Abstract
Methamphetamine (METH) is an addictive stimulant, and METH users have abnormal brain structures and function. The aims of this study were to investigate the relationships between impulsivity, brain structures, and possible sex-specific differences between METH users and non-drug using Controls. Structural MRI and the Barratt Impulsiveness Scale (BIS) questionnaire were completed in 124 subjects: 62 METH (ages 41.2 ± 1.4 years, 34 males) and 62 Controls (ages 43.3 ± 2.3 years, 36 males). Independent and interactive effects of METH use status and sex were evaluated. Relationships between METH usage characteristics, brain morphometry, and impulsivity scores were examined. METH users had higher impulsivity scores, on both the Cognitive and Behavioral Factors from the BIS (p < 0.0001–0.0001). Compared with same-sex Controls, male METH users had larger, while female METH users had smaller, right superior frontal cortex (interaction-p = 0.0005). The male METH users with larger frontal volumes and female METH users with smaller or thinner frontal cortices had greater Cognitive impulsivity (interaction-p ≤ 0.05). Only female METH users showed relatively larger nucleus accumbens (interaction-p = 0.03). Greater impulsivity and thinner frontal cortices in METH users are validated. Larger superior frontal cortex in male METH users with greater cognitive impulsivity suggest decreased dendritic pruning during adolescence might have contributed to their impulsive and drug use behaviors. In the female METH users, smaller frontal cortices and the associated greater impulsivity suggest greater neurotoxicity to these brain regions, while their relatively larger nucleus accumbens suggest an estrogen-mediated neuroprotective glial response. Men and women may be affected differently by METH use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methamphetamine (METH) is an addictive stimulant that adversely affects behaviors and the brain. In 2012, an estimated 34 million individuals used amphetamine-type stimulants (ATS) worldwide, predominantly METH (Crime UNOoDa 2014). METH users accounted for approximately 7 % of total admissions to treatment services in the United States and 30 % of total admissions in Hawaii (SAMHSA 2014), where the current study was conducted and where METH is the major drug of abuse (Naqvi et al. 2014). This drug may be taken orally as a pill, but the crystalline form, often referred to as ‘ice’ or ‘crystal meth’, is typically smoked, and may also be injected, with a subsequent euphoria that can last for hours (Newton et al. 2005). However, how chronic METH use disorders are related to persistent brain injury or behavioral alterations remain somewhat controversial.
Various abnormalities in the brain structures of METH users and METH-exposed monkeys have been reported, such as enlarged striatal structures, including the putamen (Chang et al. 2005; Ersche et al. 2012; Groman et al. 2013), globus pallidus (Chang et al. 2005) and nucleus accumbens (Jernigan et al. 2005). Paradoxically, METH users with enlarged putamen and globus pallidus had relatively normal cognitive performance, suggesting a compensatory response to maintain cognitive function (Chang et al. 2005), although worse performance was found in the exposed monkeys that had the increased putamen volumes (Groman et al. 2013). In contrast, a study of METH-dependent tobacco smokers found smaller caudate nucleus, another striatal structure, and smaller orbitofrontal and insula cortices, compared to control non-smokers (Morales et al. 2012). METH users also showed deficits in limbic and paralimbic cortices, and smaller hippocampal volumes, but white matter hypertrophy (Thompson et al. 2004), as well as greater than normal age-related decline in various cortical volumes, including the insular cortex (Nakama et al. 2011).
Greater impulsive behaviors also were documented in METH users (Hoffman et al. 2006; Rusyniak 2013; Tabibnia et al. 2011). In particular, compared to non-drug users, METH users showed greater impulsivity as measured on the Barratt Impulsiveness Scale (BIS) (Lee et al. 2009). Furthermore, METH users with greater impulsivity were younger, less educated, used greater amounts of METH, were more likely to be binge users, and scored higher on the Beck Depression Inventory (Semple et al. 2005).
The relationships between abnormal brain structures and abnormal behaviors, such as impulsivity, were evaluated in several studies. For instance, METH users with lower gray matter intensity in the right pars opercularis had worse inhibitory control (Tabibnia et al. 2011), while those with greater gray matter density in the posterior cingulate cortex and ventral striatum, but lesser gray matter in the frontal gyrus, had poorer performance on a delay-discounting task (Schwartz et al. 2010). In addition, enlarged striatal structures were associated with greater impulsivity in both stimulant users and their non-stimulant using siblings, suggesting a genetic predisposition to these phenotypes (Ersche et al. 2012).
Furthermore, sex may modulate the impact of drug use on brain morphometry. Impulsivity is typically greater in men than in women, as shown in a motor task in healthy undergraduate students (Lage et al. 2013). Conversely, a study of treatment-seeking drug users found women to be more impulsive than men (Lejuez et al. 2007). Only one study evaluated sex differences in stimulant users in relation to brain structures; female stimulant-dependent users had smaller insula while male stimulant users had larger insula relative to sex-matched non-users (Tanabe et al. 2013). However, these stimulant drug users also used significant amounts of alcohol, marijuana and heroin (Tanabe et al. 2013). Given the paucity of studies regarding sex-specific findings, and the somewhat controversial findings in brain morphometry and behaviors in previous smaller studies (Morales et al. 2012; Nakama et al. 2011; Tanabe et al. 2013), or the different drugs used amongst groups and studies (Schwartz et al. 2010; Tabibnia et al. 2011; Tanabe et al. 2013), more research that control for these potential variables is needed to evaluate the sex-specific relationship between brain morphometry and behaviors in METH users.
