Abstract
The exact relationship between solid papillary carcinoma (SPC) and invasive breast carcinoma of no special type (IBC-NST) with neuroendocrine differentiation and SPC and mucinous carcinoma (MC) of the breast remains unclear. To clarify the relationship, we conducted a comparative study of morphological and neuroendocrine features between ductal carcinoma in situ (DCIS, 72 cases) and SPC in situ (35 cases), and IBC-NST (103 cases) and invasive SPC (92 cases). We also conducted the study between MC associated with and without SPC. Synaptophysin, chromogranin A, and INSM1 were employed for the immunohistochemical study. IBC-NST had occasionally a morphological similarity with invasive SPC. While 123 of 127 cases with SPC demonstrated diffuse staining with one or more of the neuroendocrine markers, the only one case of DCIS and none of IBC-NST showed it. Type B was observed in 16 of 18 cases of MC associated with SPC and in 13 of 33 cases of MC without it. All the cases of MC with SPC and 6 of 33 cases without it showed diffuse staining for at least one of the neuroendocrine markers. In conclusion, a careful distinction between invasive SPC and IBC-NST with neuroendocrine differentiation is required. We assume that SPC in situ is a potential candidate for precursor of IBC-NST with neuroendocrine differentiation. MC of the breast is suggested to have two pathogenetic pathways through SPC in situ or non-SPC in situ. SPC in situ is thought to be less common as a precursor of MC than non-SPC in situ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Neuroendocrine neoplasm” has been recently proposed by the International Agency for Research on Cancer (IARC) and WHO as a term encompassing all tumor classes with predominant neuroendocrine differentiation, including both well-differentiated and poorly differentiated forms [1]. It is acknowledged that morphology and expression of neuroendocrine markers are key features defining these neoplasms at any specific anatomical sites [2]. The term of neuroendocrine neoplasm is applied in the breast exclusively to neuroendocrine tumor (NET) and neuroendocrine carcinoma that are practically very rare in our experience and that of other authors [3].
On the other hand, there is no complete agreement on concept of and criteria for the breast tumors showing neuroendocrine differentiation that include solid papillary carcinoma (SPC), mucinous carcinoma (MC), and invasive breast carcinoma of no special type (IBC-NST) with neuroendocrine differentiation. Although SPC and MC type B could fulfill the criteria as mammary neuroendocrine neoplasm, they are not classified as neuroendocrine neoplasm of the breast since they are “distinctive neoplasms” [1]. And definition of IBC-NST with neuroendocrine differentiation is very ambiguous to raise the diagnostic problems [4].
In our previous study of SPC of the breast, however, it was shown that the histological features of SPC were essentially compatible with those of typical NETs of the other organs [5]. It was demonstrated that almost all the cases with SPC expressed the current neuroendocrine markers. It was also supposed that SPC in situ was a precursor of NET, an invasive tumor, of the breast because the NET always coexisted with SPC in situ [5]. Accordingly, we thought that SPC should be classified as mammary neuroendocrine neoplasm.
Regarding the other breast tumors with neuroendocrine differentiation, there have been hardly ever any reports that explained a connection of SPC with IBC-NST with neuroendocrine differentiation. Additionally, we have not thoroughly described relationship between SPC and MC in relation to histologic subtype of MC and its neuroendocrine immunophenotype [5]. To elucidate true relationship between SPC and IBC-NST with neuroendocrine differentiation as well as SPC and MC, we conducted a morphological and immunohistochemical study on conventional ductal carcinoma in situ (DCIS), IBC-NST, and MC in comparison with SPC.
With this study, we want to improve the understanding and diagnostic accuracy of SPC and its related tumors with neuroendocrine differentiation and to establish their proper classification. The classification is essential for the histologic diagnosis, prediction of biological behavior, and adequate choice of treatment [6]. It will serve as a solid basis to provide invaluable materials to molecular pathology to clarify pathogenesis of the tumor and to design the targeted therapy [7].
