Abstract
The cerebellum is present in all extant gnathostomes or jawed vertebrates, of which cartilaginous fishes represent the most ancient radiation. Since the isthmic organizer induces the formation of the cerebellum, comparative genoarchitectonic analysis on the meso-isthmo-cerebellar region of cartilaginous fishes with respect to that of jawless vertebrates could reveal why the isthmic organizer acquires the ability to induce the formation of the cerebellum in gnathostomes. In the present work we analyzed the expression pattern of a variety of genes related to the cerebellar formation and patterning (ScOtx2, ScGbx2, ScFgf8, ScLmx1b, ScIrx1, ScIrx3, ScEn2, ScPax6 and ScLhx9) by in situ hybridization, and the distribution of Pax6 protein in the developing hindbrain of the shark Scyliorhinus canicula. The genoarchitectonic code in this species revealed high degree of conservation with respect to that of other gnathostomes. This resemblance may reveal the features of the ancestral condition of the gene network operating for specification of the rostral hindbrain patterning. Accordingly, the main subdivisions of the rostral hindbrain of S. canicula could be recognized. Our results support the existence of a rhombomere 0, identified as the ScFgf8/ScGbx2/ScEn2-positive and mainly negative ScIrx3 domain just caudal to the midbrain ScIrx1/ScOtx2/ScLmx1b-positive domain. The differential ScEn2 and Pax6 expression in the rhombomere 1 revealed anterior and posterior subdivisions. Interestingly, dissimilarities between S. canicula and lampreys (jawless vertebrates) were noted in the expression of Irx, Lhx and Pax genes, which could be part of significant gene network changes through evolution that caused the emergence of the cerebellum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vertebrates, two prominent external constrictions initially divide the rostral part of the neural tube into three main vesicles: hindbrain (or rhombencephalon), midbrain (or mesencephalon), and forebrain (or prosencephalon). The isthmic organizer (IsO), located at midbrain–hindbrain boundary or MHB, is a secondary organizer that controls the formation of optic tectum rostrally and the cerebellum caudally (Marín and Puelles 1994; Martínez 2001; Aroca and Puelles 2005; Nakamura et al. 2008; Martínez et al. 2013). A complex network of genes is involved in this process. Firstly, the transcription factors Otx2 and Gbx2 determine the correct positioning of the IsO at MHB (Hidalgo-Sánchez et al. 1999, 2005a, b; Simeone 2000) and Lmx1b is responsible for the initiation and maintenance of Fgf8 expression, which in turn is the main signaling molecule of the IsO (O’Hara et al. 2005; Guo et al. 2007). The Iroquois and Engrailed genes are also related to the isthmic territory and are involved in the early patterning of rostral hindbrain (for review, see Gómez-Skarmeta and Modolell 2002; Hidalgo-Sánchez et al. 2005a). These genes have intricate mutual relationships, activating and/or inhibiting each other (for review, see Liu and Joyner 2001), which appear roughly conserved throughout evolution, as revealed in studies in Drosophila (Urbach 2007).
Genetic programs homologous to that found in vertebrate signaling centers as the IsO have been reported in hemichordates, although such programs have been considered components of an ancient genetic regulatory scaffold for deuterostome body patterning, which was retained to pattern divergent structures in hemichordates and vertebrates (Pani et al. 2012; Robertshaw and Kiecker 2012). On the other hand, cephalochordates appear to have a MHB-like, but genes with organizing activity are not expressed in place at this boundary (Holland and Short 2008; Holland 2013). Conversely, urochordates showed the genetic machinery for specification of an IsO, but they have secondarily lost components of those gene programs (Ikuta and Saiga 2007; Holland et al. 2013). Thus, the emergence of an IsO with competence to induce the formation of cerebellum must have occurred during the agnathan–gnathostome transition. The cerebellum appears to be an evolutionary innovation of the common ancestor of gnathostomes or jawed vertebrates, as agnathans or jawless vertebrates do not have cerebellum (for review, see Northcutt 2002; Villar-Cerviño et al. 2010; Wullimann et al. 2011): they lack the cell types that define the cerebellum, i.e., they lack Purkinje cells (Lannoo and Hawkes 1997) and granule cells (for review, see Kuratani et al. 2002), and cerebellar connections have not been demonstrated. Owing to the fact that cartilaginous fishes represent the most ancient radiation of extant gnathostomes, they are closer to the ancestral condition of jawed vertebrates and the study of this group may shed light on the emergence of the cerebellum at the agnathan–gnathostome transition. However, in cartilaginous fishes information about the isthmic territory mainly comes from developmental studies in Scyliorhinus focused on non-nervous structures in which the expression of isthmus-related genes as Fgf8, En-1 and En-2 is circumstantially shown (Tanaka et al. 2002; Adachi et al. 2012; Compagnucci et al. 2013). Our current knowledge about the isthmic territory in gnathostomes mainly comes from studies in mammals (for review, see Joyner et al. 2000), birds (Hidalgo-Sánchez et al. 2005a), amphibians (Glavic et al. 2002) and bony fishes (Jászai et al. 2003).
The term genoarchitecture refers to the descriptions of neural structures in terms of discrete gene expression patterns (Puelles and Ferrán 2012). Because understanding the phylogenetic and ontogenetic aspects of the neural genoarchitecture of the rostral hindbrain and, in particular, of the IsO, is fundamental to advance our knowledge on the cerebellum emergence, we have analyzed the genoarchitecture of the rostral hindbrain of the shark Scyliorhinus canicula or lesser spotted dogfish, considered a model species in Evo-Devo studies (see Coolen et al. 2009). For it, we studied the expression pattern of several genes as ScOtx2, ScGbx2, ScFgf8, ScLmx1b, ScIrx1, ScIrx3, ScEn2; ScPax6 and ScLhx9, as well as the distribution of Pax6 protein at pharyngula stages. We additionally monitored the meso-isthmo-cerebellar region during later embryonic development. To analyze the degree of evolutionary conservation of the gene expression patterns found in the meso-isthmo-cerebellar area of this basal jawed vertebrate, a comparison of gnathostomes (including our new data) and agnathans was made. Similarities among gnathostomes will allow the establishment of subdivisions and early patterning of the rostral hindbrain in this shark. On the other hand, dissimilarities with respect to agnathans and non-vertebrate chordates could correspond with the causes of the cerebellar evolutionary innovation in the common ancestor of all gnathostomes.
Materials and methods
Experimental animals and tissue preparation
Embryos of the lesser spotted dogfish (Scyliorhinus canicula) were supplied by the Marine Biological Model Supply Service of the CNRS UPMC Roscoff Biological Station (France) and the Estación de Bioloxía Mariña da Graña (Galicia, Spain). Additional embryos and juveniles were kindly provided by the Aquaria of Gijón, O Grove and A Coruña (Spain). A total of 37 embryos, ranging from pharyngula stages (stages 19–27) to stage 31, were analyzed. Embryos were staged on the basis of their external features according to Ballard et al. (1993). Adequate measures were taken to minimize animal pain or discomfort. All procedures conformed to the guidelines established by the European Communities Council Directive of 22 September 2010 (2010/63/UE) and by the Spanish Royal Decree 53/2013 for animal experimentation, and were approved by the Ethics Committee of the University of Santiago de Compostela.
Specimens were anaesthetized with 0.5 % tricaine methane sulphonate (MS-222; Sigma) in seawater. Embryos were fixed by immersion in 4 % paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4) containing 1.75 % urea (elasmobranch PB). Then, the fixative was removed with PB saline. Some embryos were cryoprotected with 30 % sucrose in PB, embedded in NEG 50™ (Thermo Scientific, Kalamazoo, MI, USA), frozen with liquid nitrogen-cooled isopentane and cut on a cryostat. Parallel series of transverse and sagittal sections (18–20 µm thick) were mounted on Superfrost Plus slides (Menzel-Glässer®, Madison, WI, USA).
In situ hybridization on whole embryos and on sections
We applied in situ hybridization with cDNA probes for RNA of ScFgf8 (Compagnucci et al. 2013), ScPax6 (Rodríguez-Moldes et al. 2011); ScEn2, ScGbx2, ScIrx1, ScIrx3, ScLmx1b, ScLhx9 and ScOtx2 (Germot et al. 2001; Plouhinec et al. 2005) genes. These probes were selected from a collection of S. canicula embryonic cDNA library (mixed stages, S9–22), submitted to high-throughput EST sequencing (coord. Dr. Sylvie Mazan at the Station Biologique de Roscoff, France). The cDNA fragments were cloned in pSPORT vectors. Sense and antisense digoxigenin-UTP-labeled and fluorescein-UTP-labeled probes were synthesized directly by in vitro transcription using as templates linearized recombinant plasmid DNA (ScOtx2 and ScPax6 probes) or cDNA fragments prepared by PCR amplification of the recombinant plasmids (ScFgf8, ScEn2, ScGbx2, ScIrx1, ScIrx3, ScLmx1b and ScLhx9 probes).
