Abstract
Melatonin, through its different receptors, has pleiotropic functions in mammalian brain. Melatonin is secreted mainly by the pineal gland and exerts its effects via receptor-mediated and non-receptor-mediated actions. With recent advancement in neuroanatomical mapping, we may now understand better the localizations of the two G protein-coupled melatonin receptors MT1 and MT2. The abundance of these melatonin receptors in respective brain regions suggests that receptor-mediated actions of melatonin might play crucial roles in the functions of central nervous system. Hence, this review aims to summarize the distribution of melatonin receptors in the brain and to discuss the putative functions of melatonin in the retina, cerebral cortex, reticular thalamic nucleus, habenula, hypothalamus, pituitary gland, periaqueductal gray, dorsal raphe nucleus, midbrain and cerebellum. Studies on melatonin receptors in the brain are important because cumulative evidence has pointed out that melatonin receptors not only play important physiological roles in sleep, anxiety, pain and circadian rhythm, but might also be involved in the pathogenesis of a number of neurodegenerative diseases including Alzheimer’s disease, Parkinson’s disease and Huntington’s disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
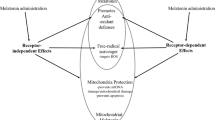
Melatonin (5-methoxy-N-acetyltryptamine) is a pleiotropic hormone that regulates a broad range of body functions. It is the main hormone synthesized and secreted by the pineal gland during the dark cycle in accordance with the 24-h sleep cycle (Pévet 2002). Once secreted, it enters into the blood circulatory system through which it travels to and acts on different regions of the body to achieve desirable physiological responses. Melatonin has high accessibility throughout the body due to its amphiphilic nature (Costa et al. 1995). The ubiquitous distribution of melatonin has allowed it to exert highly versatile functions throughout the body. The response is often mediated through specific melatonin receptors in the target cells. On top of that, melatonin also acts as an antioxidant and free radical scavenger without the involvement of its receptors (Reiter et al. 2007; Pechanova et al. 2014; Galano et al. 2013). A detailed review regarding the actions of melatonin in the periphery has been published recently (Slominski et al. 2012).
In the brain, melatonin penetrates the blood–brain barrier easily, which allows it to exert its effects onto different brain regions (Gilgun-Sherki et al. 2001). Presence of melatonin receptors in the brain, as demonstrated by a few published studies (Lacoste et al. 2015; Mazzucchelli et al. 1996; Adamah-Biassi et al. 2014), has provided evidence of involvement of melatonin in brain functions. Of recent note is the study by Lacoste et al. (2015), which reported the first neuroanatomical mapping of the distribution of melatonin receptor proteins in the rat brain. To date, a full mapping of the melatonin receptors in human brain has yet been studied. There is also a lack of comprehensive review that elaborates the role and the functions of melatonin receptors in various brain regions. Understanding the actions of melatonin in the brain is important as experimental evidence has implicated the involvement of melatonin in multiple physiological functions in the brain. This review attempts to compile the identified localizations of melatonin receptors and summarize the putative functions of melatonin in the mammalian brain. Only brain regions that have suggestive roles of melatonin supported by other studies (except pineal gland) are selected to be included in this review.
Melatonin receptors
Melatonin receptors are members of the seven transmembrane G protein-coupled receptors (GPCRs) superfamily (Witt-Enderby et al. 2003). Currently, three subtypes of melatonin receptors have been identified in mammals: MT1 (formerly Mel 1a or ML1A), MT2 (formerly Mel 1b or ML1B), and MT3 (formerly ML2) (Dubocovich et al. 2010). MT1 and MT2 remain to be the main GPCRs identified in the mammalian brain. In mammals, MT3 is identified as a melatonin-binding site located on a cytosolic enzyme, quinone reductase 2 (Nosjean et al. 2000). It generally has low binding affinity with melatonin (Luchetti et al. 2010).
MT1 receptor
The Mel1c receptor in frogs (Xenopus laevis) was the first melatonin receptor to be cloned (Ebisawa et al. 1994). Its discovery was vital in facilitating the cloning of other mammalian melatonin receptors, as the same group who cloned the Mel1c receptor was also the first to clone the MT1 receptor from the sheep and human (Reppert et al. 1994). MT1 receptor is 350 amino acids long with a molecular weight of 39,374 Da (Song et al. 1997). It is characterized as a receptor linked to a pertussis toxin-sensitive guanine nucleotide-binding protein (G protein) that mediates inhibition of adenylyl cyclase in native tissues (Carlson et al. 1989). Through the use of 2-[125l]Iodomelatonin, the MT1 receptor has been found to be expressed in various brain regions such as the pars tuberalis, hypothalamus, and peripheral tissues including the cardiovascular system, gastrointestinal tract, immune system and the kidney in mammals (Dardente et al. 2003; Wu et al. 2013; Slominski et al. 2012; Bubenik 2002; Song et al. 1992).
MT2 receptor
The expression of MT2 receptor was reported in multiple organs including the retina, brain, and the gastrointestinal tract (Wiechmann and Sherry 2013; Morgan et al. 1994; Bubenik 2002). The MT2 receptor was cloned just one year after the MT1 receptor was cloned (Reppert et al. 1995). The MT2 receptor is 362 amino acids long with a molecular weight of 40,188 Da and it shares 60% homology to the MT1 receptor (Reppert et al. 1996). Both melatonin receptors are classified under the same GPCR class and in this class, sequence homology between receptors mostly occurs within the transmembrane domains (Deupi et al. 2007; Dubocovich et al. 2010). Similarly, the MT2 receptor is also linked to G protein-coupled receptors and is involved in the inhibition of adenylyl cyclase and soluble guanylyl cyclase pathways (Petit et al. 1999).
Cellular signaling mechanism of melatonin receptors
The cellular signaling pathways for melatonin receptors vary in different species (Hardeland 2009). However, a few principal signaling pathways exist to be commonly activated upon melatonin stimulation. Both MT1 and MT2 receptors are closely linked to the pertussis toxin-sensitive Gi protein and activation of this protein inhibits the AC/cAMP/PKA/CREB pathway (Rivera-Bermudez et al. 2004; Tosini et al. 2014). Through the MT2 receptor, inhibition of the GC/cGMP/PKG pathway can also occur upon Gi activation (Petit et al. 1999). In addition, the activation of the phospholipase C pathway that leads to increase in inositol triphosphate (IP3) and 1, 2-diacylglycerol (DAG) levels has been demonstrated for both MT1 and MT2 receptors (Sharkey and Olcese 2007; Witt-Enderby et al. 2003). This activation is found to be linked to pertussis toxin-insensitive Gq protein and the MT1/MT2 heterodimer shows an augmented activation for these pathways, indicating that there are positive allosteric interactions between the two receptors (Jockers et al. 2008).
Distribution of melatonin receptors in the mammalian brain
In the past, the identification of melatonin receptors in the mammalian brain was largely performed through indirect means using non-selective radioligand 2-[125l]Iodomelatonin to identify high-affinity melatonin-binding sites in autoradiography (Weaver et al. 1993; Al-Ghoul et al. 1998; Stankov et al. 1992; Dubocovich et al. 1996; Masson-Pevet et al. 1994; Williams 1989; Williams et al. 1997; Siuciak et al. 1990). Recently, immunoreactive approach complemented with PCR analysis and in situ hybridisation has become the more popular method for the same investigation (Adi et al. 2010; Wu et al. 2006; Klosen et al. 2002; Lopez-Canul et al. 2015b; Lacoste et al. 2015; Mazzucchelli et al. 1996; Uz et al. 2005; Musshoff et al. 2002; Savaskan et al. 2002a, 2005; Brunner et al. 2006; Hunt et al. 2001; Sugden et al. 1999; Waly and Hallworth 2015; Uz et al. 2003; Poirel et al. 2002; Neu and Niles 1997). Recently a transgenic animal model which uses MT1 promoter to drive the expression of red fluorescence protein has also been developed to study the distribution of melatonin receptors (Adamah-Biassi et al. 2014).
Each of these methods has its own limitations. For instance, the autoradiographical approach is unable to discriminate the type of melatonin receptors involved. Also the binding affinity can be significantly reduced by the dimerization of melatonin receptors with their family members or other G protein-coupled receptors (Adamah-Biassi et al. 2014). Meanwhile, the transgenic animal model has unexpectedly failed to locate the MT1 receptors in the suprachiasmatic nucleus (SCN) or the striatum. The presence of MT1 receptor in SCN (Waly and Hallworth 2015) and striatum (Uz et al. 2003) of rodents has been confirmed previously in other studies. Authors have attributed this observation to the incomplete inclusion of regulatory elements crucial for the MT1 expression and also the possible integration of transgene in the chromosomal location where the gene expression is repressed in these brain regions (Adamah-Biassi et al. 2014). Nevertheless, the biggest challenge in the melatonin receptor research is the lack of reliable antibodies which would allow specific binding to the melatonin receptors with high sensitivity. In fact, many groups have resorted to complement immunoreactive approach with the results derived from PCR and in situ hybridisation to obtain a more complete set of data.
Nevertheless, as the autoradiographical approach is unable to discriminate the type of melatonin receptors involved we have listed mainly the studies that have used the immunoreactive, PCR analysis, in situ hybridisation and transgenic animal models to report the presence of melatonin receptors. The types of melatonin receptors in different brain regions and the source references are summarized in Table 1. The detailed distribution of MT1 and MT2 receptors based on these studies are summarized in Table 2.