Materials and methods
Patients’ data
We had 1708 cases with breast carcinoma that were surgically removed at our institute from 2018 to 2022. These cases included 72 cases of DCIS that were removed from May 2020 to End of 2022, 103 cases of IBC-NST from April 2022 to End of 2022, and 33 cases of MC from 2018 to 2022. We examined the 72 cases of DCIS, 103 of IBC-NST, and 33 of MC in this study. We also had 45 cases of SPC from 2018 to 2022. Thirty-three of the 45 cases of SPC had been included in our previous study of 127 cases of SPC including 35 cases with SPC in situ and 92 cases with invasive SPC [5]. Three pathologists of our department diagnosed all the cases of breast carcinoma and were involved in the present study to examine the tumors mentioned above. The patient’s clinical history, age at presentation, sex, laterality, and surgical procedure were obtained from the database of the hospital.
Histological materials and interpretation
Tissue samples were fixed in 10% buffered formalin and embedded in paraffin. The hematoxylin and eosin–stained slides of all the cases were reviewed by the authors. Nuclear grade was obtained from the clinicopathological reports [8]. When we conducted the histological study, the criteria for SPC, MC, DCIS, and IBC-NST were based on the WHO classification 2019 [1]. We compared morphologically and immunohistochemically the cases of DCIS to those of SPC in situ. We conducted the comparative study between the cases of IBC-NST and those of invasive SPC since all the cases of SPC with invasion had invasive SPC as the invasive component [1, 5]. We also compared the cases of MC associated with and without SPC (18 and 33 cases, respectively) regarding the histologic subtype and neuroendocrine immunophenotype.
In the immunohistochemical study, 4-μm-thick sections were cut from paraffin-embedded blocks, transferred onto silane-coated slides, dried at 60 °C for 1 h in the oven, and immunostained using a Ventana BenchMark XT Autostainer (Roche Diagnostics; Indianapolis, IN). To retrieve the antigens, the slides were treated with CC1 (Roche Diagnostics; Indianapolis, IN). We employed the following antibodies: synaptophysin (27H12) from Leica Biosystems, Buffalo Grove, IL; chromogranin A (LK2H10) and Insulinoma-associated Protein 1 (INSM1) (A-8) from Santa Cruz Biotechnology, Inc., Dallas, TX; estrogen receptor (SP1), progesterone receptor (IE2), and HER2 (4B5) from Roche Diagnostics, Indianapolis, IN; and Ki67 (MIB1) from Agilent, Santa Clara, CA. All the antibodies were incubated at 42 °C. An OptiView DAB IHC Detection kit (Roche Diagnostics; Indianapolis, IN) was used to detect the antigen–antibody complexes. After immunohistochemical staining, slides were processed by dehydration, cleared, and mounted in order. Positive and negative controls were included in each run.
In the present study, we categorized immunohistochemical staining for the neuroendocrine markers into three grades: diffuse, focal, and scarce or negative. The diffuse staining indicated that more than 50% of tumor cells were positive for the marker, whereas focal and scarce or negative staining meant that 10–50% and less than 10% of the cells were positive, respectively. We recognized the diffuse staining as an indicator of neuroendocrine differentiation of the tumor. As for estrogen and progesterone receptors, a positive result was defined as a positivity rate of 1% or higher of the tumor cells. The high expression level of Ki67 was defined as a positivity rate of higher than 20% of the tumor cells. Low expression was 20% or lower of them. The cut-off value for Ki67 was decided following the suggestion by the 13th St. Gallen international breast cancer conference [9].
Statistical analysis methods
Statistical analysis was performed using the statistical software ‘EZR’ (Easy R), which was based on R and R commander. EZR is freely available on a website (http://www.jichi.ac.jp/saitamasct/SaitamaHP. files/statmed.html) [10]. Chi-square/Fisher’s test was used to compare the groups, and the weighted kappa value and accuracy rate were calculated. For all the tests, a p-value of less than 0.05 was considered statistically significant.