In situ hybridization in whole embryos and on cryostat sections was carried out following standard protocols (for details, see Coolen et al. 2007; Ferreiro-Galve et al. 2012a). Briefly, sections were permeabilized with proteinase K, hybridized with sense or antisense probes overnight at 65 °C (in sections) or 70 °C (whole mount) and incubated with the alkaline phosphatase-coupled anti-digoxigenin and anti-fluorescein antibody (1:2,000, Roche Applied Science, Manheim, Germany) overnight at 4 °C. The color reaction was performed in the presence of BM-Purple and FastRed tablets (Roche). Control sense probes did not produce any detectable signal.
Immunohistochemistry
Combination of in situ hybridization on sections with immunohistochemistry for the rabbit polyclonal anti-Pax6 (Covance) and single anti-Pax6 labeling was also carried out at stages 24 and 25 following standard protocols (for details see Rodríguez-Moldes et al. 2011). For details about the specificity of Pax6 antibody in Scyliorhinus canicula, tested by pre-adsorption with the respective blocking peptide, see Ferreiro-Galve et al. (2012b).
Imaging
In toto hybridized embryos were analyzed in a Leica MZ16F stereo microscope fitted with a Leica DFC490 camera. Photomicrographs were taken with an Olympus DP70 color digital camera fitted to a Provis photomicroscope equipped for fluorescence with appropriate filter combinations. For presentation, some color photomicrographs were converted to gray scale, and brightness and contrast adjusted using Adobe Photoshop 7.0. Plate photomontage, schemes and lettering were made with Corel Draw X6 and Adobe Photoshop 7.0.
Results
We have studied the genoarchitectonic patterns at the meso-isthmo-cerebellar region during the early regionalization of the hindbrain in the lesser spotted dogfish. We have mainly focused the study on embryos at 19/20 and 24/25 stages, because they correspond to the period just before and after that the protuberance of the cerebellar primordium become anatomically evident, respectively. Of note, according to those features described by Ballard et al. (1993), we consider stages 19–23 (when pharyngeal pouches and branchial arches are forming and pharyngeal clefts start to open) and stages 24–27 (when all pharyngeal clefts become opened and opening and shaping of the mouth takes place) as early and late pharyngula stages, respectively. Equivalences between these developmental stages and those of other vertebrates are based on similarities in the reported gene expression patterns and external morphological features. Here we present the expression pattern of the ScOtx2, ScGbx2, ScFgf8, ScLmx1b, ScIrx1, ScIrx3, ScEn2, ScPax6 and ScLhx9 genes and the distribution of Pax6 protein in the caudal midbrain and rostral hindbrain at pharyngula stages.
The results obtained from in situ hybridization experiments did not yield significant differences at pharyngula stages (stages 19/20, 24/25 and 27), and even at further developmental stages (stages 29–31) as regards the expression pattern in the rostral hindbrain of most genes studied in the present work (Figs. 1, 2, 3).
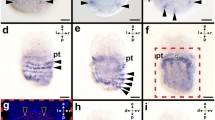
Major transcription and signal factor gene expression characterizing embryonic midbrain–hindbrain region in whole mount brains of Scyliorhinus canicula at early (stages 19–20) and late (stages 24–25) pharyngula stages. Panoramic and details of lateral views showing in situ hybridization reactions for: ScOtx2 (a, b), ScGbx2 (c, d), ScFgf8 (e, f), ScLmx1b (g, h), ScIrx1 (i, j), ScIrx3 (k, l), ScEn2 (m, n), ScPax6 (o) and ScLhx9 (p) gene expression in the meso-isthmo-cerebellar region. Black arrows in a–l, n indicate the midbrain–hindbrain boundary. Outlined arrows in k and l indicate the mesencephalic tegmentum. White arrow in l points the rostral extension of the Irx3 positive domain in the prosencephalon. Asterisks in n, p indicate the cerebellar primordium. br1–3 branchial archs 1–3, Mes mesencephalon, ot otic vesicle, Pros prosencephalon, r rhombomere, Rh rhombencephalon
Major transcription and signal factor gene expression characterizing embryonic midbrain–hindbrain region in whole mount and sagittal brain sections of Scyliorhinus canicula at the end of the pharyngula period (stage 27) and later on development (stages 29–31). Panoramic of lateral views at stage 27 showing the expression pattern of ScOtx2 (a), ScGbx2 (b), ScEn2 (c), ScIrx1 (d) and ScIrx3 (e). Panoramic and details of parasagittal sections at the level indicated in the schemes, at stages 29 (f), 30 (g) and 31 (h, i) showing the expression pattern of ScOtx2 and ScFgf8. Black arrows indicate the midbrain–hindbrain boundary. Cbp cerebellar plate, Hyp hypothalamus, IVv fourth ventricle, Mes mesencephalon, OT optic tectum, Pros prosencephalon, Rh rhombencephalon. Scale bars 200 µm (f–h); 500 µm (i)
Major transcription and signal factor gene expression characterizing embryonic midbrain–hindbrain region in sagittal brain sections of Scyliorhinus canicula at late pharyngula stages. Midsagittal (a–d, j, l) and parasagittal (e–i, k, m–p) sections of S. canicula embryos at 24 (g–n) and 25 (a–f, o, p) stages hybridized for the indicated gene markers (upper left). Black and white arrows indicate the midbrain–hindbrain boundary. a–f Details of ScGbx2, ScOtx2 and ScFgf8 positive domains. Asterisk (in e, f) indicates a negative gap for the expression of ScFgf8 and ScOtx2. g–m Details (g–i, k, m) and panoramic views (j, l) of double (g–l) or single (m) labeling of ScGbx2-ScIrx1 (g), ScGbx2 (h), ScFgf8-ScLmx1b (i, j), ScFgf8-ScIrx3 (k, l), and ScEn2 (m) genes. Insets in j and l show overlapping domains of expression. Dashed lines in j and l indicate the rostral and caudal edges of the Fgf8 expression domain. Arrowhead in k, l indicates the interface abutting ScFgf8 and ScIrx3 positive domains. Outlined arrows in l, m indicate the interface approximately abutting ScIrx3 and ScEn2 positive domains. Single labeling of the Pax6 protein (n) and double labeling of the expression of the ScEn2 gene and Pax6 protein showing colors separately (o, p). Double labeled sections were photographed after the first color development (red) and also after the second one (blue). Some double labeled sections (a′, b′, c–e) appear single labeled because of the accidental removal of red color at the end of the experiment. Note also the thin expression domain of ScFgf8 at midsagittal levels of both alar (c′ and inset in l) and basal (d′ and inset in j) plates of the isthmic domain. Yellow dashed lines in m and o tentatively indicates the caudal edge of the Fgf8 positive domain. Red dashed lines in n–p indicate the rostral edge of two areas discernible by the intensity of Pax6 labeling (showing intense labeling within rhombomere r1b and weak labeling within rhombomere r1a). Cbp cerebellar plate, Isfo isthmic fovea, Mes mesencephalon, mrc meso-rhombencephalic constriction, OT optic tectum, Pros prosencephalon, Rh rhombencephalon, RL rhombic lip. Scale bars 200 µm (a–i, k, m–p); 500 µm (j, l)
Expression pattern of isthmus and cerebellum-related genes in whole embryos
At stages 20 and 24, ScOtx2 and ScGbx2 positive domains appeared to be complementary. Strong Otx2 expression was found in the forebrain and midbrain (Fig. 1a, b), while Gbx2 was expressed in the hindbrain (Fig. 1c, d). The interface between both domains delimited the midbrain–hindbrain boundary or MHB (black arrows in Fig. 1). Caudal to this line, a conspicuous band of ScFgf8 expression was observed at stages 20 and 24 (Fig. 1e, f), similarly to that reported by Compagnucci et al. (2013). In an early pharyngula, ScLmx1b gene was also expressed in a transverse band of cells in the caudal midbrain, though a longitudinal band at dorsal-most levels was also extended towards rostral (up to the forebrain) and caudal (along the hindbrain) regions, where it partially overlapped with the ScFgf8 positive domain (Fig. 1g). Later, at stage 24, both the transverse and longitudinal ScLmx1b positive domains appeared thinner, though weak Lmx1b expression continued to be rostro-caudally extended and still partially overlapped with the ScFgf8 band in the most rostral hindbrain (compare Fig. 1f and h; see details in Fig. 2).