Putative functions of melatonin
In this section, brain regions that express melatonin receptors and have existing evidence suggestive of melatonin involvement in the functionality are selected. These include retina, cerebral cortex, reticular thalamic nucleus, habenula, hypothalamus, pituitary gland, periaqueductal gray, dorsal raphe nucleus, midbrain and cerebellum. The schematic drawings mapped the majority of nuclei which had either MT1/MT2 receptor or both receptor-positive neurons on the plates from the rat atlas (modified with permission from Paxinos and Watson 2013) (Figs. 1, 2, 3, 4). The areas where receptors have been located for both MT1 and MT2 receptors are summarized in Table 2 (modified with permission from Lacoste et al. 2015). Putative functions of these regions are compiled in Table 3.
Distribution of MT1 and MT2 receptor in the rostral forebrain. MT1 receptor immunoreactivity is weak to moderate in the cingulate cortex (A24a and A24b), and mainly in layers II/III. MT2 receptor immunoreactivity is sparse in layer V in the cingulate cortex. MT1 receptor immunoreactivity is strong in the islands of Calleja (ICj) in contrast to the weak MT2 receptor immunoreactivity. Both MT1 and MT2 receptor immunoreactivity is moderate in the ventral pallidum (VP). Only MT1 receptor immunoreactivity is present in the shell region of the nucleus accumbens (AcbSh). Medially, in the rostral septum, only weak MT1 receptor immunoreactivity is present in the septum and the nucleus of the vertical limb of the diagonal band (VDB), in contrast to the moderate immunoreactivity of MT2 receptor
Distribution of MT1 and MT2 receptor in the middle forebrain. MT1 receptor immunoreactivity is moderate to strong in the cingulate cortex (A29 and A30), and mainly in layers II/III. MT2 receptor immunoreactivity is sparse in layer V in the cingulate cortex. In the rostral hippocampus, few MT1-positive neurons are present in the CA1 region, and weak labeling in the CA2 region, whereas weak to moderate labeling in the CA3 region. MT2 receptor immunoreactivity is moderate in the CA1 region, weak in the CA2 region, and strong in the CA3 region. MT2-positive neurons are concentrated in the pyramidal layer. In the dentate gyrus (DG), MT1 immunoreactivity is sparse and positive neurons are present mainly in the polymorph layer (PoDG). MT2 receptor immunoreactivity is moderate in the dentate gyrus and positive neurons are present mainly in the dorsal portion of the granular layer (GrDG). In the rostral thalamus, MT1 receptor immunoreactivity is almost exclusively associated with dendritic processes in the thalamus except few neurons in the central medial (CM) and the rhomboid thalamic (Rh) nuclei, whereas MT2 receptor immunoreactivity is weak in the mediodorsal (MD), central medial (CM), and ventrolateral nuclei (VL), and moderate in the reticular nucleus (Rt). In the middle and posterior third of the thalamus, MT2 receptor immunoreactivity is weak in the centrolateral (CL), mediodorsal (MD), ventral posterolateral (VPL) and posterior (Po) thalamic nuclei, moderate in the caudal part of the ventrolateral thalamic nucleus (VL), strong in the reticular (Rt) and ventromedial (VM) thalamic nuclei. In the hypothalamus, MT1 receptor immunoreactivity is weak in the dorsomedial hypothalamic (DM) and perifornical (PeF) nuclei, whereas MT2 receptor immunoreactivity is moderate in the ventral part of the arcuate (Arc) hypothalamic nucleus and the medial eminence (ME). In the medial division of central amygdaloid nucleus (CeM), MT1 receptor immunoreactivity is weak to moderate. In the globus pallidus (GP), MT2 receptor immunoreactivity is weak to moderate
Distribution of MT1 and MT2 receptor in the midbrain. MT1 receptor immunoreactivity is weak in the dorsomedial (DMPAG), dorsolateral (DLPAG), and lateral periaqueductal gray (LPAG). A small number of MT1 positive neurons are present in the substantia nigra including the pars compacta (SNC) and pars reticulata (SNR), the rostral (IPR) and lateral (IPL) interpeduncular subnuclei. MT2 receptor immunoreactivity is weak in the optic nerve layer (Op) and moderate in the intermediate gray layer (InG) of the superior colliculus, but strong in the substantia nigra, pars reticulata (SNR), and the red nucleus including both the parvocellular (RPC) and magnocellular (RMC) parts
Distribution of MT1 and MT2 receptor in the caudal midbrain and rostral hindbrain. MT1 receptor immunoreactivity is weak in the dorsomedial (DMPAG), dorsolateral (DLPAG), lateral (LPAG), and ventrolateral periaqueductal gray (VLPAG), weak to moderate in the inferior colliculus (IC) except in the superficial layer, moderate in the dorsal raphe nucleus (DR), especially the ventral part of it; weak in the oral pontine reticular nucleus (PnO), and moderate in the medial paralemniscal (MPL), median raphe (MnR), and the ventral tegmental (VTg) nuclei. MT2 receptor immunoreactivity is weak in the superficial layer of the inferior colliculus, and weak to moderate in other layers of the inferior colliculus (IC), weak in the ventrolateral periaqueductal gray (VLPAG), dorsal raphe nucleus (DR), weak in the subpeduncular tegmental (SpTg), reticulotegmental (RtTg), and median raphe nuclei (MnR), moderate in the oral pontine reticular nucleus (PnO), and strong in the ventral tegmental nucleus (VTg)
Retina
The synchronization of the circadian clock to the 24-h light/dark cycle is achieved through both external and internal stimuli. Light and dark signals received by the retina act as the external stimulus to the SCN and in turn, the SCN controls circadian regulation of melatonin synthesis in the retina (Wiechmann and Sherry 2013). In mammals, RT-PCR and immunohistochemistry studies have shown that MT1 mRNA and protein are localized to the inner and outer plexiform layers (IPL and OPL), inner nuclear layer, ganglion, amacrine, horizontal cells, and rod and cone photoreceptor cells (Scher et al. 2002; Fujieda et al. 1999, 2000; Sallinen et al. 2005). MT2 receptor immunoreactivity was localized to the ganglion and bipolar cells in the inner nuclear layer of the retina, the inner segments of the photoreceptor cells and cellular processes in IPL and OPL of the retina (Savaskan et al. 2007; Sallinen et al. 2005; Yang et al. 2011; Reppert et al. 1995).
Retinal melatonin is synthesized and secreted by photoreceptors and acts as a paracrine hormone, having a localized action upon the retina. It modulates light sensitivity and neurotransmitter release, which are some of the actions retinal melatonin is involved in (Wiechmann and Sherry 2013). Regulation of neurotransmitter release appears to be mediated by MT1 receptors on amacrine cells. As melatonin is secreted from photoreceptors, it binds to melatonin receptors on specific amacrine cells and subsequent release of dopamine and GABA is regulated (Fujieda et al. 2000). Dopamine and melatonin are mutually antagonistic hormones. Binding of melatonin to receptors on dopaminergic amacrine cells inhibits the release of dopamine while dopamine binding to D2 dopamine receptors suppresses melatonin synthesis. Circadian release of melatonin results in opposing levels of melatonin and dopamine and this regulates light sensitivity of the retina (Megaw et al. 2006). For example, melatonin, synthesized at night, could increase visual sensitivity through binding to MT1 receptors. Binding of melatonin to MT1 receptors results in inhibition of dopamine release which would facilitate dark adaptation through the increase of horizontal cell coupling (Harsanyi and Mangel 1992). Therefore, it is possible that MT1 and/or MT2 melatonin receptors in amacrine and/or ganglion retinal cells modulate the retino-hypothalamic transmission of the light–dark signal via regulation of dopaminergic and GABAergic transmission (Dubocovich and Markowska 2005). Also, two studies from the same group have reported decreased immunoreactivities of MT1 and MT2 receptors in the retina of Alzheimer’s disease (AD) patients (Savaskan et al. 2002b, 2007). Nevertheless, the involvement of melatonin receptors in the pathophysiology of AD is unclear and requires further confirmation by future studies.
Melatonin and its receptors have been shown to modulate visual function and clearly demonstrated that they are indeed key players in retinal physiology. The actions of melatonin on retina seem to be primarily mediated through the regulation of selected neurotransmitters. However, the multiple targets of melatonin in the retina, such as photoreceptors and ganglion, lead to complex actions and the specific functions of each melatonin receptor subtypes in different sections of the retina remain to be investigated.
Cerebral cortex
A few studies have reported the distribution of melatonin receptors in the cerebral cortex. Lacoste et al. (2015) found that the cerebral cortex is rich in MT1 receptor, but not MT2 receptor. The majority of MT1 receptor-positive neurons were found in layers II/III and V, whereas MT2 receptor-positive neurons were mainly in deeper layers (V and VI) of cortical areas. The density of MT1 receptor-positive neurons was highest in the retrosplenial cortex and decreases rostrally and caudally immediately off the midline of the brain. MT2 receptor immunoreactivity was weak in these areas and barely detectable in the medial prefrontal cortex. The immunoreactivity of MT1 receptor was weak to moderate in the more lateral areas, which was similar to that of MT2 receptor-positive neurons. MT2 receptor-positive neurons were absent in both the entorhinal and piriform cortices, and MT1 receptor-positive neurons were barely seen in these regions. In the claustrum and the endopiriform nucleus, the immunoreactivity of MT1 and MT2 receptors was weak. In the occipital (visual) and temporal (auditory) cortices, only weak signals of MT1-positive neurons were observed.