Therefore, the current study evaluated the independent and combined effects of METH use and sex on structural brain measures and their relationship to impulsivity, using well-matched controls and a relatively large sample size. Based on prior morphometric studies (Chang et al. 2005; Ersche et al. 2012; Jernigan et al. 2005; Nakama et al. 2011; Tanabe et al. 2013), we hypothesized that METH users would have larger striatal volumes, but smaller and thinner frontal cortices, except for the insula, than sex-matched controls. We also expected METH users to have greater impulsivity, more so in females than males, relative to their sex-matched controls (Lage et al. 2013; Medina et al. 2008; Tanabe et al. 2013). Furthermore, regarding the relationship between impulsivity and brain morphometry, we expected METH users with greater impulsivity would have smaller frontal cortical but larger striatal brain measures.
Materials and methods
Participants and clinical assessments
Individuals were recruited from the community through flyers and word-of-mouth. Each participant signed a consent form approved by the institutional review boards at the University of Hawaii and at the Queen’s Medical Center. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors. Subjects who fulfilled the study criteria and completed the studies were compensated for their time and participation, with cash (for the controls) or gift cards (for the METH users). A total of 124 subjects (62 METH users: ages 41.2 ± 1.4 years, 34 men; 62 controls: ages 43.3 ± 1.9 years, 36 men) were enrolled between March 2010 and February 2014. All participants completed detailed structured neuropsychiatric and clinical evaluations to ensure they fulfilled the study criteria.
All subjects were ≥18 years of age and able to provide informed consent. Control subjects had urine toxicology negative for substances of abuse, including METH, amphetamines, cocaine, tetrahydrocannabinol (THC), benzodiazepine, and opiates. METH users also were required to have negative urine toxicology except for METH. All METH users had to have a history of METH-dependence according to DSM-IV criteria (or severe METH use disorder, DSM-V criteria) for at least 2 years. Past DSM-IV dependence or severe use disorder for marijuana and cocaine were also allowed, but the METH users could not have any current DSM-IV dependence or severe drug use disorders for other drugs, except for tobacco use. METH was the most used substance (in duration or frequency) and the “drug of choice” for all METH users.
Exclusion criteria for both groups included: (1) any neurological or psychiatric disorder that could confound brain morphometry; (2) any confounding chronic severe medical condition; (3) on medications that could confound outcome measures; (4) any contraindications for MRI (including urine test positive for pregnancy, unsafe implanted metallic objects or claustrophobia).
Detailed drug use histories and medical histories were obtained from all subjects by trained research staff. Drug use patterns included frequency, duration of use, route of use, and last use for illicit drugs, tobacco, and alcohol. Structured assessments included the Center for Epidemiologic Studies–Depression (CES–D) scale, and the Baratt impulsiveness scale (BIS) (Patton et al. 1995). The BIS is a self-administered 30-item questionnaire that assesses different personality constructs of impulsiveness; each item is rated from 1 (rarely/never) to 4 (almost always/always). The BIS scores were grouped into two factors based on the bifactor model described previously (Reise et al. 2013). Factor one will be referred to as the “Cognitive Factor” hereafter since it measures cognitive impulsivity which included questions that assessed whether the subject was “not a steady thinker”, “no concentration/self-control”, and “not planful”. Factor two will be referred to as the “Behavioral Factor” hereafter since it measures behavioral impulsivity, such as “racing thoughts”, “acting impulsively”, and “changing and moving around” frequently. Total BIS scores were also evaluated, which included additional questions that were not included in the other two factors.
MRI acquisition
All subjects were scanned on a Siemens 3T MR scanner (Tim Trio; Siemens Medical Solutions, Erlangen, Germany) with an 8- or 12-channel head coil. For structural imaging, we acquired a 3-plane localizer (TR/TE = 20/5; 3 × 3 10 mm slices), a high-resolution 3D magnetization-preparation rapid gradient echo (MP-RAGE, TR/TE/TI = 2200/4.91/1000 ms; 160 slices, 1 × 1 × 1 mm isotropic resolution), and a fluid-attenuated inversion recovery (FLAIR) sequence (9100/84/2500 ms; 44 slices, 0.9 × 0.9 × 3 mm). Structural MP-RAGE and FLAIR images were read by an experienced Neurologist to ensure the subjects did not show any significant brain lesions or abnormalities that might confound morphometric analyses.
Image analyses
Morphometric measures of brain structures were obtained from the MP-RAGE scan using the Freesurfer 5.1 software (http://surfer.nmr.mgh.harvard.edu/). The technical details are described in previous publications (Fischl and Dale 2000; Fischl et al. 2004; Han et al. 2006; Jovicich et al. 2006). After removing the skull from the MRI images, a Talairach transformation was conducted. Automated segmentation was performed for the subcortical white and gray matter structures and cortical areas using a probabilistic atlas. The process produced cortical and subcortical reconstruction and volumetric segmentation of the original MRI scans. All regions of interest (ROIs) were manually inspected to ensure accuracy.