Results
Clinical features of the cases with DCIS, IBC-NST, MC, and SPC are shown in Table 1. The mean age of the patients of DCIS was 55.7 years old, IBC-NST 59.8, MC 65.2, and SPC 66.2. All the patients in this series were women. In laterality of DCIS, 31 cases were left-sided and 41 cases were right-sided: 57 left and 46 right in IBC-NST, 15 and 18 in MC, 56 and 71 in SPC, respectively. Forty-two and 30 cases with DCIS underwent a total and partial mastectomy, respectively: 41 and 62 cases with IBC-NST, 16 and 17 with MC, and 63 and 64 with SPC, respectively.
Regarding the morphological features, DCIS exhibited a wide variety of histologic subtype (Table S1). Papillary, cribriform, and unclassifiable (not otherwise specified; NOS) subtypes were predominant. In IBC-NST, seven cases had invasive SPC-like morphology that showed solid tumor nests accompanied by thin-walled capillaries and the tumor cells of eosinophilic and granular cytoplasm (Table S2). The nuclear grading, immunohistochemical status of estrogen and progesterone receptors, and HER2, and Ki-67 index score in DCIS, IBC, and MC are listed in comparison with SPC in Table 1.
Immunohistochemical results with the neuroendocrine markers in the examined tumors are listed in Figs. 1 and 2, and Tables 1 and S1–2. The only one case with DCIS of mixed papillary and cribriform subtype showed diffuse staining (Fig. 3d). In contrast, all the cases except three with SPC in situ exhibited diffuse staining (Fig. 3m–p). Eight of 72 cases with DCIS were focally positive with INSM1 and/or synaptophysin (Fig. 3b). Histologic subtype of the eight cases included three cases of NOS, two cribriform, one papillary, one mixed papillary and solid, and one mixed cribriform and solid. All the other cases were scarcely positive or negative with all the markers (Fig. 3c).
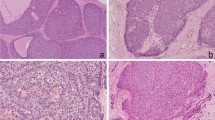
Neuroendocrine profiles on the immunohistochemistry of invasive breast carcinoma of no special type, invasive solid papillary carcinoma, and mucinous carcinoma. SYN, synaptophysin; CGA, chromogranin A; red, diffusely positive staining; yellow, focally positive staining; green, scarcely positive or negative staining
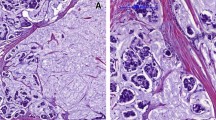
Representative histological and immunohistochemical features in all the types of breast carcinoma examined in the present study. a–d Histologic and immunohistochemical figures of conventional type of ductal carcinoma in situ. a HE × 10. b Focally positive staining for synaptophysin. c Negative staining for chromogranin A. d Diffusely positive staining for INSM1. e–h Histologic and immunohistochemical figures of invasive breast carcinoma of no special type. e HE × 10. f Focally positive staining for synaptophysin. g Negative staining for chromogranin A. h Focally positive staining for INSM1. i–l Histologic and immunohistochemical figures of mucinous carcinoma. i HE × 10. j Negative staining for synaptophysin. k Diffusely positive staining for chromogranin A. l Negative staining for INSM1. m–p Histologic and immunohistochemical figures of solid papillary carcinoma. m HE × 10. n Diffusely positive staining for synaptophysin. o Diffusely positive staining for chromogranin A. p Diffusely positive staining for INSM1
None of the cases of IBC-NST showed diffuse staining with any one of the three neuroendocrine markers. In comparison, 91 of 92 cases with invasive SPC were diffusely positive for at least one of the three markers. Eight of the 108 cases with IBC-NST displayed focal staining with the one or more neuroendocrine markers (Fig. 3e–h). Although the seven cases of IBC-NST showed invasive SPC-like morphology, all the cases except for one showed scarce or negative staining for all the neuroendocrine markers.