The gene ScIrx1 was expressed rostrally to the MHB. At stages 20 and 24, the caudal limit of the positive domain appeared to coincide with that of ScOtx2, while it extended rostrally up to the caudal forebrain (Fig. 1i, j). The expression pattern of ScIrx3, showed a conspicuous negative gap at midbrain–hindbrain domain and two positive domains, one caudal along the hindbrain, and other rostral, which extended up to the caudal forebrain at stage 20 (Fig. 1k) and more rostrally at stage 24 (white arrow in Fig. 1l). Furthermore, at stage 24 the expression in the midbrain tegmentum decreased and consequently the negative gap appeared enlarged at this level (outlined arrows in Fig. 1k, l).
The gene ScEn2 was highly expressed in the midbrain–hindbrain domain and extended in a decreasing gradient both rostrally and caudally (Fig. 1m, n). The caudal edge of the En2 positive domain was extended into the cerebellar primordium (probably corresponding to the upper rhombic lip, asterisk in Fig. 1n), which is located rostrally to the lateral recess of the fourth ventricle. The rostral edge approximately coincided with the rostral limit of the mesencephalic tegmentum (Fig. 1n).
In the rostral hindbrain, the ScPax6 gene was expressed up to the rhombomere (r) 1 at early (stage 19; Fig. 1o), and late (stage 25; Fig. 1h in Rodríguez-Moldes et al. 2011) pharyngula stages. Worth mentioning is that the r1–r2 boundary was identified on the basis of the rostral edge of the ScHoxA2 expression domain, as illustrated by Rodríguez-Moldes et al. (2011). Regarding to the ScLhx9 gene, a faint expression was observed in the cerebellar primordium in late pharyngulas (asterisk in Fig. 1p).
The meso-isthmo-cerebellar area later in development
We also aimed to advance knowledge into the conservative degree of neural genoarchitecture and to identify the rostral edge of cerebellar region throughout development. Therefore, we also studied the meso-isthmo-cerebellar region during later development. At the end of the pharyngula period, at stage 27, the expression pattern of the isthmus-related genes still appeared the same as observed from earlier pharyngula stages. The caudal and rostral edges of ScOtx2 and ScGbx2 positive domains, respectively, abutted at the MHB (Fig. 2a, b). The domain of Fgf8 expression was also observed in a band caudally to MHB (Compagnucci et al. 2013). Furthermore, though the domain of expression of ScEn2 appeared to extend more rostrally, its caudal edge remained extended into the cerebellum (Fig. 2c). Likewise, the expression of ScIrx1 and ScIrx3 genes (Fig. 2d, e) also appeared very similar to that observed at earlier stages (compare Figs. 1i–l and 2d, e). In subsequent developmental stages 29–31, the ScOtx2 and ScFgf8 expression domains still appeared to abut at MHB (arrow in Fig. 2f–i). However, contrary to that observed at pharyngula stages, the Fgf8 expression became restricted to the alar area and was excluded from the basal plate (Fig. 2g; see details in Fig. 3).
Boundaries among the expression domains of isthmus-related genes
To better discern the degree of overlapping among the expression domains of these genes at the meso-isthmo-cerebellar area, we performed single and double labeling on sections. Comparison of the expression patterns of ScGbx2 and ScOtx2 revealed that they do not overlap but just abut at MHB both at alar (compare Figs. 3a and 3a′) and basal (compare Figs. 3b and 3b′) plates, although a few cells apparently expressing both ScOtx2 and ScGbx2 genes were observed in the neuroepithelium (not shown). Likewise, ScOtx2 domain abutted with ScFgf8 domain at MHB both at alar (compare Figs. 3c and 3c′) and basal (compare Figs. 3d and 3d′) plates, except for a few weakly labeled cells in the neuroepithelium which appeared to overlap (not shown) and a conspicuous negative gap at parasagittal level (asterisk in Fig. 3e, f; see also Fig. 4b). Interestingly, the sequential analysis of sagittal sections from medial to lateral levels revealed that at some levels the isthmic or meso-rhombencephalic constriction (mrc, in Fig. 3) appeared to be located rostrally to the ScFgf8 positive area (Fig. 3l).
Schematic drawings of rostral hindbrain genoarchitecture in S. canicula early embryos. a–d Schematic drawings of sagittal (a, c) and dorsal (b, d) views of S. canicula embryos at stages 24/25 showing the expression pattern of the ScOtx2, ScGbx2, ScFgf8, ScLmx1b, ScIrx1, ScIrx3 and ScEn2 genes and the Pax6 protein. e Diagram summarizing the distribution of domains of expression in S. canicula at midbrain, midbrain–hindbrain boundary, and rostral hindbrain. Bars indicate the extension of the expression domains. Thin line in Lmx1b and Irx3 bars indicate the rostro-caudal extension of the expression pattern in the dorsal-most zone. Anterior levels correspond to the right side. br1–3 branchial archs 1–3, IVv fourth ventricle, LRL lower rhombic lip, Mes mesencephalon, MHB midbrain–hindbrain boundary, ot otic vesicle, OT optic tectum, Pros prosencephalon, r0–4 rhombomeres 0–4, Rh rhombencephalon, URL upper rhombic lip
The ScGbx2 and ScIrx1 domains were also abutting at MHB (Fig. 3g, h). The ScFgf8 and ScLmx1b transverse domains abutted at parasagittal levels (Fig. 3i). The thin longitudinal domain of Lmx1b expression, which in parasagittal sections is located at dorsal-most levels along the hindbrain and up to the forebrain (Fig. 3i; see also Fig. 1g, h), appeared dorso-ventrally extended at midsagittal levels, clearly overlaying the whole ScFgf8 domain (Fig. 3j). Interestingly, the ScFgf8 domain was narrower at midsagittal (Fig. 3c′, d′, j, l) than at lateral (Fig. 3f, i, k) levels. The ScFgf8-ScIrx3 combination showed that the rostral limit of the ScIrx3 domain in the hindbrain roughly coincided with the caudal edge of ScFgf8 domain (arrowhead in Fig. 3k, l) except for the median-alar portion, where both genes overlapped (inset in Fig. 3l). On the other hand, the caudal limit of the ScIrx3 domain in the midbrain appeared adjacent to the anterior limit of ScEn2 expression (outlined arrows in Fig. 3l, m). The ScEn2 gene showed that the limit of expression extended beyond the MHB (compare Figs. 3k and 3m) both partially in the midbrain and hindbrain. Of note, the Pax6 labeling in the hindbrain showed two discernible areas (red dashed lines in Fig. 3n–p): the most intense labeling was occupying the caudal half of r1 (see also Fig. 1h in Rodríguez-Moldes et al. 2011), while less intense labeling was observed within the rostral half of r1 (very weak at stage 24 and more patent at stage 25, compare Figs. 3n and 3p). The caudal edge of ScEn2 appeared approximately abutting with the rostral edge of Pax6 expression, as opposite gradients of expression (Fig. 3m–p). Ventrally at parasagittal levels, the ScEn2-Pax6 boundary appeared to coincide with the caudal edge of the ScFgf8 positive area (illustrated by the yellow dashed line in Fig. 3m, o; see also Rodríguez-Moldes et al. 2011), but not at the dorsal hindbrain area (RL in Fig. 3m–p).
Discussion
General considerations on the regionalization of the meso-isthmo-cerebellar area
The MHB and the most caudal midbrain and rostral hindbrain areas (or meso-isthmo-cerebellar area) express genes involved in the IsO activity, according to Hidalgo-Sánchez et al. (2005a, b), O’Hara et al. (2005) and Guo et al. (2007). Nevertheless, Fgf8 is considered as the main signaling molecule in the IsO (for review, see Nakamura et al. 2008). The position of the Fgf8-positive domain in the most rostral area of the S. canicula hindbrain has allowed us the identification (at least during part of the embryonic period) of the rhombomere 0 (r0), a region that has been defined as corresponding to the isthmus or isthmic territory (Martínez et al. 2013). This region has been considered as a pseudorhombomere or cryptorhombomere because it does not exhibit all the features of a true rhombomere (for review, see Marín et al. 2008; Watson et al. 2012; Martínez et al. 2013). According to our results, the secondary organizer in S. canicula might correspond to both the caudal midbrain region expressing ScLmx1b and the rostral hindbrain region expressing ScFgf8. However, taking into account the main organizing activity of Fgf8 and that the expression domain of ScLmx1b is rather dynamic throughout development, we consider for convenience the isthmic area in S. canicula as that expressing ScFgf8.