Subcortical regional volumes were determined in each hemisphere for the amygdala, caudate, hippocampus, globus pallidus, putamen and nucleus accumbens. The cortical volume, thickness and area were determined for each of eight a priori ROIs: insula, superior frontal, caudal anterior cingulate, rostral anterior cingulate, lateral orbitofrontal, medial orbitofrontal, caudal middle frontal and rostral middle frontal regions, determined by the Desikan–Killiany atlas in Freesurfer (Desikan et al. 2006). These eight regions were shown to be smaller and thinner in drug users and/or impulsive individuals (Kim et al. 2006; Matsuo et al. 2009; Morales et al. 2012; Nakama et al. 2011; Schwartz et al. 2010; Tanabe et al. 2013). In addition, a vertex-based analysis was conducted over the whole brain for the same cortical measures.
Statistical analysis
Statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). One-way analysis of variance (ANOVA) was used to compare demographic and clinical characteristics between male METH users, female METH users, male controls, and female controls. Student’s t tests (unpaired, two-tailed) were performed to compare METH usage characteristics between the male and female METH users. Two-way analyses of covariance (ANCOVAs) were used to test the independent and interactive effects of METH status and sex on brain morphometry and impulsivity scores. Intracranial volume (ICV) was included in the model as a covariate for subcortical and cortical volume analyses, but not for cortical thickness and surface areas, per recommendation from the Freesurfer Wiki (https://surfer.nmr.mgh.harvard.edu/fswiki/FreeSurferSupport) and similar to a previous study (Westman et al. 2013). Analyses of the BIS for group comparisons were completed with and without education and CES-D scores as covariates. p values <0.05 were considered significant for subcortical regions and impulsivity scores. One-tailed t tests were used to compare the eight a priori frontal regions based on our hypothesis that these regions would be smaller and thinner in the METH users compared to controls. Simes correction (Simes 1986) for multiple comparisons was performed on the cortical measures, dividing significance values for each hemisphere by eight. Repeated-measures ANCOVAs were used to evaluate the effects of METH across the eight frontal cortical brain regions, separately for each measure (cortical volume, cortical thickness, and surface area) and for each hemisphere. These models included two between-subject variables (METH status and age) and one within-subject factor (frontal cortical brain region), with significance at p value <0.05.
For the vertex-based analysis on cortical brain measurements, Freesurfer’s query, design, estimate, contrast (QDEC), a graphical user interface statistical engine was used. Data were smoothed using 10-mm full-width-at-half maximum Gaussian kernel, and the cluster-wise threshold was set at p < 0.05. Correction for multiple comparisons was performed using Monte Carlo Z-simulation.
Three multi-variate GLM analyses were performed to evaluate the effects of brain morphometry, sex, and drug usage on impulsivity. The first multi-variate GLM analysis included all subjects, and used the Cognitive Factor or the Behavioral Factor as dependent variables and status (four levels of status, METH-use and sex) and brain measures (six measures that showed significant METH effects in Table 2) as explanatory variables, while adjusting for age, education, and CES-D, and exploring the interaction term brain morphometry-by-status. Two additional multi-variate models were performed in METH users only. The second multi-variate GLM analysis used the two BIS factors as dependent variables, METH use characteristics (amount of METH use, age at first use, and duration of abstinence), sex, and brain morphometric measures (the same six measures as above) as explanatory variables, while adjusting for age, education, and CES-D, and exploring the interaction terms brain morphometry-by-sex and METH use characteristics-by-sex. Some METH use characteristics (days of abstinence, total amount use) were log-transformed prior to analysis to ensure normality. The third multi-variate GLM analyses included the six brain morphometric measures as dependent variables, sex and METH use characteristics as explanatory variables, while adjusting for age, and exploring the interaction terms sex-by-METH use characteristics.
Results
Clinical characteristics (Table 1)
The METH users and controls were similar in age, ethnic and racial distributions. The METH users had slightly less education than the controls (p = 0.02), but the two groups did not differ in their estimated verbal intelligence quotient (IQ) scores (p = 0.11). The METH users had more depressive symptoms with higher CES-D scores than the controls (p = 0.0002). Since education level and depressive symptoms may influence impulsivity, subsequent comparisons for impulsivity scores were co-varied for education and CES-D scores. All groups were similar in their proportion of tobacco smokers (p = 0.75), pack-years smoked (p = 0.39), lifetime alcohol used (p = 0.43), and lifetime marijuana used (p = 0.53). Male and female METH users did not differ in the amounts and patterns of their METH usage.
BIS scores
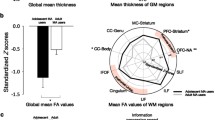
Compared to control subjects and independent of sex, METH users had higher impulsivity scores in both the Cognitive Factor (p < 0.0001) and the Behavioral Factor (p = 0.0001), as well as total BIS scores (p < 0.0001; Fig. 1a, b). Consistent with prior reports (Jaroni et al. 2004; Takahashi et al. 2008), higher impulsivity (total BIS scores) correlated with fewer years of education (p = 0.0010, r = −0.30) and higher CES-D scores (p < 0.0001, r = 0.64). Both factors also correlated with fewer years of education (Cognitive Factor-p = 0.0002, r = −0.33; Behavioral Factor-p = 0.02, r = −0.22) and higher CES-D scores (Cognitive-p < 0.0001, r = 0.53; Behavioral-p < 0.0001, r = 0.58). When education and CES-D scores were included as co-variates in all of these analyses, only the Cognitive Factor and the total BIS scores remained significant (p = 0.0005, p < 0.0001).