The physiological role of melatonin receptors in the cerebral cortex remains elusive. There is some evidence of involvement in cognitive function. For instance, genetic deletion of MT1/MT2 receptors seems to enhance murine cognitive performance, although the underlying mechanism is unclear (O’Neal-Moffitt et al. 2014). Besides that, it is also speculated that melatonin might act in concert with gamma-aminobutyric acid (GABA) receptors in the cerebral cortex. In a study conducted on rabbits, it was identified that the cortex contained abundant melatonin-binding sites, particularly in the parietal cortex (Stankov et al. 1992). Furthermore, binding of melatonin or 2-[125I]Iodomelatonin induces benzodiazepine-like and GABA-like effects by slowing neuronal firing. This action is likely to be mediated via melatonin receptors as the study showed that the receptor binding is sensitive to pertussis toxin, indicating that G protein-coupled receptors, such as melatonin receptors, are involved. This suggests that melatonin could possibly modulate the activity of GABA benzodiazepine receptors in the cerebral cortex (Marangos et al. 1981). This is supported by another study which demonstrated that subcutaneous injection of melatonin reversed the decrease in GABA benzodiazepine receptor density in the cerebral cortex of pinealectomized rats (Lowenstein et al. 1985). Apart from the pineal gland, astrocytes are possibly another source of melatonin in the cortex. This is supported by the presence of melatonin as well as the two key enzymes in melatonin synthesis—N-acetyltransferase and hydroxyindole-O-methyltransferase, in the rat cortical astrocytes and the glioma C6 cell line (Liu et al. 2007). Hence, it is possible that melatonin in the cortex is originated from the astrocytes.
Through the use of autoradiography, RT-PCR, immunohistochemistry and transgenic models, MT1 and MT2 receptor mRNAs and proteins have been found in the pyramidal and granular neurons in the hippocampus (Mazzucchelli et al. 1996; Savaskan et al. 2005; Adamah-Biassi et al. 2014). Distribution of MT1 receptor immunoreactivity was diffuse in the hippocampus, whereas MT2 receptor immunoreactivity is more distinct, especially in the CA3 region. In the CA1, MT1 receptor immunoreactivity was diffuse and few positive neurons were found in the pyramidal layer. In the most rostral part, diffuse immunoreactivity was found in the stratum oriens (Or) and radiatum (Rad). Weak MT1 immunoreactivity was observed in the CA2 region and weak to moderate in the CA3 region. In the dentate gyrus (DG), MT1 immunoreactivity was found in the hilus of the DG, scattered in the polymorph layer (PoDG), and absent in the granular layer. MT2 immunoreactivity was observed in all layers of the hippocampus, with moderate immunoreactivity in the CA1, weak in the CA2, and strong immunoreactivity in the CA3 region. Most of the MT2-positive neurons were found in the pyramidal layer. In the DG, MT2 immunoreactivity was moderate, with positive cell bodies in the hilus, the dorsal portion of the granular layer of DG (GrDG), and to a lesser extent in the polymorph layer of DG (PoDG) (Lacoste et al. 2015). This suggests a possible difference in the actions of the two receptors within the hippocampus.
Although the exact roles of melatonin receptors in the hippocampus are unclear, there is at least some evidence showing that chronic melatonin enhances hippocampal neurogenesis (Liu et al. 2013; Ramirez-Rodriguez et al. 2009, 2011). Studies on animal models of mental disorders/impaired brain functions have demonstrated beneficial effects of melatonin in attenuating inhibition of hippocampal neurogenesis induced by methamphetamine (Ekthuwapranee et al. 2015), dexamethasone (Ruksee et al. 2014), high-fat diet with streptozotocin (Wongchitrat et al. 2016). In the rats given with high-fat diet and streptozotocin, the decrease in MT1 and MT2 receptors expression levels was attenuated by melatonin, consequently leading to the restoration of the hippocampal neurogenesis and synaptic proteins (Wongchitrat et al. 2016). These studies have shown that both MT1 and MT2 receptors can enhance hippocampal neurogenesis.
In learning and memory formation, synaptic connections are strengthened through the increase of synapses and receptors. When this process is sustained after stimulation, it is called long-term potentiation (LTP) which plays an important part in memory process (Cooke and Bliss 2006). Melatonin has been known to regulate synaptic plasticity by increasing the excitability of neurons in the hippocampus (Musshoff et al. 2002). Concentration-dependent inhibition of LTP by melatonin was observed in the CA1 dendritic layer of the Schaeffer collaterals of the mouse hippocampus and this inhibition was blocked by the MT1/2 receptor antagonist luzindole, suggesting a receptor-mediated role for this action (Wang et al. 2005). Further studies with an MT2 receptor-selective antagonist 4P-PDOT also blocked the inhibiting effects, implicating the role of the MT2 receptor in LTP inhibition. Since LTP is heavily involved in memory processes, the inhibition by melatonin through the MT2 receptor should have led to memory disruption and learning impairment. However, it was also observed that MT2 KO mice have impairment in memory and LTP maintenance demonstrated by the smaller LTP magnitude in KO mice compared to that of wild-type mice, suggesting that the MT2 receptor plays a beneficial role in memory maintenance (El-Sherif et al. 2004). This is further supported by another receptor knockout (KO) study, where LTP inhibition was seen in MT2 KO, but not in MT1 KO mice (Larson et al. 2006). MT2 KO mice generally demonstrated longer transfer latencies in the short arm of the elevated plus maze, indicating a learning disability in these mice. Interestingly, MT1/MT2 double KO mice have shown improved spatial and reference learning and memory performance as well as basic memory function, in addition to displaying increased LTP and memory-related neuronal signaling (O’Neal-Moffitt et al. 2014). In view of the contrasting results, the role of the MT2 receptor in the hippocampus requires further elucidation.
Much interest gathered on the cerebral cortex and hippocampus is due to their close link with AD as susceptible brain regions. AD is an age-related, neurodegenerative disorder characterized by memory loss and cognitive deterioration. It has been reported that AD patients have reduced melatonin level in the cerebrospinal fluid (Liu et al. 1999). It was proposed at time that it was possibly a consequence of aging and neurodegeneration in AD. Later, it was identified that brain tissues from AD subjects had reduced levels of melatonin MT1 and MT2 receptors in pinealocytes and the occipital cortex compared with elderly controls (Brunner et al. 2006). Immunohistochemistry of the hippocampal regions of AD patients showed that there was a distinct decrease in MT2 receptor immunoreactivity (Savaskan et al. 2005). These results suggest the involvement of the MT2 receptor in the progression of AD although it is unknown if the loss of receptors is the consequence or the cause of AD. In contrast, the MT1 receptor immunoreactivity was found to be increased in AD (Savaskan et al. 2002a). In other regions of the brain, the MT1 receptor has been tied to melatonin’s neuroprotection abilities. Therefore, it was postulated that the increase in the MT1 receptor was a compensatory mechanism responding to neuronal damage in AD to amplify neuroprotective actions of melatonin. Interestingly, prophylactic melatonin tends to reduce amyloid peptide deposition in the hippocampus although this action of melatonin is thought to be receptor independent (O’Neal-Moffitt et al. 2015). Although current evidence has demonstrated a few AD-associated changes in the pineal gland, hippocampus and the cerebral cortex, the connection between AD and melatonin receptors is still questionable.
Overall, melatonin is reported to confer neuroprotection to the cerebral cortex. Melatonin treatment protects against the ischemic stroke-induced neuronal death of the hippocampal CA1 region of the Mongolian gerbil, and this is associated with increased MT2 immunoreactivity and protein level (Lee et al. 2010). In primarily cultured rat cortical neurons, melatonin alleviated the damage from N-methyl-d-aspartate excitotoxicity or hypoxia/reperfusion. However, it has been pointed out that this action was likely to be a non-receptor-mediated action as it was not blunted by the melatonin antagonist luzindole (Cazevieille et al. 1997). Nevertheless, there is evidence that melatonin upregulates the antioxidant enzymes in the cerebral cortex (Esparza et al. 2005; Kotler et al. 1998; Barlow-Walden et al. 1995). Results from the rat by Barlow-Walden et al. (1995) and Kotler et al. (1998) are generally in agreement with each other. They reported that melatonin increased mRNA levels of the glutathione peroxidase, copper–zinc-containing superoxide dismutase (Cu–Zn-SOD) and the manganese-containing superoxide dismutase (Mn-SOD) in the cortex of healthy rats. On the other hand, Esparza et al. (2005) have tested the effects of melatonin on aluminum-treated rats as an AD model. They observed that melatonin significantly increased activities of antioxidant enzymes including superoxide dismutase, glutathione peroxidase, and catalase in the cortices of aluminum-treated rats compared with those of the control rats. This study also confirmed that melatonin treatment alone would upregulate the expression of superoxide dismutase as well as catalase. In hippocampal slice cultures deprived of oxygen and glucose, melatonin reduces reactive oxygen species to near base levels. As this effect can be blocked by luzindole, it is thought that this action involves modulation of antioxidant genes probably through receptor-mediated transcriptional signaling (Parada et al. 2014). Interestingly, dietary supplements of melatonin resulted in significant reduction in both short and long pathogenic forms of cortical amyloid beta peptides in mice (Lahiri et al. 2004). Addition of melatonin in diet seems to increase brain melatonin levels regardless of age. However, melatonin probably does not confer any memory enhancement effect as no changes in neuronal synaptic proteins were detected in mice. Similarly, another study investigated the effects of prophylactic melatonin on a transgenic AD mouse model (O’Neal-Moffitt et al. 2015). They reported that melatonin reduced amyloid beta deposition in the frontal cortex and the hippocampus, which was receptor independent. In contrast to previous reports, they also observed that melatonin reduced gene expression of antioxidant enzymes including superoxide dismutase, catalase and glutathione peroxidase in the frontal cortex in a receptor-dependent manner. They have reasoned that this outcome was probably due to reduced oxidative stress upon long-term treatment with melatonin; hence, the need of antioxidant enzymes has reduced as well. Nevertheless, many of the above studies have the same limitation in not using melatonin agonists or KO animals to confirm the effects of receptor-mediated actions, thus it is premature to conclude that melatonin receptors confer neuroprotective effects.
