Abstract
Wnt family member 9b (Wnt9b) has been demonstrated as a valuable marker for breast cancer diagnosis in surgical pathology. In this study, we examined the utility of Wnt9b in diagnosing metastatic breast carcinoma in cytology samples. Cell blocks from fine needle aspirations (FNA) and fluid specimens of 96 metastatic breast carcinomas and 123 primary and metastatic non-breast neoplasms from various organ systems were evaluated by Wnt9b and GATA3 immunohistochemistry (IHC). Wnt9b and GATA3 were positive in 81.3% and 92.7% of metastatic breast carcinomas, respectively. Conversely, 93.5% and 90.0% of non-breast, non-urothelial carcinomas were negative for Wnt9b and GATA3, respectively. Wnt9b expression was positive in rare gastrointestinal, gynecological, lung, pancreas, and salivary gland tumors. All twenty-eight urothelial carcinomas were negative for Wnt9b, while twenty-six (92.9%) were positive for GATA3. Wnt9b was slightly less sensitive but more specific than GATA3 in diagnosing metastatic breast cancer in cytology samples. Particularly, Wnt9b shows higher specificity in differentiating breast and urothelial primaries. The combined use of Wnt9b and GATA3 may increase diagnostic accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common cancer worldwide, surpassing lung cancer for the first time in 2020. An estimated 2,261,419 new breast cancer cases were diagnosed in women worldwide in 2020. Breast cancer is the most common cancer type in women in the United States after skin cancer and remains the second cause of cancer death in women, despite many advances in treatment and a significant decline in mortality [1]. Despite the decrease in mortality, breast cancer incidence rates continue to increase annually by about 0.5% [2]. Core needle biopsy (CNB) is the method of choice for initial breast cancer diagnosis. However, cytology methods such as fine needle aspiration (FNA) and effusion cytology are used to diagnose most metastatic breast cancers. Cytology methods are generally less invasive with lower cost and fewer complications than core needle biopsy [3]. However, cytology diagnosis is often limited by the lack of histologic architecture and the low quantity of available tumor cells [4,5,6]. Given these limitations, immunohistochemical markers with high sensitivity and specificity are particularly useful in the cytologic diagnosis of breast origin.
Traditionally, cytokeratin 7 (CK7), estrogen receptor (ER), gross cystic disease fluid protein-15 (GCDFP15), and mammaglobin have been used as immunohistochemical markers of breast cancer. However, given their expression in many other organ systems, these markers are not sensitive or specific [7,8,9,10,11,12,13,14]. Later, GATA binding protein 3 (GATA3) emerged as the best marker to confirm the breast origin. GATA3 is more sensitive than ER, GCDFP15, and mammaglobin [15,16,17] and has been widely utilized in routine pathology practice. However, GATA3 is expressed in non-breast neoplasms, such as urothelial carcinomas, renal cell carcinomas, and paragangliomas [18,19,20,21]. Recently, Trichorhinophalangeal syndrome GATA-binding type 1 (TRPS1), another GATA-binding transcription factor, has gained increased attention as a new sensitive and specific marker for breast cancer with sensitivity ranging from 86 to 98% [22,23,24,25,26] since its utility in breast cancer diagnosis was first introduced in 2010 [27].
Wnt9b protein has recently gained attention as a possible breast cancer marker due to its association with cancer development since its discovery. Wnt family proteins are encoded by 19 different Wnt genes that have vital roles in embryonal development and homeostasis [28]. They play a role in activating many downstream genes by nuclear localization of β-catenin that results from the binding of Wnt proteins to Frizzled (FZD) and LRP5/6 receptors as a part of the canonical pathway. Wnt proteins can also increase intracellular calcium by binding to the FZD receptor without affecting β-catenin as part of the non-canonical pathway. Abnormal Wnt signaling promotes cancer by affecting cell proliferation, angiogenesis, metastasis, tumor immunology, genome stability, and glycolysis [29]. Although their exact roles in tumorigenesis and tumor progression of each cancer type are not fully understood, different types of Wnt protein overexpression are reported in cervical (Wnt6), colorectal (Wnt6b and Wnt10a), and breast carcinomas (Wnt9b) [30,31,32,33]. In our previous study, we demonstrated that Wnt9b has sensitivity similar to GATA3 but greater specificity in establishing breast primary in surgical pathology [34]. In this current study, we aimed to evaluate Wnt9b expression in cytology samples with tumors of breast and non-breast origin and examine the utility of Wnt9b as a diagnostic marker in cytology side by side with GATA3.