Barratt impulsivity scores in METH users and controls (p values are from two-way ANOVA). a Meth users, regardless of sex, showed higher impulsivity on the Barratt impulsivity scale, as shown in the two factors, the Cognitive Factor and Behavior Factor. b METH users, regardless of sex, showed higher impulsivity on the Barratt impulsivity scale on the total scores. On two-way ANCOVA, with METH and Sex as main effects, and education and CES-D scores were added as co-variates, the Cognitive Factor and total BIS scores remained significant
Vertex-based analysis of cortical measurements
On vertex-based analyses, compared to controls, METH users had smaller left superior frontal volumes (cluster-wise-p value = 0.0003, data not shown) and thinner lateral orbitofrontal cortices (cluster-wise-p = 0.00006; Fig. 2a–c). These findings remained significant after correction for multiple comparisons using Monte Carlo simulation.
Left lateral orbitofrontal cortical thickness in METH users and controls. a Statistical p value maps showing METH users had thinner left orbitofrontal cortex on the vertex-based analysis (cluster-wise-p value = 0.00006 after corrections for multiple comparisons). b Difference maps between METH users and controls showing the thinner cortices in the METH users (blue regions). c Cortical thickness extracted from the vertex maximum shown in (a) is illustrated, showing METH users had thinner left lateral orbitofrontal cortices than controls (p < 0.0001). Similarly thinner left lateral orbitofrontal cortices in METH users than controls were also found in the ROI analyses (p = 0.04, not shown). All analyses were performed using two-way ANCOVA on thickness measurements at max vertices, with METH and sex status as main effects and age as a co-variate
ROI cortical brain measurements (Table 2; Fig. 3)
Regardless of sex, in the left hemisphere, METH users had smaller rostral middle frontal (−2.2 %, p = 0.04), superior frontal (−1.8 %, p = 0.02), and caudal middle frontal volumes (−4.9 %, p = 0.01) compared to controls, but these contrasts did not remain significant after Simes correction (Table 2). In the right hemisphere, several regions were also smaller in the METH users compared to controls, but only the rostral middle frontal volume (−3.0 %, p = 0.01) and the superior frontal volume (-1.5%, p = 0.02) remained significant after Simes correction. METH users also tended to have larger right insula volumes compared to controls (+4.1 %, p = 0.06; not significant after Simes).
Thinner left frontal cortices in METH users than controls. a Regions of interests in the frontal cortices. b METH users showed thinner frontal cortices than controls, regardless of sex, in six of seven frontal regions of interests (repeated measures ANCOVA p = 0.002, age as a co-variate). p values for shown for Rostral anterior cingulate, medial orbitofrontal, lateral orbitofrontal, and superior frontal regions indicate that these regions remained significant after Simes correction in two-way ANCOVA analysis for METH effect (age as co-variate)
In addition, METH users consistently had thinner cortices than controls across all eight frontal regions in the left hemisphere (repeated measure-ANCOVA-p = 0.002), especially in the medial orbitofrontal (−2.8 %, p = 0.003) and rostral anterior cingulate regions (−4.0 %, p = 0.0008) (Fig. 3; Table 2). METH users also had thinner right frontal cortices (repeated-measure ANCOVA-p = 0.01), especially the medial orbital frontal cortex (−3.2 %, p = 0.006) (Table 2). Conversely, regional surface areas were not different between METH users and controls.
METH by-sex interactions
In the cortical ROI analyses, only the right superior frontal volume showed a METH-by-sex interaction (p = 0.02; Fig. 4a), with female METH users showing relatively smaller volumes (−6 %), but male METH users showing relatively larger volumes (+3 %), compared to sex-matched controls. This region also showed a METH-by-sex interaction in the vertex-based analysis (cluster-wise corrected p = 0.00005, Fig. 4b).
METH-by-sex interaction and association with duration of METH abstinence in right superior frontal cortical volume. a ROI analysis showing significant interaction effect (p = 0.02), with the female METH users showing 6 % smaller right superior frontal volumes compared to female controls (post hoc p = 0.06), while the male METH users had about 3 % larger volumes compared to male controls (post hoc not significant). Furthermore, female METH users had 14 % smaller right superior frontal volumes compared to male METH users (post hoc p < 0.0001). b Statistical significance p value maps showing METH-by-sex interaction in the superior frontal region on the vertex based analysis (cluster-wise-p = 0.00005). Age and ICV were included as co-variates. c Longer duration of abstinence was associated with small right superior frontal volumes in the METH users (multi-variate GLM analysis p = 0.03; Pearson-p = 0.006, r = −0.5)
Subcortical volumes
Amongst the seven subcortical brain regions, only the nucleus accumbens showed group differences (ANCOVA, co-varied for age and ICV, Fig. 5). In the left nucleus accumbens, the METH users had larger volumes than the controls (+7.3 %, p = 0.03), mostly due to the 13 % larger volume in female METH users than female controls (p = 0.03), although the METH-by-sex interaction did not reach significance (p = 0.18) (Fig. 5a). The right accumbens volume was also larger in the female METH users than female controls (+10 %, p = 0.02), but the difference amongst males was only 1 %, resulting in a METH-by-sex interaction (p = 0.03, Fig. 5c). While the nucleus accumbens volumes were smaller in female than male controls (right: −12 %; left: −12 %), female and male METH users had similar volumes.