Evolutionary conservation of rostral hindbrain neural genoarchitecture among gnathostomes
To know the degree of evolutionary conservation in the early cerebellar development, we carried out a comparison between the neural genoarchitecture observed in S. canicula and that described in other gnathostomes. The striking morphological resemblance between early embryos of the lesser spotted dogfish (stages 19–25), and those of chick (HH14–HH19) and mouse (8.5 and 9.5/10.5), greatly facilitates the comparison at these stages. Therefore, pharyngula stages would correspond to the phylotypic stage, when the embryos of all vertebrates are more similar one each other and show a basic common bauplan (reviewed in: Slack et al. 1993; Kimmel et al. 1995; Kuratani and Horigome 2000; Kuratani et al. 2001; Mueller et al. 2006). Comparison to other anamniota, as bony fishes and amphibians, was also carried out. In general, we observed that the expression pattern of the isthmus-related genes in the lesser spotted dogfish is quite similar to that reported in mouse (see Allen Developing Mouse Brain Atlas [Internet] 2009) and other gnathostomes (see below). Accordingly, we have accurately identified the MHB and the main subdivisions of the rostral-most hindbrain by identifying the r0 as the ScFgf8/ScGbx2/ScEn2-positive and mainly negative ScIrx3 domain, just caudal to the midbrain ScIrx1/ScOtx2/ScLmx1b-positive domain (see Fig. 4).
Regarding to the MHB, it is defined as the interface between the Otx2-Gbx2 expression domains (Simeone 2000). In the lesser spotted dogfish, we observed that the caudal edge of ScOtx2 positive domain abutted with the rostral edge of ScGbx2 positive domain (see Fig. 4, and also see Fig. 3 in Mazan et al. 2000 and Fig. 2 in Plouhinec et al. 2005), as has been described in other gnathostomes [Xenopus (Glavic et al. 2002), zebrafish (Jászai et al. 2003; Kikuta et al. 2003; Rhinn et al. 2003), chick (Hidalgo-Sánchez et al. 2005a) and mouse (for review, see Joyner et al. 2000)]. Another evidence for the identification of the MHB was the gene Irx1, which is also required in the formation of the MHB (for review, see Aroca and Puelles 2005). In fact, the caudal edge of ScIrx1 coincided with that of ScOtx2. Similarly, in most jawed vertebrates this gene is not expressed at isthmic area (Cohen et al. 2000; Cheng et al. 2001, 2007; present results). However, the Irx1 positive domain of expression described in the hindbrain of mouse and zebrafish (Cohen et al. 2000; Cheng et al. 2001, 2007) was absent in the lesser spotted dogfish. This dissimilarity might be related to secondarily derived features.
In the lesser spotted dogfish, during early development, the isthmic constriction appeared to be located more rostrally than the anterior edge of the ScFgf8 expression domain. However, later in development, the isthmic constriction coincided with the MHB, which would imply that, in this species, the isthmic constriction changes its position caudalwards as development proceeds, and not rostralwards as in amniotes (Puelles et al. 1995; for review, see also Martínez et al. 2013).
The r0 is recognized by the Fgf8 positive band in the rostral hindbrain abutting with the MHB at the caudal edge of Otx2 (as described Aroca and Puelles 2005), which is clearly identified in the lesser spotted dogfish at pharyngula stages and even later on development (Compagnucci et al. 2013; present results) and matches well with what was described in all groups of gnathostomes (Glavic et al. 2002; Inoue et al. 2008; Hidalgo-Sánchez et al. 2005a; Joyner et al. 2000). Additionally, the apparent colocalization of a few ScFgf8-ScOtx2 expressing cells we observed in the lesser spotted dogfish (not shown) was also previously described in chick (Hidalgo-Sánchez et al. 2005a). Furthermore, the negative gap between ScFgf8 and ScOtx2 (asterisk in Fig. 2e, f) could either correspond to that observed in chick, where it has been related with the morphogenesis of the isthmic constriction (Adams et al. 2000; see Fig. 2 in Sotelo 2004), or could be equivalent to the transient area for free cell intermixing between midbrain and hindbrain described in mouse (Zervas et al. 2004). The interface abutting ScIrx3 and the caudal edge of the ScFgf8 domain observed in S. canicula (present results), also supports the r0 existence. A negative gap for Irx3 expression in the isthmic area was also found in other gnathostomes (Fig. 2 in Kobayashi et al. 2002; see also; Bosse et al. 1997; Tan et al. 1999; Cohen et al. 2000). Contrary, in Xenopus, the ortholog Xiro3 was expressed in the isthmic area at early stages, but its expression becomes reduced in this area later in development (Bellefroid et al. 1998; Rodríguez-Seguel et al. 2009).
The maintenance of Fgf8 expression in S. canicula throughout development could be crucial, as in amniotes, for the specification of different structures at the meso-isthmo-cerebellar region at different developmental stages (Sato and Joyner 2009). The apparent decrease of the extension of the ScFgf8 domain later in development (after pharyngula stages), which became excluded from the basal portion and reduced to a thin ring close to the MHB in the alar plate, also occurs in amniota species (Aroca and Puelles 2005). According to Vaage subdivisions of the rostral hindbrain (isthmus, plus r1 and r2; Vaage 1969, 1973) and its current interpretation in chick embryos (Aroca and Puelles 2005), the Fgf8 positive domain might also correspond in S. canicula to certain extension of the pro-rhombomere A1, but larger at pharyngula stages than at late developmental stages (when it became reduced to its rostral-most part). Moreover, the ScFgf8 expression domain would be a reliable marker of r0 only early on development, as described in chick (Aroca and Puelles 2005). Nevertheless, the topographic boundary between the ScFgf8 and ScOtx2 expression domains allows the truthful identification of the MHB also at late developmental stages, and consequently, the accurate location of diverse structures in the correct brain subdivisions according to the segmental model of the brain (for review, see Nieuwenhuys 2011). This may be useful for further evolutionary studies on the cerebellum and/or hindbrain in this basal gnathostome.
Other remarkable isthmus-related gene is the Lmx1b, which is important in the maintenance of the secondary organizer. It is involved in the regulation of Wnt1 and Fgf8 expression and is coexpressed with the Wnt1 positive domain in the caudal-most midbrain (see Aroca and Puelles 2005; Guo et al. 2007), and therefore, it abuts with the Fgf8 domain. Furthermore, it is involved in the formation of roof plate structures (Chizhikov and Millen 2004) and in the process of morphogenesis of the isthmic constriction (Adams et al. 2000). This gene in S. canicula could play the same roles, because the expression pattern of ScLmbx1b also appears similar to that of its orthologs in other jawed vertebrates (Adams et al. 2000; Haldin et al. 2003; O’Hara et al. 2005; Cheng et al. 2007; Mishima et al. 2009; Liu et al. 2010). Moreover, in chick and mouse, the Lmx1b positive domain temporarily covers the MHB and isthmic area and progressively becomes approximately restricted to the caudal midbrain (Adams et al. 2000; Guo et al. 2007; Mishima et al. 2009). Similarly, a reduction in the extension of Lmx1b positive domain throughout development was also observed in S. canicula (present results).
These findings not only show the existence of the MHB and r0 in a basal lineage of gnathostomes, but also show the high degree of evolutionary conservation of topographic relationships among the expression patterns of a broad set of isthmus-related genes. Therefore, it supports the hypothesis that it would correspond to the gene basic and basal network necessary for the formation of the isthmic territory, and consequently the cerebellum.
Subdivision of the rhombomere 1 in S. canicula
Once the signaling activity from the IsO starts, the onset of the formation of the cerebellum, which is derivative from r1 plus part of r0, takes place (for review, see Martínez et al. 2013). A more detailed analysis of r1, which presents larger size and greater degree of complexity than other rhombomeres, has led to the identification of two sub-rhombomeres (r1a and r1b) in amniotes, which would be nearly consistent with that firstly described by Vaage (1969) in chick embryos (reviewed in Aroca and Puelles 2005; Moreno-Bravo et al. 2014). On the other hand, subdivisions of r1 identified in early embryos were associated with prospective different cerebellar domains at mature developmental stages (Sgaier et al. 2005).