In Table 2, we summarized morphological and immunohistochemical characteristics of MC that was associated with and without SPC. Eighteen cases of MC associated with SPC had two cases of type A and 16 cases of type B. All the cases with SPC were diffusely positive for at least one of the three neuroendocrine markers. The cases associated without SPC, by contrast, exhibited 20 cases of type A and 13 cases of type B. And they showed the various immunohistochemical staining results. Five of the 33 cases expressed diffuse staining for at least one of the two markers (synaptophysin, chromogranin A). None of them displayed diffuse staining for INSM1. They had three cases of type A and two of type B. The nine cases manifested focal staining for at least one of the three markers (Fig. 3i–l). The other 19 cases were scarcely positive or negative for all the three.
Discussion
Our first study of SPC showed that the morphology was reminiscent of NET in the systemic organs, since all the cases displayed rosette or pseudo-rosette, focal streaming pattern, and cytoplasmic eosinophilic granularity [11]. In our recent clinicopathological study of 127 cases of SPC, we indicated that the characteristic morphology of SPC represents a typical histology of NET in the systemic organs [5]. Cytologically, we found that SPC shared many specific features with NET of the breast and the other organs [12, 13]. Morphological diagnosis of SPC is, therefore, not difficult in most of the cases, but nonetheless it is occasionally challenging to make a histologic distinction between SPC and the other type of breast carcinoma, in particular DCIS of mixed type and IBC-NST with invasive SPC-like pattern. Hence, immunohistochemistry with neuroendocrine markers is sometimes needed to make a proper diagnosis of the challenging cases [3].
There have been only a few reports that conducted a comparative study of neuroendocrine features between SPC in situ and DCIS of the breast. With positive cut-off value of 1% of tumor cells, Tan et al. reported that almost all the cases with SPC in situ showed positivity for synaptophysin and chromogranin A, while more than 95% and half of the cases with conventional DCIS revealed positivity for synaptophysin and chromogranin A, respectively [14]. Moritani et al. reported that 67% of solid intraductal papillary carcinoma and 8% of the nonsolid type showed positive staining with synaptophysin and/or chromogranin A in more than 70% of the tumor cells [15]. In our study with the positive cut-off value of more than 50% of tumor cells, conventional DCIS hardly displayed diffuse staining whereas SPC in situ typically showed it. Accordingly, SPC in situ almost always offered clear evidence for neuroendocrine differentiation. A wide range of the positive rate with DCIS was probably attributable to the variety of immunohistochemical antibodies, positive cut-off values, and histologic criteria.
Bogina et al. reported that IBC-NST presented diffuse staining with synaptophysin and/or chromogranin A in 58 of the 940 cases (6.2%) with positive cut-off value of more than 50% of tumor cells [4]. On the other hand, we had no cases of IBC-NST with diffuse staining. The same cut-off value notwithstanding, number of the cases with diffuse staining evidently differed in the two reports. We suspect that a main reason for the difference is a complete lack of morphological definition of IBC-NST with neuroendocrine differentiation [4, 16]. It is suggested that IBC-NST with neuroendocrine differentiation has occasionally a similar histology to invasive SPC when it is a malignancy of low or intermediate grade [1, 6]. Ki-67 proliferation index may help to differentiate invasive SPC from the IBC-NST of high grade and neuroendocrine carcinoma. Although coexistence of SPC in situ is important to diagnose invasive SPC, it is sometimes difficult to identify SPC in situ if the invasive tumor is predominant.
Moreover, we have no certain agreement about pathogenesis of IBC-NST with neuroendocrine differentiation [3]. In the present study, none of 72 cases except one with DCIS presented with neuroendocrine differentiation. It suggests that IBC-NST with neuroendocrine differentiation rarely arises from conventional DCIS. Accordingly, we assume that the tumor instead arises from SPC in situ. This assumption is supported by the fact that breast carcinomas with neuroendocrine differentiation or neuroendocrine breast tumors display distinctive mutational profiles compared with common type of breast carcinoma [7, 17]. The further genetic study of SPC and its related tumors with neuroendocrine differentiation is required to clarify the precise pathogenesis. The proper classification of these tumors is essential for the molecular research as well.