Nucleus accumbens volumes in four subject groups. a Two-way ANCOVA showing METH status main effect (p = 0.03). METH users had 8 % larger left accumbens volume compared to the controls, driven mostly by the 13 % larger volume in the female METH users compared to the female controls (post hoc p = 0.03). b 2-D coronal slice of the brain, displaying location of N. Accumbens. c Two-way ANCOVA METH-by-sex interaction (p = 0.03). Right accumbens volume was 10 % larger in the female METH users than female controls (post hoc p = 0.02), compared to a 1 % difference in the two male groups. METH users: the men and women had similar right accumbens volume, controls: females had 12 % smaller volumes compared to the male controls (post hoc p = 0.006). All ANCOVA tests were co-varied for age and ICV
Relationships between METH usage, impulsivity and brain morphometry
In our multi-variate analyses, significant interactions were observed between several brain measures, METH use status and sex on the impulsivity Factors across the four subject groups (Fig. 6a–c). The relationship between medial orbitofrontal cortical thickness and the impulsivity scores, on the Cognitive Factor, were different across subject groups (interaction-p = 0.03), with the female METH users showing an opposite relationship from the other three groups (Fig. 6a). In addition, the Cognitive Factor scores decrease with larger right superior frontal volume (volume-p = 0.05), but at different levels of Cognitive Factor scores across groups (group status-p = 0.02, Fig. 6b). Furthermore, thicker left lateral orbitofrontal cortices were associated with higher impulsivity on the Behavioral Factor scores across all groups (Thickness-p = 0.04, Fig. 6c). When these relationships were explored only in METH users, thicker medial orbitofrontal cortices was associated with greater impulsivity Cognitive Factor scores in male METH users, but lower Cognitive Factor scores in the female users (interaction-p = 0.05, Fig. 6d). Furthermore, shorter duration of abstinence was associated with larger right superior frontal cortical volume (p = 0.03, Fig. 4c). In contrast, thinner left superiorfrontal cortices were associated with higher impulsivity Cognitive Factor scores in both METH user groups (Thickness-p = 0.05, Fig. 6e). Lastly, female METH users with earlier Age of first use had higher impulsivity on the Behavioral Factor scores, while male METH users showed no such relationship (Age of first use × Sex-p = 0.05, Fig. 6f).
Multivariate GLM analyses showing significant relationships between brain measures or METH use characteristics on impulsivity factors across subject groups. a The relationship between medial orbitofrontal cortical thickness and the impulsivity scores, on the Cognitive Factor, are different across subject groups (interaction-p = 0.03), with the female METH users showing an opposite relationship from the other three groups. b The Cognitive Factor scores decrease with larger right superior frontal volume (volume-p = 0.05) and the groups differed on the cognitive scores (group status-p = 0.02). c Thicker left lateral orbitofrontal cortices were associated with higher impulsivity on the Behavioral Factor Scores across all groups (Thickness-p = 0.04). d Thicker medial orbitofrontal cortices was associated with greater impulsivity Cognitive Factor scores in male METH users, but lower Cognitive Factor scores in the female users (interaction-p = 0.05). e Thinner left superiorfrontal thickness was associated with higher impulsivity Cognitive Factor scores in both METH user groups (Thickness-p = 0.05). f Female METH users with earlier Age of first use had higher impulsivity on the Behavioral Factor scores, while male METH users showed no such relationship (Age of first use × Sex-p = 0.05)
Discussion
Consistent with prior reports (Jernigan et al. 2005; Lee et al. 2009; Nakama et al. 2011), this study found greater impulsivity and alterations in several brain structures in METH users, particularly in the nucleus accumbens and frontal cortex. However, the current study extends and clarifies prior reports by showing sex-specific alterations in brain structures associated with METH use, such that female METH users showed relatively larger nucleus accumbens volume. Male METH users showed relatively larger frontal cortices while female METH users showed smaller superior frontal cortices compared to sex-matched controls. The sex-specific brain alterations in the female METH users were also associated with greater behavioral impulsivity.
Impulsivity in METH users
As a group, our METH users were more impulsive than controls, as shown in both the Cognitive and Behavioral Factors and the total BIS scores. This finding is consistent with previous reports of higher total scores on the BIS in METH users (Lee et al. 2009), as well as other studies in stimulant users (Liu et al. 2011; Perry et al. 2013). Contrary to our hypothesis and the greater impulsiveness in men that might have rendered them more susceptible to alcohol use disorders than women (Stoltenberg et al. 2008), there was no difference in impulsivity between the male and female METH users. However, the female METH users who started using METH at an earlier age had higher impulsivity scores, consistent with previous reports of younger METH users having significantly higher levels of impulsivity (Semple et al. 2005). Our findings, together with an earlier report of greater brain alterations and executive dysfunction in adolescent METH users (Lyoo et al. 2015) suggest that the developing brain may be more vulnerable to the neurotoxic effects of METH. Furthermore, earlier onset of METH use may increase the risk of progression to development of METH use disorders, as reported in alcohol users. (DeWit et al. 2000).