In S. canicula we identified r1 as the area in between the caudal edge of the ScFgf8 expression domain (present results) and the rostral edge of the ScHoxA2 expression domain (see Rodríguez-Moldes et al. 2011). Searching for the existence of sub-rhombomeres in the r1 in this species, we have thoroughly analyzed the expression pattern of ScEn2 at pharyngula stages, as in chick and mouse the caudal edge of En2 expression domain seemed to coincide with the limit between r1a and r1b (Aroca and Puelles 2005; see also Fig. 13 in Alonso et al. 2013). The expression pattern of Engrailed genes at the meso-isthmo-cerebellar area appeared highly conserved among cartilaginous fishes and with respect to other gnathostomes (Liu et al. 1999; Tanaka et al. 2002; Lekven et al. 2003; Hidalgo-Sánchez et al. 2005a; Koenig et al. 2010; Adachi et al. 2012; present results). Moreover, the caudal limit of the ScEn2 positive domain allowed us to distinguish rostral region in the r1 (r1a) at dorsal-most levels, while differential expression of Pax6 allow us to distinguish it at ventral-most levels (see Fig. 4). In zebrafish, En2 also appears approximately abutting with Pax6 (Scholpp et al. 2003). Other evidence for r1 subdivision are the GAD-DCX double labeled cells previously described at the most rostral part of r1 (or r0–r1 boundary), which may correspond to migrating neuroblasts from upper rhombic lip or part of the r1 (Rodríguez-Moldes et al. 2011; Pose-Méndez et al. 2014), as they appear to abut with the caudal edge of ScFgf8 expression domain and to overlap with the ScEn2 positive domain in the rostral half of the r1 (present results).
The identification of these two sub-rhombomeres (r1a and r1b) in S. canicula may help us to determine in further studies whether this subdivision would be related to the formation of different regions within the cerebellum. In that case, it might be associated with different functional domains of the cerebellum also in basal gnathostomes.
Basis for understanding the emergence of the cerebellum by comparing with non-vertebrates and agnathans
Despite the portions of gene networks related to a IsO-like regulatory program have been described in hemichordates (Pani et al. 2012; Robertshaw and Kiecker 2012; Holland et al. 2013), a true IsO inducing the cerebellar formation appears to be present only in gnathostomes. Therefore, dissimilarities on the gene expression patterns between the lesser spotted dogfish with respect to non-vertebrates and agnathans could explain why this secondary organizer acquired the ability of inducing the formation of the cerebellum, for the first time in evolution, probably in the common ancestor of all jawed vertebrates (see Fig. 5).
Comparative aspects in relation to the midbrain–hindbrain boundary and isthmic organizer throughout evolution. Cladogram summarizing the main aspects of the potential evolutionary origin of isthmus-related genes and their genetic program in different groups of extant animals. Information about urbilateria and prochordates is based on data from: Hirth et al. (2003), Urbach (2007), Irimia et al. (2010), Steinmetz et al. (2011), Pani et al. (2012), Holland et al. (2013). Topographic similarities and dissimilarities in the expression pattern of different isthmus-related genes between agnathans (in lampreys) and gnathostomes (in the lesser spotted dogfish, see text for details) are also illustrated. Presence/absence of the cerebellum and jaws in the different animal groups were taken into account. Scheme of gene expression patterns in lampreys was elaborated based on Kuratani et al. (2002), Suda et al. (2009), Takio et al. (2007), Sugahara et al. (2011), Matsuura et al. (2008), Jiménez-Guri and Pujades (2011), Osório et al. (2005), Murakami et al. (2005). Data about millions of years in the divergence nodes of different taxa and time of evolutionary emergence of diverse groups (based on the molecular dating) were taken from: Blair and Hedges (2005), Lu et al. (2012). On the other hand, data about fossil dating in millions of years (in the box below the cladogram) were taken from: Janvier (2008), Friedman and Brazeau (2013), Zhu et al. (2013)
Differently from that observed in the lesser spotted dogfish and other vertebrates (see above), the edge of the Fgf8 positive band and the Otx and Gbx boundary are reversely located in hemichordates (Pani et al. 2012). Conversely, in cephalochordates (amphioxus) and tunicates (ascidians), the topographic boundaries of certain isthmus-related genes show higher resemblance to those of vertebrates (including S. canicula) than in the case of hemichordates (see Pani et al. 2012; Holland et al. 2013; present results). The amphioxus show an Otx2-Gbx2 interface as in S. canicula and other vertebrates, but other isthmus-related genes are not expressed at this boundary. On the other hand, urochordates show more of the machinery for an IsO-like in place than cephalochordates, but they have lost the Gbx2 gene. So, our results would support the hypothesis proposed by Pani et al. (2012) and Holland et al. (2013) that at least a partial IsO-like signaling center predates vertebrates despite that it is not entirely clear whether it occurred in the ancestor of deuterostomes (Pani et al. 2012) or at the base of the chordates lineage (Holland et al. 2013). A summary of comparative aspects in relation to the MHB and IsO throughout evolution is shown in Fig. 5.
In agnathans (see Fig. 5), the expression pattern of an array of genes related to the MHB appears consistent with that of other vertebrates (Kuratani et al. 2002) including the lesser spotted dogfish (present results). This is the case for the expression domains of Otx (Tomsa and Langeland 1999; Murakami et al. 2001; Murakami and Watanabe 2009; Suda et al. 2009), Gbx (Takio et al. 2007), Fgf8 (Rétaux and Kano 2010; Sugahara et al. 2011) and En (Matsuura et al. 2008; Hammond et al. 2009) genes in lampreys. Nevertheless, the IrxA gene in lampreys, ortholog to the Irx1/3 of gnathostomes, does not show a negative gap of expression at MHB and r0 (Fig. 2s–x in Jiménez-Guri and Pujades 2011), differently from that observed in the lesser spotted dogfish (present results). In fact, mice deficient for the expression of Irx2 (which is involved in the formation of the cerebellum, Matsumoto et al. 2004), did not present the negative gap of Irx3 expression at MHB (Lebel et al. 2003). Besides, ziro3 in zebrafish appears expressed in the MHB, but only after the formation of the cerebellum (Tan et al. 1999). Additionally, a close observation of the ortholog to Lhx9 gene in lampreys reveals that, differently from S. canicula and other gnathostomes (Wang et al. 2005; Sun et al. 2008; present results), it does not appear expressed in the r1 (see Figs. 2, 3, 6 in Osório et al. 2005). Of note, in mouse Lhx9 is a marker of deep cerebellar nuclei. However, in chick and Xenopus this gene is not expressed in the cerebellum, though some of Lhx9-expressing structures may be rhombic lip derivatives (Moreno et al. 2005; Liu et al. 2010; Green and Wingate 2014; Green et al. 2014). Therefore, the possibility that this gene was not so relevant for the evolutionary emergence of the cerebellum cannot be ruled out.
The findings obtained in the present work would support several potential causes to explain the emergence of the cerebellum, probably in the common ancestor of all gnathostomes. Firstly, the fact that in the lesser spotted dogfish, as in other gnathostomes, Pax6 is expressed in the rhombic lip and cerebellum (Rodríguez-Moldes et al. 2008, 2011; present results) together with the absence of Pax6 in the dorsal part of r1 in lampreys, supports previously proposed hypothesis that Pax6 was co-opted in this area (Kuratani et al. 2002; Murakami et al. 2001, 2005; Murakami and Watanabe 2009). A relationship between co-option of genes and innovation of structures was also described by Holland (2013). Furthermore, the possibility cannot be ruled out that the induction of the cerebellum may be directly or indirectly related to the appearance of new Iroquois or Lhx isoforms due to genetic duplications in gnathostomes (Kerner et al. 2009) and/or to the down-regulation of Irx3 at the MHB and r0. Genetic duplications and/or changes in developmental programs that result in the duplication of a structure are considered necessary for evolutionary innovations (Wagner 2008; Montgomery et al. 2012). However, some authors have proposed that the novelty in evolution is more influenced by changes in regulatory mechanisms than by genetic duplications (Carroll 2008). Alternatively, the novelty in the evolution of the cerebellum may depend on the threshold of expression of particular genes. Thus, some genes must reach a high threshold of expression and must be expressed long time enough to activate the pathways involved in the formation of the cerebellum, as in the case of Fgf8 (Sato and Nakamura 2004; Sato and Joyner 2009) or Gbx2 (Waters and Lewandoski 2006) genes. Therefore, though the expression pattern of some isthmus-related genes appears very similar in agnathans and gnathostomes, it could be possible that the level of expression of these genes was not high enough before the evolutionary innovation of the cerebellum. Certainly, the cause of the evolutionary emergence of the cerebellum could be a sum of all the factors cited above.
