Paris 109(1):38–52
Schlichting ML, Preston AR (2015) Memory integration: neural mechanisms and implications for behavior. Curr Opin Behav Sci 1:1–8
Schonberg T, O‘Doherty JP, Joel D, Inzelberg R, Segev Y, Daw ND (2010). Selective impairment of prediction error signaling in human dorsolateral but not ventral striatum in Parkinson’s disease patients: evidence from a model-based fMRI study. NeuroImage, 49(1), 772 – 81
Schönberger AR, Barbe MT, Hagelweide K, Kühn AB, Fink GR, Schubotz RI (2013) Joint principles of motor and cognitive dysfunction in Parkinson’s disease. Neuropsychologia 51(8):1417–1425
Schott BH, Niehaus L, Wittmann BC, Schütze H, Seidenbecher CI, Heinze HJ, Düzel E (2007) Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain 130(9):2412–2424
Schubotz RI (2007) Prediction of external events with our motor system: towards a new framework. Trends Cognitive Sci 11(5):211–218
Schultz W, Dickinson A (2000) Neuronal coding of prediction errors. Annu Rev Neurosci 23:473–500
Shergill SS, Brammer MJ, Fukuda R, Bullmore E, Amaro E Jr, Murray RM, McGuire PK (2002) Modulation of activity in temporal cortex during generation of inner speech. Hum Brain Mapp 16:219–227
Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, Ota T, Asahina M, Fukushi K, Kuwabara S, Hattori T, Suhara T, Irie T (2009) Mapping of brain acetylcholinesterase alterations in Lewy body disease by PET. Neurology 73:273–278
Snodgrass JG, Corwin J (1988) Pragmatics of measuring recognition memory: applications to dementia and amnesia. J Exp Psychol Gen 117(1):34
Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ (2010) Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci 30(42):14205–14212
Tessitore A, Santangelo G, De Micco R, Vitale C, Giordano A, Raimo S, Corbo D, Amboni M, Barone P, Tedeschi G (2016) Cortical thickness changes in patients with Parkinson’s disease and impulse control disorders. Parkinsonism Rel Disord 24:119–125
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Movement Disord 25(15):2649–2653
Trempler I, Schiffer AM, El-Sourani N, Ahlheim C, Fink GR, Schubotz RI (2017) Frontostriatal contribution to the interplay of flexibility and stability in serial prediction. J Cogn Neurosci 29(2):298–309
Vaillancourt DE, Schonfeld D, Kwak Y, Bohnen NI, Seidler R (2013) Dopamine overdose hypothesis: evidence and clinical implications. Movement Disord 28(14):1920–1929
Vazey EM, Aston-Jones G (2012). The emerging role of norepinephrine in cognitive dysfunctions of Parkinson’s disease. Front Behav Neurosci, 6
Villain N, Fouquet M, Baron JC, Mézenge F, Landeau B, de La Sayette V, Viader F, Eustache F, Desgranges B, Chételat G (2010) Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain 133(11):3301–3314
Zimmermann P, Fimm B (1993) Testbatterie zur Aufmerksamkeitsprüfung (TAP). Psytest, Freiburg
Acknowledgements
We sincerely thank the participants involved in the current investigation and Paul Reker for the UPDRS III video rating. We also acknowledge Klara Hagelweide for proof-reading and commenting on earlier versions of the manuscript. We are grateful to Anna Kuhns, Pascasie Leonie Dombert, Alexander Geiger, and Eileen Oberwelland for their assistance in data acquisition. We thank the German Research Foundation (Clinical Research Group KFO219 “Basal-Ganglia-Cortex-Loops: Mechanisms of Pathological Interactions and Therapeutic Modulation”, SCHU 1439/5 − 2) for financially supporting the project. Finally, we are thankful for the insightful and productive comments by our reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Trempler, I., Binder, E., El-Sourani, N. et al. Association of grey matter changes with stability and flexibility of prediction in akinetic-rigid Parkinson’s disease. Brain Struct Funct 223, 2097–2111 (2018). https://doi.org/10.1007/s00429-018-1616-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-018-1616-2