Enlarged striatal structures in METH users
The nucleus accumbens was the only striatal structure that was larger in the METH users in the current study. Larger than normal nucleus accumbens volumes in METH users was also reported in a previous study (Jernigan et al. 2005), but other studies found larger putamen (Chang et al. 2005; Ersche et al. 2012; Groman et al. 2013) and globus pallidus (Chang et al. 2005) instead. However, post hoc analyses showed that only the female METH users showed the enlarged nucleus accumbens. The larger than normal nucleus accumbens in METH users, as well as the enlarged striatal structures in other studies, may be due to neuroinflammation in these structures. For instance, METH-mediated increased and activated microglia was observed in preclinical studies (LaVoie et al. 2004; Thomas et al. 2004) and postmortem studies of METH users (Kitamura 2009). Also, METH users showed elevated binding for [11C](R)-PK11195, a radiotracer for microglia in the striatum of METH users (Sekine et al. 2008). Furthermore, an enhanced astrocytic response may promote neuronal survival in the nucleus accumbens, similar to previous findings in female mice but not male mice in the nigrostriatal dopaminergic system (Dluzen et al. 2003). Estrogen also could diminish METH-induced neurodegeneration of striatal dopamine (Dluzen and McDermott 2006). Hence, the larger accumbens in the female METH users may indicate an estrogen-mediated protective glial response.
Abnormal frontal cortical measures in METH users
METH users, and specifically male users, had larger right superior frontal volumes compared to non-users. These alterations were associated with a tendency for greater lack of self-control and shorter duration of abstinence from METH. The thinner frontal cortices in the METH users, especially in the medial orbitofrontal and rostral anterior cingulate cortex, are similar to prior findings in amphetamine-type stimulant users (Koester et al. 2012). The orbitofrontal cortex is well known for its impulse control function, and plays a major role in drug addiction; this brain region may be more prone to the neurotoxic effects of METH, as shown by its relationship with lower levels of dopamine D2 receptor availability in METH users compared to controls (Volkow et al. 2001). The smaller caudal middle frontal volumes in our METH users are consistent with the smaller frontal gray matter volumes found in previous studies of METH users (Morales et al. 2012; Nakama et al. 2011).
Consistent with a prior report (Tanabe et al. 2013), the right insula tended to be larger than normal in our METH users. The insula has been implicated in different modalities of self-control (Dambacher et al. 2014) and decision-making with risk-taking (Paulus et al. 2003; Xue et al. 2010), suggesting an important role in drug addiction (Naqvi et al. 2014). However, prior studies showed mixed results regarding the relationship between insula volume and drug addiction. Higher gray matter density in the left anterior insula was found in cigarette smokers compared to nonsmokers (Zhang et al. 2011), while smaller left insula was found in METH-dependent subjects, who likely were cigarette smokers also, than in controls (Morales et al. 2012). These inconsistencies may in part be due to methodological differences. In our study, automated segmentation of cortical brain regions was performed using FreeSurfer; which showed similar findings as the larger insulae found in the substance-dependent men, assessed by the same technique (Tanabe et al. 2013).
Sex differences in frontal cortical structures in METH users
The larger volumes in the right superior frontal cortex in the male but smaller volumes in the female METH users, compared to sex-matched controls, suggest that sex may modulate the effects of METH on brain morphometry. Likewise, decreased cerebral glucose metabolism in the right superior frontal white matter and impairment in executive function was found only in abstinent male METH users (Kim et al. 2005). Larger right superior frontal volumes in our male METH users are also similar to the larger prefrontal cortex in male adolescents with alcohol use disorder, which might be due to decreased dendritic pruning during adolescence (Medina et al. 2008). Selective pruning results in cortical gray matter thinning and age-related improvements in performance on executive function tasks (Kharitonova et al. 2013). Conversely, the male METH users in the current study with larger superior frontal volumes had more impulsive behavior with higher Cognitive Factor scores (i.e., less self control and concentration). Therefore, abnormal synaptic pruning during adolescence in these male METH users may also account for their lack of self-control, which might have led to their drug-using behaviors. Additionally, since METH users with larger right superior frontal volumes had shorter duration of abstinence, greater neuroinflammation in this brain region might have resulted from more recent METH use and might lead to greater impulsivity, especially in the male METH users.
Another study of METH users found a negative correlation between the left superior frontal gyrus gray matter density and impulsivity on a delay-discounting task (Schwartz et al. 2010), which is consistent with the thinner orbitofrontal cortex and greater impulsivity on the Cognitive Factor in our female METH users. Similarly, the smaller right superior frontal volumes in the female METH users with greater impulsivity further suggest that the frontal regions in the females might be more susceptible to the neurotoxic effects of METH.
Finally, prior studies found inconsistent morphometry with and without correction for ICV, with one study finding opposites results when adding sexual dimorphic covariates to their ANCOVA analysis (Pintzka et al. 2015). To address this, we ran our analyses with and without correcting for ICV and the majority of our results remained significant when co-varying for ICV.
Limitations
Several limitations in our study might confound our interpretations. First, the current study was cross-sectional; therefore, we cannot preclude premorbid brain structural abnormalities in these METH users, such as the possibility that the male METH users had lesser pruning. Second, since the BIS is a self-reported measure of impulsivity, findings may not reflect the actual degree of impulsivity of our subjects due to the variable subjective interpretations or truthfulness. Third, METH users often use other substances concurrently or had other substance use disorders, which may confound the interpretation of the findings in relation to METH use. We attempted to minimize these confounding effects by recruiting an equal proportion and amounts of tobacco and alcohol use in all groups. Furthermore, we excluded subjects with past dependence according to DSM-IV criteria for other illicit drugs and alcohol. A larger sample size and to include METH users without other substances are needed for future studies since some of the brain measures showed only trends for group differences (e.g. the insula) and to validate our findings.