Conclusions
Similarities observed between the lesser spotted dogfish and other gnathostomes show the high degree of conservation of the expression pattern of isthmus-related genes. Furthermore, due to so divergent lineages, features in common between them might correspond to those already present in the common ancestor of all gnathostomes, which supports the hypothesis that the chondrichthyans bauplan might reveal the ancestral condition of the cerebellar formation. Additionally, this comparative analysis allowed the accurate recognition of the boundaries between midbrain–hindbrain, r0–r1 and r1a–r1b. On the other hand, the dissimilarities found with respect to other gnathostome species reveal some features that could be secondarily derived.
While non-vertebrates present particular combinations of various isthmus-related genes, only vertebrates present a whole set of isthmus-related genes with conserved expression patterns. Finally, though more neural genoarchitectonic studies in basal gnathostomes and agnathans would be necessary, small dissimilarities we observed between them might give a clue to clarify why the isthmic organizer acquired the ability to induce the formation of the cerebellum in the common ancestor of all jawed vertebrates.
References
Adachi N, Takechi M, Hirai T, Kuratani S (2012) Development of the head and trunk mesoderm in the dogfish, Scyliorhinus torazame: II. Comparison of gene expression between the head mesoderm and somites with reference to the origin of the vertebrate head. Evol Dev 14:257–276
Adams KA, Maida JM, Golden JA, Riddle RD (2000) The transcription factor Lmx1b maintains Wnt1 expression within the isthmic organizer. Development 127:1857–1867
Allen Developing Mouse Brain Atlas [Internet] (2009) Allen Institute for Brain Science, Seattle. http://developingmouse.brain-map.org
Alonso A, Merchán P, Sandoval JE, Sánchez-Arrones L, Garcia-Cazorla A, Artuch R, Ferrán JL, Martínez-de-la-Torre M, Puelles L (2013) Development of the serotonergic cells in murine raphe nuclei and their relations with rhombomeric domains. Brain Struct Funct 218:1229–1277
Aroca P, Puelles L (2005) Postulated boundaries and differential fate in the developing rostral hindbrain. Brain Res Rev 49:179–190
Ballard WW, Mellinger J, Lechenault H (1993) A series of normal stages for development of Scyliorhinus canicula, the lesser spotted dogfish (Chondrichthyes: Scyliorhinidae). J Exp Zool 267:318–336
Bellefroid EJ, Kobbe A, Gruss P, Pieler T, Gurdon JB, Papalopulu N (1998) Xiro3 encodes a Xenopus homolog of the Drosophila Iroquois genes and functions in neural specification. EMBO J 17:191–203
Blair JE, Hedges SB (2005) Molecular phylogeny and divergence times of deuterostome animals. Mol Biol Evol 22:2275–2284
Bosse A, Zülch A, Becker MB, Torres M, Gómez-Skarmeta JL, Modolell J, Gruss P (1997) Identification of the vertebrate Iroquois homeobox gene family with overlapping expression during early development of the nervous system. Mech Dev 69:169–181
Carroll SB (2008) Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell 134:25–36
Cheng CW, Hui C, Strähle U, Cheng SH (2001) Identification and expression of zebrafish Iroquois homeobox gene irx1. Dev Genes Evol 211:442–444
Cheng CW, Yan CH, Choy SW, Hui MN, Hui CC, Cheng SH (2007) Zebrafish homologue irx1a is required for the differentiation of serotonergic neurons. Dev Dyn 236:2661–2667
Chizhikov VV, Millen KJ (2004) Control of roof plate development and signaling by Lmx1b in the caudal vertebrate CNS. J Neurosci 24:5694–56703
Cohen DR, Cheng CW, Cheng SH, Hui CC (2000) Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech Dev 91:317–321
Compagnucci C, Debiais-Thibaud M, Coolen M, Fish J, Griffin JN, Bertocchini F, Minoux M, Rijli FM, Borday-Birraux V, Casane D, Mazan S, Depew MJ (2013) Pattern and polarity in the development and evolution of the gnathostome jaw: both conservation and heterotopy in the branchial arches of the shark, Scyliorhinus canicula. Dev Biol 377:428–448
Coolen M, Sauka-Spengler T, Nicolle D, Le-Mentec C, Lallemand Y, Da Silva C, Plouhinec JL, Robert B, Wincker P, Shi DL, Mazan S (2007) Evolution of axis specification mechanisms in jawed vertebrates: insights from a chondrichthyan. PLoS ONE 2:e374
Coolen M, Menuet A, Chassoux D, Compagnucci C, Henry S, Lévèque L, Da Silva C, Gavory F, Samain S, Wincker P, Thermes C, D’Aubenton-Carafa Y, Rodriguez-Moldes I, Naylor G, Depew M, Sourdaine P, Mazan S (2009) The dogfish Scyliorhinus canicula, a reference in jawed vertebrates. In: Behringer RR, Johnson AD, Krumlauf RE (eds) Emerging model organisms. A laboratory manual, vol 1. CSHL Press, Cold Spring Harbor, pp 431–446
Ferreiro-Galve S, Candal E, Rodríguez-Moldes I (2012a) Dynamic expression of Pax6 in the shark olfactory system: evidence for the presence of Pax6 cells along the olfactory nerve pathway. J Exp Zool B (Mol Dev Evol) 318:79–90
Ferreiro-Galve S, Rodríguez-Moldes I, Candal E (2012b) Pax6 expression during retinogenesis in sharks: comparison with markers of cell proliferation and neuronal differentiation. J Exp Zool (Mol Dev Evol) 318:91–108
Friedman M, Brazeau MD (2013) A jaw-dropping fossil fish. Nature 502:175–177
Germot A, Lecointre G, Plouhinec JL, Le Mentec C, Girardot F, Mazan S (2001) Structural evolution of Otx genes in craniates. Mol Biol Evol 18:1668–1678
Glavic A, Gómez-Skarmeta JL, Mayor R (2002) The homeoprotein Xiro1 is required for midbrain–hindbrain boundary formation. Development 129:1609–1621
Gómez-Skarmeta JL, Modolell J (2002) Iroquois genes: genomic organization and function in vertebrate neural development. Curr Opin Genet Dev 12:403–408
Green MJ, Wingate RJ (2014) Developmental origins of diversity in cerebellar output nuclei. Neural Dev 9:1
Green MJ, Myat AM, Emmenegger BA, Wechsler-Reya RJ, Wilson LJ, Wingate RJ (2014) Independently specified Atoh1 domains define novel developmental compartments in rhombomere 1. Development 141:389–398
Guo C, Qiu HY, Huang Y, Chen H, Yang RQ, Chen SD, Johnson RL, Chen ZF, Ding YQ (2007) Lmx1b is essential for Fgf8 and Wnt1 expression in the isthmic organizer during tectum and cerebellum development in mice. Development 134:317–325
Haldin CE, Nijjar S, Massé K, Barnett MW, Jones EA (2003) Isolation and growth factor inducibility of the Xenopus laevis Lmx1b gene. Int J Dev Biol 47:253–262
Hammond KL, Baxendale S, McCauley DW, Ingham PW, Whitfield TT (2009) Expression of patched, prdm1 and engrailed in the lamprey somite reveals conserved responses to Hedgehog signaling. Evol Dev 11:27–40
Hidalgo-Sánchez M, Millet S, Simeone A, Alvarado-Mallart RM (1999) Comparative analysis of Otx2, Gbx2, Pax2, Fgf8 and Wnt1 gene expressions during the formation of the chick midbrain/hindbrain domain. Mech Dev 81:175–178
Hidalgo-Sánchez M, Millet S, Bloch-Gallego E, Alvarado-Mallart RM (2005a) Specification of the meso-isthmo-cerebellar region: the Otx2/Gbx2 boundary. Brain Res Rev 49:134–149
Hidalgo-Sánchez M, Martínez de la Torre M, Alvarado-Mallart RM, Puelles L (2005b) A distinct preisthmic histogenetic domain is defined by overlap of Otx2 and Pax2 gene expression in the avian caudal midbrain. J Comp Neurol 483:17–29
Hirth F, Kammermeier L, Frei E, Walldorf U, Noll M, Reichert H (2003) An urbilaterian origin of the tripartite brain: developmental genetic insights from Drosophila. Development 130:2365–2373