Reticular thalamic nucleus
MT2 receptor, but not MT1 receptor, is found abundant in the reticular thalamic nucleus (Lacoste et al. 2015). A recent study has also shown the involvement of melatonin receptors in the reticular thalamic nucleus on sleep regulation, where spindle formation during transition to deeper sleep stages is found to be promoted by MT2 receptor agonist (Ochoa-Sanchez et al. 2011). These findings suggest a possible distinct involvement of melatonin receptors in sleep phases. MT2 receptor is involved in the NREM sleep through modulating the reticular thalamic nucleus and other areas involved in NREM, whereas MT1 receptor is involved in REM sleep through acting on brain areas such as the hypothalamus. However, more studies are still needed to understand the role played by MT1 and MT2 in different regions involved in sleep regulation and homeostasis.
Habenula
Habenula is a brain microstructure that has been thought to play roles in major depression, addiction, schizophrenia and pain processing (Boulos et al. 2016). Habenula expresses high level of MT1 receptor but not MT2 receptor (Lacoste et al. 2015; Adamah-Biassi et al. 2014). Habenular commissure and medial habenula are the areas particularly rich in MT1 receptor. There is emerging evidence that medial habenula involves not only in pain processing (Shelton et al. 2012), but also in depression (Ranft et al. 2010) and addiction (Ranft et al. 2010). High levels of MT1 receptor in the medial habenula might implicate the involvement of MT1 receptor in these processes. As a matter of fact, MT1 receptor KO mice has demonstrated depressive behaviour and interruption in sensorimotor gating in the prepulse inhibition test, which suggests a role of MT1 receptor in depression (Weil et al. 2006; Comai et al. 2015). A recent animal study has also shown that the activation of presynaptic melatonin receptors increases the strength of glutamatergic synapses within the habenula (Evely et al. 2016). This has provided us some insight into the mechanism of how melatonin controls the function of habenula neurons.
Hypothalamus
The area of note within the hypothalamus with regards to melatonin receptor distribution is the SCN. In human, MT1 receptor protein content within the SCN is the highest. In rat, it is mainly localized on the dendrites and somas of SCN neurons (Waly and Hallworth 2015). For the MT2 receptor, its level in SCN is comparatively low. Most of it was identified in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) instead (Wu et al. 2013).
SCN is the main circadian clock in mammals and it regulates various circadian rhythms in the body when an external stimulus is received. These rhythms include diverse physiological, metabolic and behavioral ones such as the sleep cycle and levels of different hormones in mammals (Herbert 1994). One of the best known rhythms controlled by the SCN is the synthesis and secretion of melatonin from the pineal gland. The light–dark cycle of the environment acts as an external stimulus that entrains the SCN to trigger the synthesis and secretion of melatonin (Liu et al. 1997). However, melatonin holds another role in the system as endogenous melatonin is further involved in the regulation of the circadian rhythms through feedback to the SCN (Pévet 2002). In this case, melatonin may act as an internal stimulus in the circadian timing system. Furthermore, melatonin has an important action in the circadian thermoregulatory adjustment of body temperature by sending signals to the preoptic area of the anterior hypothalamus (Saarela and Reiter 1994). In short, the synthesis and secretion of melatonin is regulated by the SCN and in turn, melatonin is also able to regulate the circadian clock in set phases.
The action of melatonin upon SCN is linked to the circadian system. In fact, a recent study shows that the expression level of melatonin receptor mRNAs changes diurnally in accordance with the melatonin release cycle. Expression of MT1 receptor mRNA reaches the peak at dusk compared to at dawn and midnight (Waly and Hallworth 2015). This result indicates that melatonin receptors are synthesized during the dark cycle and degrades during the light cycle. This result corresponds well to other studies on the rat SCN, in which a similar pattern of expression was discovered (Poirel et al. 2002; Neu and Niles 1997). Nevertheless, a study has reported contrary results with no significant change of MT1 receptor mRNA expression with time of the day in SCN (Sugden et al. 1999). Meanwhile, the expression of MT2 receptor is thought to be independent of the circadian rhythm. Waly and Hallworth (2015) did not find any circadian pattern of MT2 receptor in the SCN. Moreover, MT2, unlike MT1 receptor, is a receptor that desensitizes very rapidly in the presence of a full agonist of melatonin receptors (Witt-Enderby et al. 2003). Evidence from an in vitro study that used an MT2 selective antagonist to study the role of MT2 receptor in circadian rhythm is inconclusive (Hunt et al. 2001), mainly because it is not practical to study the circadian rhythm using in vitro techniques. Therefore, expression of MT1 receptor is strictly linked to circadian rhythm and SCN function, but not MT2 receptor. This is confirmed by studies on the KO animals—phase shift effect in sleep rhythm was not seen in the absence of MT1 receptor (Liu et al. 1997; Dubocovich et al. 2005), but it can occur in the absence of MT2 receptor (Weaver et al. 1996).
A particular effect melatonin has on the SCN is in sleep regulation, mainly in sleep promotion and sleep onset phase shift. The sleep/wake cycle is one of the many body rhythms regulated by the circadian system and is closely synchronized with the 24 h circulating melatonin level whose peak levels coincide with maximum sleepiness (Reiter et al. 2007). A study shows that MT1 receptor in the SCN mediates inhibition of neuronal firing, leading to promotion of sleep, as evidenced by MT1 KO mice demonstrating a reduction in rapid eye movement (REM) sleep (Ochoa-Sanchez et al. 2011). MT2 receptor, on the other hand, is implicated in the induction of sleep onset, mainly affecting non-rapid eye movement (NREM) sleep. MT2 KO studies revealed that NREM sleep was decreased while REM sleep remained unaffected in MT2 KO mice. This is supported by pharmacological studies using MT2 selective agonists, in which lengthened NREM sleep was observed (Comai and Gobbi 2014; Ochoa-Sanchez et al. 2011, 2014; Fisher and Sugden 2009). This indicates that MT2 receptor affects NREM sleep and the changes are related to the light phase of the light/dark sleep cycle (Comai and Gobbi 2014). In fact, sleep recordings in mice with genetic deletions of MT1, MT2 or both receptors have suggested that REM sleep is regulated by MT1 receptor, whereas NREM sleep is largely regulated by MT2 receptor (Comai et al. 2013).
A study reveals that MT1 receptor colocalizes with vasopressin in neurons of SCN, parvocellular and magnocellular parts of PVN, with oxytocin in neurons of the SON, and with corticotropin-releasing hormone in neurons of the parvocellular part of PVN (Wu et al. 2006). The presence of MT1 receptor in these neurons suggests that perhaps melatonin is involved in the regulation of a few pituitary hormones. In vitro experiments using melatonin receptor ligands and receptor KO animals have shown that activation of MT1 and MT2 in the SCN, respectively, leads to distinctive responses. An in vitro study on rodent SCN slices showed that there is a concentration-dependent effect of melatonin on neuronal firing inhibition (Shibata et al. 1989). Further studies with targeted disruption of the MT1 receptor in transgenic mice revealed that the inhibitory action on neuronal firing in the SCN is absent in MT1 KO mice (Liu et al. 1997; Jin et al. 2003). These findings suggest that inhibitory effect on neuronal activity in the SCN is primarily mediated by the MT1 receptor. Meanwhile, an in vitro study using an MT2 selective antagonist, 4-phenyl-2-propionamidotetralin (4P-PDOT), found that the circadian phase shifting effect on neuronal firing was mediated by the MT2 receptor (Hunt et al. 2001). However, this effect does not seem to be solely regulated by the MT2 receptor as there is evidence of interactions between MT1 and MT2 receptors to result in this outcome. The phase shift effect, while still present in MT1 KO mice, is reduced compared to wild-type mice, indicating a possible role of MT1 receptors in phase shift within the SCN (Liu et al. 1997). The phase shifting effects on circadian rhythm is observed in animals lacking the MT2 receptor in the SCN hence it is likely that these effects are mediated by MT1 receptor (Weaver et al. 1996). Taking these together, phase shifting in the SCN is mediated mainly by the MT1 receptor.
Over the years, it is believed that dopaminergic neurons in the hypothalamus are well reserved in Parkinson’s disease (PD) and thus is concluded that hypothalamus might not be a susceptible brain region in PD (Matzuk and Saper 1985). However, a very recent study has pointed out that this notion may not be fully representative because hypothalamic gray matter volume loss was observed in PD patients and this was significantly associated with the reduced serum melatonin level in PD (Breen et al. 2016). This reduced systemic level of melatonin was also seen in early PD (Breen et al. 2014), suggesting that reduced melatonin level may lead to the disruption of hypothalamic functions and results in sleep dysfunction, a commonly reported non-motor symptom in PD. Level of melatonin might serve as a potential biomarker in PD diagnosis.
Pituitary gland
Consistent with findings from animal studies (Lacoste et al. 2015; Klosen et al. 2002; Adamah-Biassi et al. 2014), MT1 receptor was identified to be the main melatonin receptor in the pituitary gland in the human brain (Wu et al. 2006; Williams et al. 1997; Weaver et al. 1993). The pars tuberalis (PT) within the pituitary expresses a high level of MT1 receptor (Adamah-Biassi et al. 2014). In the rat PT, MT1 receptor is mainly expressed in a subpopulation of endocrine cells which also expresses alpha-glycoprotein subunit and beta-thyroid-stimulating hormone (Klosen et al. 2002). Colocalization of MT1 receptor and retinoid-related orphan receptor beta (ROR beta) was reported in these cells (Klosen et al. 2002). It was speculated that melatonin possibly controls the transcriptional activity of ROR receptors indirectly via the MT1 receptor. ROR beta is a nuclear receptor belonging to the RZR/ROR family. It was once suggested that melatonin was an endogenous ligand to ROR beta based on a study using in vitro transient transfection assay (Becker-Andre et al. 1994). However, the authors failed to reproduce the results later. Therefore, it is still inconclusive whether melatonin is a native ligand to ROR beta in PT.