Conclusions
The larger superior frontal cortices in the male METH users suggests decreased dendritic pruning during adolescence, and the even larger volumes in those with more recent METH use suggests greater neuroinflammation with more recent use, which in turn might exacerbate the impulsivity. The larger nucleus accumbens in the female, but not male, METH users suggest that estrogen might have mediated a protective glial response. Contrary to the male METH users, females METH had smaller and thinner frontal cortices that were associated with greater impulsivity, suggesting greater neurotoxicity to these brain regions. Together, these results suggest that METH may affect men and women differently, with sex-specific regional differences; therefore, sex should be accounted for in brain morphometry studies. Future studies using diffusion-tensor imaging and MR spectroscopy, measuring microscopic diffusivity and the glial marker myo-inositol in these frontal regions, may provide further insights regarding the tissue characteristics and neuroinflammation in METH users.
References
Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T (2005) Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol Psychiatry 57:967–974. doi:10.1016/j.biopsych.2005.01.039
Crime UNOoDa (2014) World drug report. United Nations Publications, New York
Dambacher F, Sack AT, Lobbestael J, Arntz A, Brugman S, Schuhmann T (2014) Out of control evidence for anterior insula involvement in motor impulsivity and reactive aggression. Social Cognit Affect Neurosci. doi:10.1093/scan/nsu077
Desikan RS et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. doi:10.1016/j.neuroimage.2006.01.021
DeWit DJ, Adlaf EM, Offord DR, Ogborne AC (2000) Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 157:745–750. doi:10.1176/appi.ajp.157.5.745
Dluzen DE, McDermott JL (2006) Estrogen, testosterone, and methamphetamine toxicity. Ann N Y Acad Sci 1074:282–294. doi:10.1196/annals.1369.025
Dluzen DE, Tweed C, Anderson LI, Laping NJ (2003) Gender differences in methamphetamine-induced mRNA associated with neurodegeneration in the mouse nigrostriatal dopaminergic system. Neuroendocrinology 77:232–238. doi:10.1159/000070278
Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET (2012) Abnormal brain structure implicated in stimulant drug addiction. Science 335:601–604. doi:10.1126/science.1214463
Fischl B, Dale AM (2000) Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. doi:10.1073/pnas.200033797
Fischl B et al (2004) Automatically parcellating the human cerebral cortex. Cerebral cortex (New York, NY: 1991) 14:11–22
Groman SM, Morales AM, Lee B, London ED, Jentsch JD (2013) Methamphetamine-induced increases in putamen gray matter associate with inhibitory control. Psychopharmacology 229:527–538. doi:10.1007/s00213-013-3159-9
Han X et al (2006) Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. NeuroImage 32:180–194. doi:10.1016/j.neuroimage.2006.02.051
Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH (2006) Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology 188:162–170. doi:10.1007/s00213-006-0494-0
Jaroni JL, Wright SM, Lerman C, Epstein LH (2004) Relationship between education and delay discounting in smokers. Addict Behav 29:1171–1175. doi:10.1016/j.addbeh.2004.03.014
Jernigan T et al (2005) Effects of methamphetamine dependence and hiv infection on cerebral morphology. Am J Psychiatry 162:1461–1472
Jovicich J et al (2006) Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage 30:436–443. doi:10.1016/j.neuroimage.2005.09.046
Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA (2013) Cortical gray-matter thinning is associated with age-related improvements on executive function tasks Developmental cognitive neuroscience 6:61–71. doi:10.1016/j.dcn.2013.07.002
Kim SJ et al (2005) Frontal glucose hypometabolism in abstinent methamphetamine users. Off Publ Am College Neuropsychopharmacol 30:1383–1391. doi:10.1038/sj.npp.1300699
Kim SJ et al (2006) Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol 9:221–228. doi:10.1017/s1461145705005699
Kitamura O (2009) Detection of methamphetamine neurotoxicity in forensic autopsy cases. Legal Med (Tokyo, Japan) 11 Suppl 1:S63–S65. doi:10.1016/j.legalmed.2009.01.003
Koester P, Tittgemeyer M, Wagner D, Becker B, Gouzoulis-Mayfrank E, Daumann J (2012) Cortical thinning in amphetamine-type stimulant users. Neuroscience 221:182–192. doi:10.1016/j.neuroscience.2012.06.049
Lage GM, Albuquerque MR, Fuentes D, Correa H, Malloy-Diniz LF (2013) Sex differences in dimensions of impulsivity in a non-clinical sample. Percept Motor Skills 117:601–607. doi:10.2466/15.19.PMS.117x18z2
LaVoie MJ, Card JP, Hastings TG (2004) Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol 187:47–57. doi:10.1016/j.expneurol.2004.01.010
Lee B et al (2009) Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci 29:14734–14740. doi:10.1523/JNEUROSCI.3765-09.2009
Lejuez CW, Bornovalova MA, Reynolds EK, Daughters SB, Curtin JJ (2007) Risk factors in the relationship between gender and crack/cocaine. Exp Clin Psychopharmacol 15:165–175. doi:10.1037/1064-1297.15.2.165
Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG (2011) Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse 37:117–122. doi:10.3109/00952990.2010.543204