Holland LZ (2013) Evolution of new characters after whole genome duplications: insights from amphioxus. Semin Cell Dev Biol 24:101–109
Holland LZ, Short S (2008) Gene duplication, co-option and recruitment during the origin of the vertebrate brain from the invertebrate chordate brain. Brain Behav Evol 72:91–105
Holland LZ, Carvalho JE, Escriva H, Laudet V, Schubert M, Shimeld SM, Yu JK (2013) Evolution of bilaterian central nervous systems: a single origin? EvoDevo 4:27
Ikuta T, Saiga H (2007) Dynamic change in the expression of developmental genes in the ascidian central nervous system: revisit to the tripartite model and the origin of the midbrain–hindbrain boundary region. Dev Biol 312:631–643
Inoue F, Parvin MS, Yamasu K (2008) Transcription of fgf8 is regulated by activating and repressive cis-elements at the midbrain–hindbrain boundary in zebrafish embryos. Dev Biol 316:471–486
Irimia M, Piñeiro C, Maeso I, Gómez-Skarmeta JL, Casares F, García-Fernández J (2010) Conserved developmental expression of Fezf in chordates and Drosophila and the origin of the Zona Limitans Intrathalamica (ZLI) brain organizar. EvoDevo 1:7
Janvier P (2008) The brain in the early fossil jawless vertebrates: evolutionary information from an empty nutshell. Brain Res Bull 75:314–318
Jászai J, Reifers F, Picker A, Langenberg T, Brand M (2003) Isthmus-to-midbrain transformation in the absence of midbrain–hindbrain organizer activity. Development 130:6611–6623
Jiménez-Guri E, Pujades C (2011) An ancient mechanism of hindbrain patterning has been conserved in vertebrate evolution. Evol Dev 13:38–46
Joyner AL, Liu A, Millet S (2000) Otx2, Gbx2 and Fgf8 interact to position and maintain a mid-hindbrain organizer. Curr Opin Cell Biol 12:736–741
Kerner P, Ikmi A, Coen D, Vervoort M (2009) Evolutionary history of the iroquois/Irx genes in metazoans. BMC Evol Biol 9:74
Kikuta H, Kanai M, Ito Y, Yamasu K (2003) gbx2 Homeobox gene is required for the maintenance of the isthmic region in the zebrafish embryonic brain. Dev Dyn 228:433–450
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Kobayashi D, Kobayashi M, Matsumoto K, Ogura T, Nakafuku M, Shimamura K (2002) Early subdivisions in the neural plate define distinct competence for inductive signals. Development 129:83–93
Koenig SF, Brentle S, Hamdi K, Fichtner D, Wedlich D, Gradl D (2010) En2, Pax2/5 and Tcf-4 transcription factors cooperate in patterning the Xenopus brain. Dev Biol 340:318–328
Kuratani S, Horigome N (2000) Developmental morphology of branchiomeric nerves in a catshark, Scylorhinus torazame, with special reference to rhombomeres, cephalic mesoderm, and distribution patterns of cephalic crest cells. Zool Sci 17:893–909
Kuratani S, Nobusada Y, Horigome N, Shigetani Y (2001) Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Philos Trans R Soc Lond B Biol Sci 356:1615–1632
Kuratani S, Kuraku S, Murakami Y (2002) Lamprey as an evo-devo model: lessons from comparative embryology and molecular phylogenetics. Genesis 34:175–183
Lannoo MJ, Hawkes R (1997) A search for primitive Purkinje cells: zebrin II expression in sea lampreys (Petromyzon marinus). Neurosci Lett 237:53–55
Lebel M, Agarwal P, Cheng CW, Kabir MG, Chan TY, Thanabalasingham V, Zhang X, Cohen DR, Husain M, Cheng SH, Bruneau BG, Hui CC (2003) The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain–hindbrain boundary in mice. Mol Cell Biol 23:8216–8225
Lekven AC, Buckles GR, Kostakis N, Moon RT (2003) Wnt1 and wnt10b function redundantly at the zebrafish midbrain–hindbrain boundary. Dev Biol 254:172–187
Liu A, Joyner AL (2001) EN and GBX2 play essential roles downstream of FGF8 in patterning the mouse mid/hindbrain region. Development 128:181–191
Liu A, Losos K, Joyner AL (1999) FGF8 can activate Gbx2 and transform regions of the rostral mouse brain into a hindbrain fate. Development 126:4827–4838
Liu ZR, Shi M, Hu ZL, Zheng MH, Du F, Zhao G, Ding YQ (2010) A refined map of early gene expression in the dorsal rhombomere 1 of mouse embryos. Brain Res Bull 82:74–82
Lu J, Zhu M, Long JA, Zhao W, Senden TJ, Jia L, Quiao T (2012) The earliest known stem-tetrapod from the Lower Devonian of China. Nat Commun 3:1160
Marín F, Puelles L (1994) Patterning of the embryonic avian midbrain after experimental inversions: a polarizing activity from the isthmus. Dev Biol 163:19–37
Marín F, Aroca P, Puelles L (2008) Hox gene colinear expression in the avian medulla oblongata is correlated with pseudorhombomeric domains. Dev Biol 323:230–247
Martínez S (2001) The isthmic organizer and brain regionalization. Int J Dev Biol 44:367–371
Martínez S, Andreu A, Mecklenburg N, Echevarría D (2013) Cellular and molecular basis of cerebellar development. Front Neuroanat 7:18
Matsumoto K, Nishihara S, Kamimura M, Shiraishi T, Otoguro T, Uehara M, Maeda Y, Ogura K, Lumsden A, Ogura T (2004) The prepattern transcription factor Irx2, a target of the FGF8/MAP kinasecascade, is involved in cerebellum formation. Nat Neurosci 7:605–612
Matsuura M, Nishihara H, Onimaru K, Kokubo N, Kuraku S, Kusakabe R, Okada N, Kuratani S, Tanaka M (2008) Identification of four Engrailed genes in the Japanese lamprey, Lethenteron japonicum. Dev Dyn 237:1581–1589
Mazan S, Jaillard D, Baratte B, Janvier P (2000) Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol Dev 2:186–193
Mishima Y, Lindgren AG, Chizhikov VV, Johnson RL, Millen KJ (2009) Overlapping function of Lmx1a and Lmx1b in anterior hindbrain roof plate formation and cerebellar growth. J Neurosci 29:11377–11384
Montgomery JC, Bodznick D, Yopak KE (2012) The cerebellum and cerebellum-like structures of cartilaginous fishes. Brain Behav Evol 80:152–165
Moreno N, Bachy I, Rétaux S, González A (2005) LIM-homeodomain genes as territory markers in the brainstem of adult and developing Xenopus laevis. J Comp Neurol 485:240–254
Moreno-Bravo JA, Perez-Balaguer A, Martinez-Lopez JE, Aroca P, Puelles L, Martinez S, Puelles E (2014) Role of Shh in the development of molecularly characterized tegmental nuclei in mouse rhombomere 1. Brain Struct Funct 219:777–792. doi:10.1007/s00429-013-0534-6
Mueller T, Vernier P, Wullimann MF (2006) A phylotypic stage in vertebrate brain development: gABA cell patterns in zebrafish compared with mouse. J Comp Neurol 494:620–634
Murakami Y, Watanabe A (2009) Development of the central and peripheral nervous systems in the lamprey. Dev Growth Differ 51:197–205
Murakami Y, Ogasawara M, Sugahara F, Hirano S, Satoh N, Kuratani S (2001) Identification and expression of the lamprey Pax6 gene: evolutionary origin of the segmented brain of vertebrates. Development 128:3521–3531
Murakami Y, Uchida K, Rijli FM, Kuratani S (2005) Evolution of the brain developmental plan: insights from agnathans. Dev Biol 280:249–259
Nakamura H, Sato T, Suzuki-Hirano A (2008) Isthmus organizer for mesencephalon and metencephalon. Dev Growth Differ 50(Suppl 1):S113–S118
Nieuwenhuys R (2011) The structural, functional and molecular organization of the brainstem. Front Neuroanat 5:33
Northcutt RG (2002) Understanding vertebrate brain evolution. Integr Comp Biol 42:743–756
O’Hara FP, Beck E, Barr LK, Wong LL, Kessler DS, Riddle RD (2005) Zebrafish Lmx1b.1 and Lmx1b.2 are required for maintenance of the isthmic organizer. Development 132:3163–3173
Osório J, Mazan S, Rétaux S (2005) Organisation of the lamprey (Lampetra fluviatilis) embryonic brain: insights from LIM-homeodomain, Pax and hedgehog genes. Dev Biol 288:100–112