The neurocrine function of the pituitary gland is closely associated with the hypothalamus. MT1 receptor colocalizes with vasopressin, oxytocin, and corticotropin-releasing hormone-secreting neurons in the pituitary, respectively, suggesting a role of melatonin in regulating these pituitary hormones (see 5.6) although this functional link is not well understood (Wu et al. 2006). Interestingly, several reports have found that melatonin is also involved in regulating prolactin secretion (Williams et al. 1997; Jilg et al. 2005). In mammals, it is widely accepted that photoperiod changes alter patterns of pineal melatonin production, which results in changes of pituitary hormone secretion. In a study using hypothalamo-pituitary disconnected rams, binding sites of 2-[125l]Iodomelatonin were unaltered compared to the control rams, but secretion of many pituitary hormones, except prolactin, was affected by the disrupted hypothalamus–pituitary axis. This shows that prolactin is regulated by melatonin rather than the hypothalamus–pituitary axis. Despite this finding, colocalization of the MT1 receptor with prolactin was not observed in the PT. Hence, it is postulated that melatonin acts on another site within the pituitary gland to control prolactin secretion and this action is independent of external influence. Later this site was identified to be the pars distalis (Jilg et al. 2005). This study also demonstrated that binding of melatonin to the MT1 receptor triggers the change of mRNA expression of a few circadian proteins such as Per1, Per2 and Cry1, which ultimately results in the change of seasonal cycle in prolactin secretion. Furthermore, it was reported that melatonin regulates expression of clock genes, particularly Per1, Cry1, Clock, and Bmal1, through the MT1 receptor (Dardente et al. 2003; von Gall et al. 2005). Interestingly, evidence yielded from an in vitro study using GT1-7 gonadotrophin-releasing hormone (GnRH) secreting neurons also suggests that melatonin might regulate gene expression of GnRH in the pituitary gland (Roy and Belsham 2002).
Periaqueductal gray
The antinociceptive effect of melatonin has been documented previously (Ambriz-Tututi et al. 2009). Melatonin is able to attenuate nociceptive responses to noxious stimuli such as heat, inflammation and neuropathic pain (Lopez-Canul et al. 2015a, b). This effect is often described as potent and longlasting in the animal model (Lakin et al. 1981). The periaqueductal gray in the midbrain is known to be involved in analgesia via the action of enkephalins on opioid receptors (Graeff 2012). The presence of melatonin receptors in the periaqueductal gray (mostly MT2 receptors, even if MT1 are also present), as evidenced by several animal studies, suggests that melatonin may play a pivotal role in pain processing (Lacoste et al. 2015; Lopez-Canul et al. 2015b; Adamah-Biassi et al. 2014). In fact, it has been reported that melatonin receptors, at least MT2, modulate antinociception via the glutamatergic pathway (Lopez-Canul et al. 2015b). This action of melatonin potentially results in the secretion of opioid peptide beta-endorphin but not enkephalin in the periaqueductal gray, which presumably leads to the analgesic effect (Yu et al. 2000).
Dorsal raphe nucleus
Moderate immunoreactivity of the MT1 receptor in the dorsal raphe nucleus (DR) has suggested the possible involvement of the MT1 receptor in the pathogenesis of depression (Lacoste et al. 2015). This is supported by a study which has demonstrated behavioral changes that mimic the symptoms of melancholic depression in MT1 KO mice (Comai et al. 2015). On top of that, there is evidence of melatonin imposing tonic inhibitory control over a subpopulation of serotonin-containing neurons in DR (Dominguez-Lopez et al. 2012). Changes in serotonin levels in DR have been implicated in mood disorders such as depression. Hence, this study has suggested a possible link between melatonin and serotonin neurotransmission in depression.
Midbrain
The striatum and substantia nigra are important in the production of movement. These two regions are connected through the nigrostriatal pathway, one of the main dopaminergic pathways located within the brain. Several evidence has supported that melatonin may have a receptor-mediated role in the nigrostriatal dopaminergic pathways. First, mRNA expression for both MT1 and MT2 receptors has been observed in the striatum of mice, rats and human (Uz et al. 2003). Also, MT1 receptor is found in caudate–putamen and it colocalizes with D2 receptor (Uz et al. 2005). Furthermore, neuroanatomical mapping of rat’s SN revealed a high level of the MT1 receptor in the SN pars compacta and the MT2 receptor in the SN pars reticulata (Lacoste et al. 2015). The presence of melatonin receptors in these regions implies the involvement of melatonin in the movement initiation process. It is likely that melatonin acts by inhibiting the secretion of dopamine via MT1 receptor in this pathway (Zisapel 2001). Abnormality in nigrostriatal dopaminergic pathway is heavily implicated in PD. There was a report that both melatonin receptors were found to be downregulated in the amygdala and SN of idiopathic PD patients (Adi et al. 2010). Along with the reported systemic changes of melatonin levels in PD patients (Bordet et al. 2003; Videnovic et al. 2014), this intriguing finding would indicate a possible involvement of melatonin in the progression of PD, although more studies are mandatory to confirm this association.
There is evidence that MT1, but not MT2 receptor, in striatum is involved in the pathophysiology of Huntington’s disease (HD). In HD patients, mRNA and protein expression level of MT1, but not MT2 receptor, are decreased in the striatum (Wang et al. 2011). However, this deficit can be partially reversed by treatment with melatonin in R6/2 mouse model of HD (Wang et al. 2011). This mechanism by melatonin is likely mediated by blockage of mitochondrial cell death pathway. Hence, melatonin might confer neuroprotection in HD via MT1-mediated action.
On the other hand, melatonin receptor might be involved in the mesocorticolimbic projections too. Dopaminergic neurons in ventral tegmental area and nucleus accumbens express MT1 mRNA (Uz et al. 2005). In nucleus accumbens, MT1 receptor colocalizes with D2 receptor. These regions are central to the mesocorticolimbic dopaminergic system. The presence of the MT1 receptor in these areas suggests possible involvement of melatonin in the reward system, possibly via modulation of the dopaminergic system.
Meanwhile, there have been reports that suggest, instead of modulating the dopaminergic system, melatonin protects neurons against devastating conditions such as elevated oxidative stress in the dopaminergic neurons as depicted in studies on a PD model (Srinivasan et al. 2011; Saravanan et al. 2007). It is possible that melatonin confers neuroprotection via receptor-mediated actions in PD. Nevertheless, current evidence is too scarce and further functional research, such as KO studies, is required to warrant the exact roles of melatonin and its receptors.
Cerebellum
While consensus on the exact role of the cerebellum has yet to be reached, this region is heavily implicated in motor control, execution and learning and non-motor functions such as cognition (Manto et al. 2012; Buckner 2013). In situ hybridisation on human cerebellum revealed that MT1 receptor mRNA was expressed in granule cells and basket–stellate cells while MT2 receptor mRNA was found in Bergmann glia and astrocytes (Al-Ghoul et al. 1998). Presence of MT1 receptor in the cerebellum has also been confirmed in a transgenic mouse model (Adamah-Biassi et al. 2014).
Cerebellum plays a supporting role in various brain functions such as motor control and cognition. As such, elucidating the specific pathways regulated by melatonin receptors in this region has been difficult. One of the earliest correlations found between melatonin and the cerebellum was in balance control, where administration of melatonin impaired sensorimotor performance (Fraschini et al. 1999). Posture control relies on multisensory integration, which occurs mainly in the cerebellum. Administration of melatonin could have activated cerebellar melatonin receptors to lead to an inhibitory effect on cerebellar sensory integration or on pathways from the cerebellum to the vestibular system. One study has demonstrated the neuroprotective effect of melatonin on the cerebellum (Manda et al. 2008), which was abolished by the melatonin receptor antagonist luzindole. Moreover, melatonin upregulates the gene expressions of antioxidant enzymes catalase and superoxide dismutase in cerebellum in aluminum-treated mice (Esparza et al. 2005). While the exact pathways or melatonin receptor subtypes involved are yet to be elucidated, the findings indicate that the presence of melatonin receptors in the cerebellum may confer additional neuroprotection to that region. In a recent in vivo study by Pinato et al. (2015), the cerebellum was shown to be able to synthesize melatonin during acute neuroinflammation induced by intracerebroventricular lipopolysaccharide injection in male Wistar rats. This local production of melatonin induced by lipopolysaccharide was only observed in the cerebellum but not in the hippocampus and the cortex. The increase in cerebellar melatonin confers neuroprotection specifically to that region, where neuronal damage was less pronounced after lipopolysaccharide injection, compared to the hippocampus and the cortex.
Concluding remarks
In this review, we have discussed the distribution of melatonin receptors in the brain. Based on the fact that certain variation across species has been noticed for the location of melatonin receptors (Stankov et al. 1991), we have only included findings from mammals for discussion in hope that these findings can be extrapolated to human. Though melatonin was discovered over half a century ago, its actions are still largely unknown. While melatonin receptors are abundant in the brain, their functions and underlying pathways remain unclear due to the complexity of the brain. With findings from a number of studies, we speculate that melatonin is an important mediator of a wide range of physiological functions, and alterations in the melatonin system are implicated in the etiopathogenesis of diverse neurodegenerative diseases such as AD, PD and HD. Therefore, future investigation on the actions of melatonin receptors in the brain should be warranted. Development of selective MT1 and MT2 receptor ligands should also be emphasized to facilitate studies of these receptors.