In the present study with random selection of MC associated without SPC, we had 20 cases of type A and 13 of type B. In the previous study of MC associated with SPC, however, we had an overwhelming majority of type B (16 cases) over type A (2cases) [5]. And neuroendocrine immunophenotype differs in MC of type B associated with and without SPC: All of the 16 cases with SPC showed diffuse staining for one or more of neuroendocrine markers, while only two of the 13 cases without SPC demonstrated it. Interestingly, chromogranin A is the least sensitive of the three neuroendocrine markers in the cases with SPC, whereas INSM1 is the least in the cases without SPC. Taken together, we suggest that MC has two different precursors: the one is SPC in situ and the other is non-SPC in situ. MC arising from SPC in situ is frequently of type B and diffusely positive with the neuroendocrine markers. In contrast, MC from non-SPC in situ has no preference for the subtype and often shows scarcely positive or negative staining for the neuroendocrine markers. And we also believe that SPC in situ is less common as precursor of MC than non-SPC in situ [5].
In conclusion, based on the results of this study, it is absolutely necessary to make a careful differential diagnosis between invasive SPC and IBC-NST with neuroendocrine differentiation because of their morphological similarity. We assume that SPC in situ is a potential candidate for a precursor of IBC-NST with neuroendocrine differentiation instead of conventional DCIS. We suggest that MC of the breast has two pathogenetic pathways through SPC in situ and non-SPC in situ. It is probably true that SPC in situ is less frequently a precursor of MC than non-SPC in situ is.
Data availability
Data supporting the findings of this study are included in this published article and its supplementary information files.
References
WHO Classification of Tumours Editorial Board (2019) WHO Classification of Tumours, 5th edn., Volume 2, Breast Tumours. IARC, Lyon
WHO Classification of Tumours Editorial Board (2019) WHO Classification of Tumours, 5th edn., Volume 1, Digestive System Tumours. IARC, Lyon
Wachter DL, Hartmann A, Beckmann MW, Fasching PA, Hein A, Bayer CM, Agaimy A (2014) Expression of neuroendocrine markers in different molecular subtypes of breast carcinoma. Biomed Res Int 2014:408459. https://doi.org/10.1155/2014/408459
Bogina G, Munari E, Brunelli M, Bortesi L, Marconi M, Sommaggio M, Lunardi G, Gori S, Massocco A, Pegoraro MC, Zamboni G (2016) Neuroendocrine differentiation in breast carcinoma: clinicopathological features and outcome. Histopathology 68:422–432. https://doi.org/10.1111/his.12766
Otsuki Y, Suwa K, Ohtsuka S, Mori N, Yoshida M, Serizawa A, Shimizu SI, Kobayashi H (2023) A large-scale clinicopathological and long-term follow-up study of solid papillary carcinoma of the breast. Virchow Arch 482:687–695. https://doi.org/10.1007/s00428-023-03489-7
Metovic J, Cascardi E, Uccella S, Maragliano R, Querzoli G, Osella-Abate S, Pittaro A, La Rosa S, Bogina G, Cassoni P, Marchiò C, Sapino A, Castellano I, Papotti M (2022) Neuroendocrine neoplasms of the breast: diagnostic agreement and impact on outcome. Virchow Arch 481:839–846. https://doi.org/10.1007/s00428-022-03426-0
Karihtala P, Porvari K, Roininen N, Voutilainen S, Mattson J, Heikkilä P, Haapasaari KM, Selander K (2022) Comparison of the mutational profiles of neuroendocrine breast tumours, invasive ductal carcinomas and pancreatic neuroendocrine carcinomas. Oncogenesis 11:53. https://doi.org/10.1038/s41389-022-00427-1