Lyoo IK et al (2015) Predisposition to and effects of methamphetamine use on the adolescent brain. Mole Psychiatry 20:1516–1524. doi:10.1038/mp.2014.191
Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC (2009) A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Human Brain Mapp 30:1188–1195. doi:10.1002/hbm.20588
Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF (2008) Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res 32:386–394. doi:10.1111/j.1530-0277.2007.00602.x
Morales AM, Lee B, Hellemann G, O’Neill J, London ED (2012) Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend 125:230–238. doi:10.1016/j.drugalcdep.2012.02.017
Nakama H, Chang L, Fein G, Shimotsu R, Jiang CS, Ernst T (2011) Methamphetamine users show greater than normal age-related cortical gray matter loss. Addiction 106:1474–1483. doi:10.1111/j.1360-0443.2011.03433.x
Naqvi NH, Gaznick N, Tranel D, Bechara A (2014) The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann New York Acad Sci 1316:53–70. doi:10.1111/nyas.12415
Newton TF, De La Garza R, Kalechstein AD, Nestor L (2005) Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav 82:90–97. doi:10.1016/j.pbb.2005.07.012
Patton JH, Stanford MS, Barratt ES (1995) Factor structure of the Barratt impulsiveness scale. J Clin Psychol 51:768–774
Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB (2003) Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage 19:1439–1448
Perry RI, Krmpotich T, Thompson LL, Mikulich-Gilbertson SK, Banich MT, Tanabe J (2013) Sex modulates approach systems and impulsivity in substance dependence. Drug Alcohol Depend 133:222–227. doi:10.1016/j.drugalcdep.2013.04.032
Pintzka CW, Hansen TI, Evensmoen HR, Haberg AK (2015) Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: a HUNT MRI study. Front Neurosci 9:238. doi:10.3389/fnins.2015.00238
Reise SP, Moore TM, Sabb FW, Brown AK, London ED (2013) The Barratt impulsiveness scale-11: reassessment of its structure in a community sample. Psychol Assess 25:631–642. doi:10.1037/a0032161
Rusyniak DE (2013) Neurologic manifestations of chronic methamphetamine abuse. Psychiatric Clin North Am 36:261–275. doi:10.1016/j.psc.2013.02.005
SAMHSA (2014) Treatment Episode Data Set—Admissions (TEDS-A), 2012. Inter-university Consortium for Political and Social Research, Ann Arbor
Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF (2010) Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage 50:1392–1401. doi:10.1016/j.neuroimage.2010.01.056
Sekine Y et al (2008) Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci 28:5756–5761. doi:10.1523/jneurosci.1179-08.2008
Semple SJ, Zians J, Grant I, Patterson TL (2005) Impulsivity and methamphetamine use. J Subst Abuse Treat 29:85–93. doi:10.1016/j.jsat.2005.05.001
Simes RJ (1986) An improved Bonferroni procedure for multiple tests of significance. Biometrika 73:751–754
Stoltenberg SF, Batien BD, Birgenheir DG (2008) Does gender moderate associations among impulsivity and health-risk behaviors? Addict Behav 33:252–265. doi:10.1016/j.addbeh.2007.09.004
Tabibnia G et al (2011) Different forms of self-control share a neurocognitive substrate. J Neurosci 31:4805–4810. doi:10.1523/JNEUROSCI.2859-10.2011
Takahashi T et al (2008) Depressive patients are more impulsive and inconsistent in intertemporal choice behavior for monetary gain and loss than healthy subjects–an analysis based on Tsallis’ statistics. Neuro Endocrinol Lett 29:351–358
Tanabe J et al (2013) Insula and orbitofrontal cortical morphology in substance dependence is modulated by sex. AJNR Am J Neuroradiol 34:1150–1156. doi:10.3174/ajnr.A3347
Thomas DM, Francescutti-Verbeem DM, Liu X, Kuhn DM (2004) Identification of differentially regulated transcripts in mouse striatum following methamphetamine treatment–an oligonucleotide microarray approach. J Neurochem 88:380–393
Thompson PM et al (2004) Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028–6036. doi:10.1523/jneurosci.0713-04.2004
Volkow ND et al (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158:2015–2021
Westman E, Aguilar C, Muehlboeck JS, Simmons A (2013) Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr 26:9–23. doi:10.1007/s10548-012-0246-x
Xue G, Lu Z, Levin IP, Bechara A (2010) The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage 50:709–716. doi:10.1016/j.neuroimage.2009.12.097
Zhang X, Salmeron BJ, Ross TJ, Geng X, Yang Y, Stein EA (2011) Factors underlying prefrontal and insula structural alterations in smokers. NeuroImage 54:42–48. doi:10.1016/j.neuroimage.2010.08.008
Acknowledgments
We thank all of our research participants and the clinical and technical staff of the Neuroscience and MR Program at UH, and the Queens’ Medical Center in Honolulu, Hawaii. We are supported by the following NIH Grants, which made this research possible: R24DA-27318, K24DA-16170, U54NS56883, G12-MD007601.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kogachi, S., Chang, L., Alicata, D. et al. Sex differences in impulsivity and brain morphometry in methamphetamine users. Brain Struct Funct 222, 215–227 (2017). https://doi.org/10.1007/s00429-016-1212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-016-1212-2