Pani AM, Mullarkey EE, Aronowicz J, Assimacopoulos S, Grove EA, Lowe CJ (2012) Ancient deuterostome origins of vertebrate brain signaling centres. Nature 483:289–294
Plouhinec JL, Leconte L, Sauka-Spengler TS, Bovolenta P, Mazan S, Saule S (2005) Comparative analysis of gnathostome Otx gene expression patterns in the developing eye: implications for the functional evolution of the multigene family. Dev Biol 278:560–575
Pose-Méndez S, Candal E, Adrio F, Rodríguez-Moldes I (2014) Development of the cerebellar afferent system in the shark Scyliorhinus canicula: insights into the basal organization of precerebellar nuclei in gnathostomes. J Comp Neurol 522:131–168
Puelles L, Ferrán JL (2012) Concept of neural genoarchitecture and its genomic fundament. Front Neuroanat 6:47
Puelles L, Marín F, MartínezdelaTorre S, Martínez S (1995) The midbrain–hindbrain junction: a model system for brain regionalization through morphogenetic neuroepithelial interactions. In: Lonai P (ed) Towards analysis of vertebrate development. Harwood Publishers, Chur, pp 173–197
Rétaux S, Kano S (2010) Midline signaling and evolution of the forebrain in chordates: a focus on the lamprey Hedgehog case. Integr Comp Biol 50:98–109
Rhinn M, Lun K, Amores A, Yan YL, Postlethwait JH, Brand M (2003) Cloning, expression and relationship of zebrafish gbx1 and gbx2 genes to Fgf signaling. Mech Dev 120:919–936
Robertshaw E, Kiecker C (2012) Phylogenetic origins of brain organisers. Scientifica 2012:475017
Rodríguez-Moldes I, Ferreiro-Galve S, Carrera I, Sueiro C, Candal E, Mazan S, Anadón R (2008) Development of the cerebellar body in sharks: spatiotemporal relations of Pax6-expression, cell proliferation and differentiation. Neurosci Lett 432:105–110
Rodríguez-Moldes I, Carrera I, Pose-Méndez S, Quintana-Urzainqui I, Candal E, Anadón R, Mazan S, Ferreiro-Galve S (2011) Regionalization of the shark hindbrain: a survey of an ancestral organization. Front Neuroanat 5:16
Rodríguez-Seguel E, Alarcón P, Gómez-Skarmeta JL (2009) The Xenopus Irx genes are essential for neural patterning and define the border between prethalamus and thalamus through mutual antagonism with the anterior repressors Fezf and Arx. Dev Biol 329:258–268
Sato T, Joyner AL (2009) The duration of Fgf8 isthmic organizer expression is key to patterning different tectal-isthmo-cerebellum structures. Development 136:3617–3626
Sato T, Nakamura H (2004) The Fgf8 signal causes cerebellar differentiation by activating the Ras-ERK signaling pathway. Development 131:4275–4285
Scholpp S, Lohs C, Brand M (2003) Engrailed and Fgf8 act synergistically to maintain the boundary between diencephalon and mesencephalon. Development 130:4881–4893
Sgaier SK, Millet S, Villanueva MP, Berenshteyn F, Song C, Joyner AL (2005) Morphogenetic and cellular movements that shape the mouse cerebellum; insights from genetic fate mapping. Neuron 45:27–40
Simeone A (2000) Positioning the isthmic organizer where Otx2 and Gbx2 meet. Trends Genet 16:237–240
Slack JM, Holland PW, Graham CF (1993) The zootype and the phylotypic stage. Nature 361:490–492
Sotelo C (2004) Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol 72:295–339
Steinmetz PR, Kostyuchenko RP, Fischer A, Arendt D (2011) The segmental pattern of otx, gbx, and Hox genes in the annelid Platynereis dumerilii. Evol Dev 13:72–79
Suda Y, Kurokawa D, Takeuchi M, Kajikawa E, Kuratani S, Amemiya C, Aizawa S (2009) Evolution of Otx paralogue usages in early patterning of the vertebrate head. Dev Biol 325:282–295
Sugahara F, Aota S, Kuraku S, Murakami Y, Takio-Ogawa Y, Hirano S, Kuratani S (2011) Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development 138:1217–1226
Sun X, Saitsu H, Shiota K, Ishibashi M (2008) Expression dynamics of the LIM-homeobox genes, Lhx1 and Lhx9, in the diencephalon during chick development. Int J Dev Biol 52:33–41
Takio Y, Kuraku S, Murakami Y, Pasqualetti M, Rijli FM, Narita Y, Kuratani S, Kusakabe R (2007) Hox gene expression patterns in Lethenteron japonicum embryos-Insights into the evolution of vertebrate Hox code. Dev Biol 308:606–620
Tan JTY, Korzh V, Gong Z (1999) Expression of a zebrafish iroquois homeobox gene, Ziro3, in the midline axial structures and central nervous system. Mech Dev 87:165–168
Tanaka M, Münsterberg A, Anderson WG, Prescott AR, Hazon N, Tickle C (2002) Fin development in a cartilaginous fish and the origin of vertebrate limbs. Nature 416:527–531
Tomsa JM, Langeland JA (1999) Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw. Dev Biol 207:26–37
Urbach R (2007) A procephalic territory in Drosophila exhibiting similarities and dissimilarities compared to the vertebrate midbrain/hindbrain boundary region. Neural Dev 2:23
Vaage S (1969) The segmentation of the primitive neural tube in chick embryos (Gallus domesticus). A morphological histochemical and autoradiographic investigation. In: Brodal HOH et al (eds) Advances in anatomy, embryology and cell biology. Springer, Berlin, pp 5–21
Vaage S (1973) The histogenesis of the isthmic nuclei in chick embryos (Gallus domesticus). Z Anat Entwicklungsgesch 142:283–314
Villar-Cerviño V, Barreiro-Iglesias A, Rodicio MC, Anadón R (2010) D-serine is distributed in neurons in the brain of the sea lamprey. J Comp Neurol 518:1688–1710
Wagner A (2008) Gene duplications, robustness and evolutionary innovations. BioEssays 30:367–373
Wang VY, Rose MF, Zoghbi HY (2005) Math1 expression redefines the rhombic lip derivatives and reveals novel lineages within the brainstem and cerebellum. Neuron 48:31–43
Waters ST, Lewandoski M (2006) A threshold requirement for Gbx2 levels in hindbrain development. Development 133:1991–2000
Watson C, Paxinos G, Puelles L (2012) The mouse nervous system. Elsevier Academic Press, London
Wullimann MF, Mueller T, Distel M, Babaryka A, Grothe B, Köster RW (2011) The long adventurous journey of rhombic lip cells in jawed vertebrates: a comparative developmental analysis. Front Neuroanat 5:27
Zervas M, Millet S, Ahn S, Joyner A (2004) Cell behaviors and genetic lineages of the mesencephalon and rhombomere 1. Neuron 43:345–357
Zhu M, Yu X, Ahlberg PE, Choo B, Lu J, Qiao T, Qu Q, Zhao W, Jia L, Blom H, Zhu Y (2013) A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502:188–193
Acknowledgments
We thank Prof. Dr. R. Anadón for the valuable comments made during the preparation of this paper and his critical reading of the manuscript. We also thank Dr. S. Ferreiro-Galve for her helpful support and contribution in some experimental procedures. This work was supported by grants from the Spanish Dirección General de Investigación-FEDER (BFU2010-15816), the Xunta de Galicia (10PXIB200051PR, CN 2012/237), and European Community-Research Infrastructure Action under the FP7 “Capacities” Specific Programme (ASSEMBLE 227799).
Conflict of interest
The authors declare that they have no conflict of interest
Ethical standard
The manuscript does not contain clinical studies or patient data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pose-Méndez, S., Candal, E., Mazan, S. et al. Genoarchitecture of the rostral hindbrain of a shark: basis for understanding the emergence of the cerebellum at the agnathan–gnathostome transition. Brain Struct Funct 221, 1321–1335 (2016). https://doi.org/10.1007/s00429-014-0973-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-014-0973-8