References
Adamah-Biassi EB, Zhang Y, Jung H, Vissapragada S, Miller RJ, Dubocovich M (2014) Distribution of MT1 melatonin receptor promoter-driven RFP expression in the brains of BAC C3H/HeN transgenic mice. J Histochem Cytochem Off J Histochem Soc 62(1):70–84. doi:10.1369/0022155413507453
Adi N, Mash DC, Ali Y, Singer C, Shehadeh L, Papapetropoulos S (2010) Melatonin MT1 and MT2 receptor expression in Parkinson’s disease. Med Sci Monit 16(2):Br61–Br67
Al-Ghoul WM, Herman MD, Dubocovich ML (1998) Melatonin receptor subtype expression in human cerebellum. NeuroReport 9(18):4063–4068
Ambriz-Tututi M, Rocha-Gonzalez HI, Cruz SL, Granados-Soto V (2009) Melatonin: a hormone that modulates pain. Life Sci 84(15–16):489–498. doi:10.1016/j.lfs.2009.01.024
Barlow-Walden LR, Reiter RJ, Abe M, Pablos M, Menendez-Pelaez A, Chen LD, Poeggeler B (1995) Melatonin stimulates brain glutathione peroxidase activity. Neurochem Int 26(5):497–502
Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C (1994) Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J Biol Chem 269(46):28531–28534
Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, Destee A (2003) Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol 26(2):65–72
Boulos LJ, Darcq E, Kieffer BL (2016) Translating the habenula—from rodents to humans. Biol Psychiatry. doi:10.1016/j.biopsych.2016.06.003
Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, Barker RA (2014) Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 71(5):589–595. doi:10.1001/jamaneurol.2014.65
Breen DP, Nombela C, Vuono R, Jones PS, Fisher K, Burn DJ, Brooks DJ, Reddy AB, Rowe JB, Barker RA (2016) Hypothalamic volume loss is associated with reduced melatonin output in Parkinson’s disease. Mov Disord Off J Mov Disord Soc. doi:10.1002/mds.26592
Brunner P, Sozer-Topcular N, Jockers R, Ravid R, Angeloni D, Fraschini F, Eckert A, Muller-Spahn F, Savaskan E (2006) Pineal and cortical melatonin receptors MT1 and MT2 are decreased in Alzheimer’s disease. Eur J Histochem EJH 50(4):311–316
Bubenik GA (2002) Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 47(10):2336–2348
Buckner RL (2013) The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80(3):807–815. doi:10.1016/j.neuron.2013.10.044
Carlson LL, Weaver DR, Reppert SM (1989) Melatonin signal transduction in hamster brain: inhibition of adenylyl cyclase by a pertussis toxin-sensitive G protein. Endocrinology 125(5):2670–2676. doi:10.1210/endo-125-5-2670
Cazevieille C, Safa R, Osborne NN (1997) Melatonin protects primary cultures of rat cortical neurones from NMDA excitotoxicity and hypoxia/reoxygenation. Brain Res 768(1–2):120–124
Comai S, Gobbi G (2014) CCNP award paper: unveiling the role of melatonin MT(2) receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci 39(1):6–21. doi:10.1503/jpn.130009
Comai S, Ochoa-Sanchez R, Gobbi G (2013) Sleep-wake characterization of double MT(1)/MT(2) receptor knockout mice and comparison with MT(1) and MT(2) receptor knockout mice. Behav Brain Res 243:231–238. doi:10.1016/j.bbr.2013.01.008
Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, Gobbi G (2015) Melancholic-like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. doi:10.1093/ijnp/pyu075
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129(Pt 7):1659–1673. doi:10.1093/brain/awl082
Costa EJ, Lopes RH, Lamy-Freund MT (1995) Permeability of pure lipid bilayers to melatonin. J Pineal Res 19(3):123–126
Dardente H, Klosen P, Pevet P, Masson-Pevet M (2003) MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: effect of photoperiod. J Neuroendocrinol 15(8):778–786
Deupi X, Dolker N, Lopez-Rodriguez ML, Campillo M, Ballesteros JA, Pardo L (2007) Structural models of class a G protein-coupled receptors as a tool for drug design: insights on transmembrane bundle plasticity. Curr Top Med Chem 7(10):991–998
Dominguez-Lopez S, Mahar I, Bambico FR, Labonte B, Ochoa-Sanchez R, Leyton M, Gobbi G (2012) Short-term effects of melatonin and pinealectomy on serotonergic neuronal activity across the light–dark cycle. J Psychopharmacol 26(6):830–844. doi:10.1177/0269881111408460
Dubocovich ML, Markowska M (2005) Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 27(2):101–110. doi:10.1385/endo:27:2:101
Dubocovich ML, Benloucif S, Masana MI (1996) Melatonin receptors in the mammalian suprachiasmatic nucleus. Behav Brain Res 73(1–2):141–147
Dubocovich ML, Hudson RL, Sumaya IC, Masana MI, Manna E (2005) Effect of MT1 melatonin receptor deletion on melatonin-mediated phase shift of circadian rhythms in the C57BL/6 mouse. J Pineal Res 39(2):113–120. doi:10.1111/j.1600-079X.2005.00230.x
Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J (2010) International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 62(3):343–380. doi:10.1124/pr.110.002832
Ebisawa T, Karne S, Lerner MR, Reppert SM (1994) Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci USA 91(13):6133–6137
Ekthuwapranee K, Sotthibundhu A, Govitrapong P (2015) Melatonin attenuates methamphetamine-induced inhibition of proliferation of adult rat hippocampal progenitor cells in vitro. J Pineal Res 58(4):418–428. doi:10.1111/jpi.12225
El-Sherif Y, Witt-Enderby P, Li PK, Tesoriero J, Hogan MV, Wieraszko A (2004) The actions of a charged melatonin receptor ligand, TMEPI, and an irreversible MT2 receptor agonist, BMNEP, on mouse hippocampal evoked potentials in vitro. Life Sci 75(26):3147–3156. doi:10.1016/j.lfs.2004.06.009
Esparza JL, Gomez M, Rosa Nogues M, Paternain JL, Mallol J, Domingo JL (2005) Melatonin reduces oxidative stress and increases gene expression in the cerebral cortex and cerebellum of aluminum-exposed rats. J Pineal Res 39(2):129–136. doi:10.1111/j.1600-079X.2005.00225.x
Evely KM, Hudson RL, Dubocovich ML, Haj-Dahmane S (2016) Melatonin receptor activation increases glutamatergic synaptic transmission in the rat medial lateral habenula. Synapse (New York, NY) 70(5):181–186. doi:10.1002/syn.21892
Fisher SP, Sugden D (2009) Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett 457(2):93–96. doi:10.1016/j.neulet.2009.04.005
Fraschini F, Cesarani A, Alpini D, Esposti D, Stankov BM (1999) Melatonin influences human balance. Biol Signals Recept 8(1–2):111–119. doi:10.1159/000014578
Fujieda H, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM (1999) Expression of mt1 melatonin receptor in rat retina: evidence for multiple cell targets for melatonin. Neuroscience 93(2):793–799
Fujieda H, Scher J, Hamadanizadeh SA, Wankiewicz E, Pang SF, Brown GM (2000) Dopaminergic and GABAergic amacrine cells are direct targets of melatonin: immunocytochemical study of mt1 melatonin receptor in guinea pig retina. Vis Neurosci 17(1):63–70
Galano A, Tan DX, Reiter RJ (2013) On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. J Pineal Res 54(3):245–257. doi:10.1111/jpi.12010
Gilgun-Sherki Y, Melamed E, Offen D (2001) Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 40(8):959–975
Graeff FG (2012) New perspective on the pathophysiology of panic: merging serotonin and opioids in the periaqueductal gray. Braz J Med Biol Res Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica [et al] 45(4):366–375
Hardeland R (2009) Melatonin: signaling mechanisms of a pleiotropic agent. BioFactors 35(2):183–192. doi:10.1002/biof.23
Harsanyi K, Mangel SC (1992) Activation of a D2 receptor increases electrical coupling between retinal horizontal cells by inhibiting dopamine release. Proc Natl Acad Sci USA 89(19):9220–9224
Herbert J (1994) The suprachiasmatic nucleus. The mind’s clock. J Anat 184(Pt 2):431
Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML (2001) Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol 280(1):C110–C118
Jilg A, Moek J, Weaver DR, Korf HW, Stehle JH, von Gall C (2005) Rhythms in clock proteins in the mouse pars tuberalis depend on MT1 melatonin receptor signalling. Eur J Neurosci 22(11):2845–2854. doi:10.1111/j.1460-9568.2005.04485.x
Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR (2003) Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol Cell Biol 23(3):1054–1060
Jockers R, Maurice P, Boutin JA, Delagrange P (2008) Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br J Pharmacol 154(6):1182–1195. doi:10.1038/bjp.2008.184
Klosen P, Bienvenu C, Demarteau O, Dardente H, Guerrero H, Pevet P, Masson-Pevet M (2002) The mt1 melatonin receptor and RORbeta receptor are co-localized in specific TSH-immunoreactive cells in the pars tuberalis of the rat pituitary. J Histochem Cytochem Off J Histochem Soc 50(12):1647–1657
Kotler M, Rodriguez C, Sainz RM, Antolin I, Menendez-Pelaez A (1998) Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res 24(2):83–89
Lacoste B, Angeloni D, Dominguez-Lopez S, Calderoni S, Mauro A, Fraschini F, Descarries L, Gobbi G (2015) Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J Pineal Res 58(4):397–417. doi:10.1111/jpi.12224