Otsuki Y, Shimizu S, Suwa K, Yoshida M, Kanzaki M, Kobayashi H (2007) Which is the better pathological prognostic factor, the Nottingham histological grade or the Japanese nuclear grade? A large- scale study with a long-term follow-up. Jpn Clin Oncol 37:266–274. https://doi.org/10.1093/jjco/hym026
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel members (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol 24:2206–23. https://doi.org/10.1093/annonc/mdt303
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48:452–458. https://doi.org/10.1038/bmt.2012.244
Otsuki Y, Yamada M, Shimizu S, Suwa K, Yoshida M, Tanioka F, Ogawa H, Nasuno H, Serizawa A, Kobayashi H (2007) Solid-papillary carcinoma of the breast: clinicopathological study of 20 cases. Pathol Int 57:421–429. https://doi.org/10.1111/j.1440-1827.2007.02118.x
Yamada M, Otsuki Y, Shimizu S, Tanioka F, Ogawa H, Kobayashi H (2007) Cytological study of 20 cases of solid-papillary carcinoma of the breast. Diagn Cytopathol 35:417–422. https://doi.org/10.1002/dc.20668
Yamada M, Otsuki Y, Ikeya T, Shimizu SI, Tanioka F, Ogawa H, Kobayashi H (2023) Cytological study of 44 cases with solid papillary carcinoma and a systemic review of solid papillary carcinoma and neuroendocrine tumor of the breast. Diagn Cytopathol 51:341–348. https://doi.org/10.1002/dc.25112
Tan BY, Thike AA, Ellis IO, Tan PH (2016) Am J Surg Pathol 40:1334-42. https://doi.org/10.1097/PAS.0000000000000702
Moritani S, Ichihara S, Kushima R, Okabe H, Bamba M, Kobayashi TK, Hattori T (2007) Myoepithelial cells in solid variant of intraductal papillary carcinoma of the breast: a potential diagnostic pitfall and a proposal of an immunohistochemical panel in the differential diagnosis with intraductal papilloma with usual ductal hyperplasia. Virchows Arch 450:539–547. https://doi.org/10.1007/s00428-007-0402-y
Rindi G, Klimstra D, Abedi-Ardekani B, Asa S, Bosman F, Brambilla E, Busam K, de Krijger R, Dietel M, El-Naggar A, Fernandez-Cuesta L, Klőppel G, McCluggage W, Moch H, Ohgaki H, Rakha E, Reed N, Rous B, Sasano H, Scarpa A, Scoazec J-Y, Travis W, Tallini G, Trouillas J, van Krieken J, Cree I (2018) A common classification framework for neuroendocrine neoplasms: an international Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol 31:1770–1786. https://doi.org/10.1038/s41379-018-0110-y
Marchio C, Geyer F, Ng C, Piscuoglio S, De Fillippo M, Cupo M, Schultheis A, Lim R, Burke K, Guerini-Rocco E, Rapotti M, Norton L, Sapino A, Weigelt B, Reis-Filho J (2018) The genetic landscape of breast carcinomas with neuroendocrine differentiation. J Pathol 241:405–419. https://doi.org/10.1002/path.4837
Acknowledgements
The authors thank all the medical technologists at the Department of Clinical Laboratory, Seirei Hamamatsu General Hospital, Hamamatsu, Japan, for their excellent technical assistance.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yoshiro Otsuki and Hiroshi Kobayashi. Yoshiro Otsuki, Yuki Asano, and Hiroshi Kobayashi were involved in evaluating the immunohistochemistry. The first draft of the manuscript was written by Yoshiro Otsuki and Hiroshi Kobayashi, and all the authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Otsuki, Y., Asano, Y., Ikeya, T. et al. A morphological and immunohistochemical study of ductal carcinoma in situ, invasive breast carcinoma of no special type, and mucinous carcinoma of the breast in comparison with solid papillary carcinoma regarding neuroendocrine marker expression. Virchows Arch 485, 547–555 (2024). https://doi.org/10.1007/s00428-024-03857-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-024-03857-x