Lahiri DK, Chen D, Ge YW, Bondy SC, Sharman EH (2004) Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res 36(4):224–231. doi:10.1111/j.1600-079X.2004.00121.x
Lakin ML, Miller CH, Stott ML, Winters WD (1981) Involvement of the pineal gland and melatonin in murine analgesia. Life Sci 29(24):2543–2551
Larson J, Jessen RE, Uz T, Arslan AD, Kurtuncu M, Imbesi M, Manev H (2006) Impaired hippocampal long-term potentiation in melatonin MT2 receptor-deficient mice. Neurosci Lett 393(1):23–26. doi:10.1016/j.neulet.2005.09.040
Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kwon YG, Kim YM, Won MH (2010) Melatonin’s protective action against ischemic neuronal damage is associated with up-regulation of the MT2 melatonin receptor. J Neurosci Res 88(12):2630–2640. doi:10.1002/jnr.22430
Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19(1):91–102
Liu RY, Zhou JN, van Heerikhuize J, Hofman MA, Swaab DF (1999) Decreased melatonin levels in postmortem cerebrospinal fluid in relation to aging, Alzheimer’s disease, and apolipoprotein E-epsilon4/4 genotype. J Clin Endocrinol Metab 84(1):323–327. doi:10.1210/jcem.84.1.5394
Liu YJ, Zhuang J, Zhu HY, Shen YX, Tan ZL, Zhou JN (2007) Cultured rat cortical astrocytes synthesize melatonin: absence of a diurnal rhythm. J Pineal Res 43(3):232–238. doi:10.1111/j.1600-079X.2007.00466.x
Liu J, Somera-Molina KC, Hudson RL, Dubocovich ML (2013) Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J Pineal Res 54(2):222–231. doi:10.1111/jpi.12023
Lopez-Canul M, Comai S, Dominguez-Lopez S, Granados-Soto V, Gobbi G (2015a) Antinociceptive properties of selective MT(2) melatonin receptor partial agonists. Eur J Pharmacol 764:424–432. doi:10.1016/j.ejphar.2015.07.010
Lopez-Canul M, Palazzo E, Dominguez-Lopez S, Luongo L, Lacoste B, Comai S, Angeloni D, Fraschini F, Boccella S, Spadoni G, Bedini A, Tarzia G, Maione S, Granados-Soto V, Gobbi G (2015b) Selective melatonin MT2 receptor ligands relieve neuropathic pain through modulation of brainstem descending antinociceptive pathways. Pain 156(2):305–317. doi:10.1097/01.j.pain.0000460311.71572.5f
Lowenstein PR, Rosenstein R, Cardinali DP (1985) Melatonin reverses pinealectomy-induced decrease of benzodiazepine binding in rat cerebral cortex. Neurochem Int 7(4):675–681
Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F (2010) Melatonin signaling and cell protection function. Faseb J 24(10):3603–3624. doi:10.1096/fj.10-154450
Manda K, Ueno M, Anzai K (2008) Melatonin mitigates oxidative damage and apoptosis in mouse cerebellum induced by high-LET 56Fe particle irradiation. J Pineal Res 44(2):189–196. doi:10.1111/j.1600-079X.2007.00507.x
Manto M, Bower JM, Conforto AB, Delgado-García Jé M, da Guarda SNF, Gerwig M, Habas C, Hagura N, Ivry RB, Mariën P, Molinari M, Naito E, Nowak DA, Ben Taib NO, Pelisson D, Tesche CD, Tilikete C, Timmann D (2012) Consensus paper: roles of the cerebellum in motor control—the diversity of ideas on cerebellar involvement in movement. Cerebellum 11(2):457–487. doi:10.1007/s12311-011-0331-9
Marangos PJ, Patel J, Hirata F, Sondhein D, Paul SM, Skolnick P, Goodwin FK (1981) Inhibition of diazepam binding by tryptophan derivatives including melatonin and its brain metabolite N-acetyl-5-methoxy kynurenamine. Life Sci 29(3):259–267
Masson-Pevet M, George D, Kalsbeek A, Saboureau M, Lakhdar-Ghazal N, Pevet P (1994) An attempt to correlate brain areas containing melatonin-binding sites with rhythmic functions: a study in five hibernator species. Cell Tissue Res 278(1):97–106
Matzuk MM, Saper CB (1985) Preservation of hypothalamic dopaminergic neurons in Parkinson’s disease. Ann Neurol 18(5):552–555. doi:10.1002/ana.410180507
Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM (1996) The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res 39(1–2):117–126
Megaw PL, Boelen MG, Morgan IG, Boelen MK (2006) Diurnal patterns of dopamine release in chicken retina. Neurochem Int 48(1):17–23. doi:10.1016/j.neuint.2005.08.004
Meyer P, Pache M, Loeffler KU, Brydon L, Jockers R, Flammer J, Wirz-Justice A, Savaskan E (2002) Melatonin MT-1-receptor immunoreactivity in the human eye. Br J Ophthalmol 86(9):1053–1057
Morgan PJ, Barrett P, Howell HE, Helliwell R (1994) Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24(2):101–146
Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ (2002) Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus 12(2):165–173. doi:10.1002/hipo.1105
Neu JM, Niles LP (1997) A marked diurnal rhythm of melatonin ML1A receptor mRNA expression in the suprachiasmatic nucleus. Brain Res Mol Brain Res 49(1–2):303–306
Nosjean O, Ferro M, Coge F, Beauverger P, Henlin JM, Lefoulon F, Fauchere JL, Delagrange P, Canet E, Boutin JA (2000) Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem 275(40):31311–31317. doi:10.1074/jbc.M005141200
Ochoa-Sanchez R, Comai S, Lacoste B, Bambico FR, Dominguez-Lopez S, Spadoni G, Rivara S, Bedini A, Angeloni D, Fraschini F, Mor M, Tarzia G, Descarries L, Gobbi G (2011) Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J Neurosci 31(50):18439–18452. doi:10.1523/jneurosci.2676-11.2011
Ochoa-Sanchez R, Comai S, Spadoni G, Bedini A, Tarzia G, Gobbi G (2014) Melatonin, selective and non-selective MT1/MT2 receptors agonists: differential effects on the 24-h vigilance states. Neurosci Lett 561:156–161. doi:10.1016/j.neulet.2013.12.069
O’Neal-Moffitt G, Pilli J, Kumar SS, Olcese J (2014) Genetic deletion of MT(1)/MT(2) melatonin receptors enhances murine cognitive and motor performance. Neuroscience 277:506–521. doi:10.1016/j.neuroscience.2014.07.018
O’Neal-Moffitt G, Delic V, Bradshaw PC, Olcese J (2015) Prophylactic melatonin significantly reduces Alzheimer’s neuropathology and associated cognitive deficits independent of antioxidant pathways in AbetaPP(swe)/PS1 mice. Mol Neurodegener 10:27. doi:10.1186/s13024-015-0027-6
Parada E, Buendia I, Leon R, Negredo P, Romero A, Cuadrado A, Lopez MG, Egea J (2014) Neuroprotective effect of melatonin against ischemia is partially mediated by alpha-7 nicotinic receptor modulation and HO-1 overexpression. J Pineal Res 56(2):204–212. doi:10.1111/jpi.12113
Paxinos G, Watson C (2013) The rat brain in stereotaxic coordinates, 7th edn. Elsevier Academic Press, San Diego
Pechanova O, Paulis L, Simko F (2014) Peripheral and central effects of melatonin on blood pressure regulation. Int J Mol Sci 15(10):17920–17937. doi:10.3390/ijms151017920
Petit L, Lacroix I, de Coppet P, Strosberg AD, Jockers R (1999) Differential signaling of human Mel1a and Mel1b melatonin receptors through the cyclic guanosine 3′–5′-monophosphate pathway. Biochem Pharmacol 58(4):633–639
Pévet P (2002) Melatonin. Dialogues Clin Neurosci 4(1):57–72
Pinato L, da Silveira Cruz-Machado S, Franco DG, Campos LMG, Cecon E, Fernandes P, Bittencourt JC, Markus RP (2015) Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct Funct 220(2):827–840. doi:10.1007/s00429-013-0686-4
Poirel VJ, Masson-Pevet M, Pevet P, Gauer F (2002) MT1 melatonin receptor mRNA expression exhibits a circadian variation in the rat suprachiasmatic nuclei. Brain Res 946(1):64–71
Ramirez-Rodriguez G, Klempin F, Babu H, Benitez-King G, Kempermann G (2009) Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 34(9):2180–2191. doi:10.1038/npp.2009.46
Ramirez-Rodriguez G, Ortiz-Lopez L, Dominguez-Alonso A, Benitez-King GA, Kempermann G (2011) Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res 50(1):29–37. doi:10.1111/j.1600-079X.2010.00802.x
Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG (2010) Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med 40(4):557–567. doi:10.1017/s0033291709990821
Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S (2007) Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci 52:11–28
Reppert SM, Weaver DR, Ebisawa T (1994) Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13(5):1177–1185
Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF (1995) Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA 92(19):8734–8738
Reppert SM, Weaver DR, Ebisawa T, Mahle CD, Kolakowski LF Jr (1996) Cloning of a melatonin-related receptor from human pituitary. FEBS Lett 386(2–3):219–224
Rivera-Bermudez MA, Masana MI, Brown GM, Earnest DJ, Dubocovich ML (2004) Immortalized cells from the rat suprachiasmatic nucleus express functional melatonin receptors. Brain Res 1002(1–2):21–27. doi:10.1016/j.brainres.2003.12.008
Roy D, Belsham DD (2002) Melatonin receptor activation regulates GnRH gene expression and secretion in GT1-7 GnRH neurons. Signal transduction mechanisms. J Biol Chem 277(1):251–258. doi:10.1074/jbc.M108890200
Ruksee N, Tongjaroenbuangam W, Mahanam T, Govitrapong P (2014) Melatonin pretreatment prevented the effect of dexamethasone negative alterations on behavior and hippocampal neurogenesis in the mouse brain. J Steroid Biochem Mol Biol 143:72–80. doi:10.1016/j.jsbmb.2014.02.011
Saarela S, Reiter RJ (1994) Function of melatonin in thermoregulatory processes. Life Sci 54(5):295–311
Sallinen P, Saarela S, Ilves M, Vakkuri O, Leppaluoto J (2005) The expression of MT1 and MT2 melatonin receptor mRNA in several rat tissues. Life Sci 76(10):1123–1134. doi:10.1016/j.lfs.2004.08.016
Saravanan KS, Sindhu KM, Mohanakumar KP (2007) Melatonin protects against rotenone-induced oxidative stress in a hemiparkinsonian rat model. J Pineal Res 42(3):247–253. doi:10.1111/j.1600-079X.2006.00412.x
Savaskan E, Olivieri G, Meier F, Brydon L, Jockers R, Ravid R, Wirz-Justice A, Muller-Spahn F (2002a) Increased melatonin 1a-receptor immunoreactivity in the hippocampus of Alzheimer’s disease patients. J Pineal Res 32(1):59–62
Savaskan E, Wirz-Justice A, Olivieri G, Pache M, Krauchi K, Brydon L, Jockers R, Muller-Spahn F, Meyer P (2002b) Distribution of melatonin MT1 receptor immunoreactivity in human retina. J Histochem Cytochem Off J Histochem Soc 50(4):519–526. doi:10.1177/002215540205000408
Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Muller-Spahn F, Jockers R (2005) Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res 38(1):10–16. doi:10.1111/j.1600-079X.2004.00169.x
Savaskan E, Jockers R, Ayoub M, Angeloni D, Fraschini F, Flammer J, Eckert A, Muller-Spahn F, Meyer P (2007) The MT2 melatonin receptor subtype is present in human retina and decreases in Alzheimer’s disease. Curr Alzheimer Res 4(1):47–51
Scher J, Wankiewicz E, Brown GM, Fujieda H (2002) MT(1) melatonin receptor in the human retina: expression and localization. Investig Ophthalmol Vis Sci 43(3):889–897
Sharkey J, Olcese J (2007) Transcriptional inhibition of oxytocin receptor expression in human myometrial cells by melatonin involves protein kinase C signaling. J Clin Endocrinol Metab 92(10):4015–4019. doi:10.1210/jc.2007-1128
Shelton L, Pendse G, Maleki N, Moulton EA, Lebel A, Becerra L, Borsook D (2012) Mapping pain activation and connectivity of the human habenula. J Neurophysiol 107(10):2633–2648. doi:10.1152/jn.00012.2012
Shibata S, Cassone VM, Moore RY (1989) Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci Lett 97(1–2):140–144
Siuciak JA, Fang JM, Dubocovich ML (1990) Autoradiographic localization of 2-[125I]iodomelatonin binding sites in the brains of C3H/HeN and C57BL/6J strains of mice. Eur J Pharmacol 180(2–3):387–390
Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS, Slominski AT (2012) Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351(2):152–166. doi:10.1016/j.mce.2012.01.004
Song Y, Ayre EA, Pang SF (1992) The identification and characterization of 125I-labelled iodomelatonin-binding sites in the duck kidney. J Endocrinol 135(2):353–359
Song Y, Chan CW, Brown GM, Pang SF, Silverman M (1997) Studies of the renal action of melatonin: evidence that the effects are mediated by 37 kDa receptors of the Mel1a subtype localized primarily to the basolateral membrane of the proximal tubule. Faseb J 11(1):93–100
Srinivasan V, Cardinali DP, Srinivasan US, Kaur C, Brown GM, Spence DW, Hardeland R, Pandi-Perumal SR (2011) Therapeutic potential of melatonin and its analogs in Parkinson’s disease: focus on sleep and neuroprotection. Ther Adv Neurol Disord 4(5):297–317. doi:10.1177/1756285611406166
Stankov B, Cozzi B, Lucini V, Fumagalli P, Scaglione F, Fraschini F (1991) Characterization and mapping of melatonin receptors in the brain of three mammalian species: rabbit, horse and sheep. A comparative in vitro binding study. Neuroendocrinology 53(3):214–221
Stankov B, Biella G, Panara C, Lucini V, Capsoni S, Fauteck J, Cozzi B, Fraschini F (1992) Melatonin signal transduction and mechanism of action in the central nervous system: using the rabbit cortex as a model. Endocrinology 130(4):2152–2159. doi:10.1210/endo.130.4.1312448
Sugden D, McArthur AJ, Ajpru S, Duniec K, Piggins HD (1999) Expression of mt(1) melatonin receptor subtype mRNA in the entrained rat suprachiasmatic nucleus: a quantitative RT-PCR study across the diurnal cycle. Brain Res Mol Brain Res 72(2):176–182
Tosini G, Owino S, Guillaume JL, Jockers R (2014) Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. BioEssays 36(8):778–787. doi:10.1002/bies.201400017
Uz T, Akhisaroglu M, Ahmed R, Manev H (2003) The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology 28(12):2117–2123. doi:10.1038/sj.npp.1300254
Uz T, Arslan AD, Kurtuncu M, Imbesi M, Akhisaroglu M, Dwivedi Y, Pandey GN, Manev H (2005) The regional and cellular expression profile of the melatonin receptor MT1 in the central dopaminergic system. Brain Res Mol Brain Res 136(1–2):45–53. doi:10.1016/j.molbrainres.2005.01.002
Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, Rademaker AW, Simuni T, Zadikoff C, Zee PC (2014) Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 71(4):463–469. doi:10.1001/jamaneurol.2013.6239
von Gall C, Weaver DR, Moek J, Jilg A, Stehle JH, Korf HW (2005) Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann N Y Acad Sci 1040:508–511. doi:10.1196/annals.1327.105
Waly N, Hallworth R (2015) Circadian Pattern of melatonin MT1 and MT2 receptor localization in the rat suprachiasmatic nucleus. J Circadian Rhythms 13:Art. 1
Wang LM, Suthana NA, Chaudhury D, Weaver DR, Colwell CS (2005) Melatonin inhibits hippocampal long-term potentiation. Eur J Neurosci 22(9):2231–2237. doi:10.1111/j.1460-9568.2005.04408.x
Wang X, Sirianni A, Pei Z, Cormier K, Smith K, Jiang J, Zhou S, Wang H, Zhao R, Yano H, Kim JE, Li W, Kristal BS, Ferrante RJ, Friedlander RM (2011) The melatonin MT1 receptor axis modulates mutant Huntingtin-mediated toxicity. J Neurosci 31(41):14496–14507. doi:10.1523/jneurosci.3059-11.2011
Weaver DR, Stehle JH, Stopa EG, Reppert SM (1993) Melatonin receptors in human hypothalamus and pituitary: implications for circadian and reproductive responses to melatonin. J Clin Endocrinol Metab 76(2):295–301. doi:10.1210/jcem.76.2.8381796
Weaver DR, Reppert SM (1996) The Mel1a melatonin receptor gene is expressed in human suprachiasmatic nuclei. Neuroreport 8(1):109–112
Weaver DR, Liu C, Reppert SM (1996) Nature’s knockout: the Mel1b receptor is not necessary for reproductive and circadian responses to melatonin in Siberian hamsters. Mol Endocrinol 10(11):1478–1487. doi:10.1210/mend.10.11.8923472
Weil ZM, Hotchkiss AK, Gatien ML, Pieke-Dahl S, Nelson RJ (2006) Melatonin receptor (MT1) knockout mice display depression-like behaviors and deficits in sensorimotor gating. Brain Res Bull 68(6):425–429. doi:10.1016/j.brainresbull.2005.09.016
Wiechmann AF, Sherry DM (2013) Role of melatonin and its receptors in the vertebrate retina. Int Rev Cell Mol Biol 300:211–242. doi:10.1016/b978-0-12-405210-9.00006-0
Williams LM (1989) Melatonin-binding sites in the rat brain and pituitary mapped by in vitro autoradiography. J Mol Endocrinol 3(1):71–75
Williams LM, Lincoln GA, Mercer JG, Barrett P, Morgan PJ, Clarke IJ (1997) Melatonin receptors in the brain and pituitary gland of hypothalamo-pituitary disconnected Soay rams. J Neuroendocrinol 9(8):639–643
Witt-Enderby PA, Bennett J, Jarzynka MJ, Firestine S, Melan MA (2003) Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci 72(20):2183–2198
Wongchitrat P, Lansubsakul N, Kamsrijai U, Sae-Ung K, Mukda S, Govitrapong P (2016) Melatonin attenuates the high-fat diet and streptozotocin-induced reduction in rat hippocampal neurogenesis. Neurochem Int 100:97–109. doi:10.1016/j.neuint.2016.09.006
Wu YH, Zhou JN, Balesar R, Unmehopa U, Bao A, Jockers R, Van Heerikhuize J, Swaab DF (2006) Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol 499(6):897–910. doi:10.1002/cne.21152
Wu YH, Ursinus J, Zhou JN, Scheer FA, Ai-Min B, Jockers R, van Heerikhuize J, Swaab DF (2013) Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord 148(2–3):357–367. doi:10.1016/j.jad.2012.12.025
Yang XF, Miao Y, Ping Y, Wu HJ, Yang XL, Wang Z (2011) Melatonin inhibits tetraethylammonium-sensitive potassium channels of rod ON type bipolar cells via MT2 receptors in rat retina. Neuroscience 173:19–29. doi:10.1016/j.neuroscience.2010.11.028
Yu CX, Wu GC, Xu SF, Chen CH (2000) Melatonin influences the release of endogenous opioid peptides in rat periaqueductal gray. Sheng li xue bao [Acta physiologica Sinica] 52(3):207–210
Zisapel N (2001) Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol 21(6):605–616
Acknowledgements
This work is supported by MOSTI eScience Research Grant (02-02-10-SF0109) from Ministry of Science, Technology and Innovation of Malaysia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ng, K.Y., Leong, M.K., Liang, H. et al. Melatonin receptors: distribution in mammalian brain and their respective putative functions. Brain Struct Funct 222, 2921–2939 (2017). https://doi.org/10.1007/s00429-017-1439-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-017-1439-6