Materials and methods
The study was approved by the Institutional Review Board of Lifespan Health System in Rhode Island.
Patient selection
Ninety-six metastatic breast cancers and one hundred and twenty-three primary and metastatic non-breast cancers accessioned from 2011 to 2019 were retrieved from the Department of Pathology archives of Lifespan Health Care System (Rhode Island Hospital and The Miriam Hospital). Concurrent lymph node aspirates at the time of the initial breast cancer diagnosis were excluded. The breast group consisted of 36 samples fixed in 10% neutral buffered formalin, and 60 fixed in CytoLyt® solution. The specimens included fine needle aspiration biopsies of bone and soft tissue, (non-axillary) lymph nodes, lung, mediastinum, liver, and fluids (pericardial, pleural, ascitic, and cerebrospinal). In sixty-eight cases, there was a high clinical suspicion of breast cancer (e.g., a recent history of high-stage breast carcinoma). Twenty-three were diagnosed with an intermediate suspicion (e.g., remote history of breast cancer or history of a second malignancy) in which confirmatory immunohistochemistry was helpful. Five cases were “cancers of unknown primary” with no documented history of breast cancer, and IHC was essential for establishing breast primary. The non-breast cancers consisted of 123 samples (41 primary and 82 metastatic tumors), 39 of which were fixed in 10% neutral buffered formalin, and 84 of which were fixed in CytoLyt® solution. The specimens included fine needle aspiration biopsies of bone and soft tissue, lymph nodes, lung, esophagus, pancreas, kidney, adrenal, liver, and fluids (pleural and ascitic). The tumor types included lung (58), gastrointestinal tract (12), gynecologic tract (8), genitourinary tract (28), kidney (4), liver (4), pancreas (4), prostate (3), salivary gland (1), and soft tissue (1) tumors.
It is not always feasible to have a concurrent surgical specimen as the gold standard for all the cytology cases (e.g., effusions), especially in patients presenting with advanced disease. 80.21% of our breast cancer cases had a corresponding surgical pathology specimen. 88.6% of the non-breast cases had a corresponding surgical pathology specimen during the workup, either as a concurrent sample (48.7%) or as previous or follow-up specimens (39.9%), which confirmed the cytologic and immunohistochemical impressions.
Immunohistochemistry
Wnt9b immunostain was performed on the cell blocks of all breast and non-breast cancers. GATA3 stain was performed on all the cases except for five non-breast cancers due to limited tissue in the block.
Immunohistochemical staining was performed on the Dako Autostainer Plus using EnVision Dual Link detection reagent (DAKO, Carpinteria, CA) with DAB (DAKO). The following primary antibodies were used: mouse monoclonal GATA3 antibody (clone L50-823, 1:100 dilution; Biocare Medical, Pacheco, CA) and rabbit polyclonal Wnt9b antibody (clone HPA058361, 1:100; MilliporeSigma, St. Louis, MO).
Wnt9b and GATA3 expression were considered positive if ≥ 1% of tumor cells showed at least weak nuclear staining intensity. To further stratify the Wnt9b positive cases we used the Allred scoring scheme [35], similar to our previous study [34]. Specifically, the staining intensity score was graded as 0 for negative, 1 for weak, 2 for moderate, and 3 for strong staining. The percentage score was rated as 0 for 0%, 1 for 0- < %1, 2 for 1–10%, 3 for 11–33%, 4 for 34–66%, and 5 for 67–100% of tumor nuclei stained. A combined intensity and percentage score of ≥ 3 was considered positive for Wnt9b. Wnt9b staining was reviewed by a cytopathologist (SM), and a consensus was made among other pathologists if there was any ambiguity. Breast cancer receptor status and other clinicopathologic features were obtained from the pathology reports.
Statistical methods
All data were collected and analyzed using Microsoft Excel (Microsoft Office Professional Plus 2016), SAS 9.4, and JMP Pro 16 (SAS Institute, Cary, NC). We used Fisher’s exact test to compare the categorical variables (i.e., comparison of the specificity of GATA3 and Wnt9b and the accuracy of the aforementioned immunohistochemical markers in formalin-fixed versus CytoLyt®-fixed cell blocks). A p-value of < 0.05 was considered statistically significant.
Results
Expression of Wnt9b and GATA3 in breast carcinoma
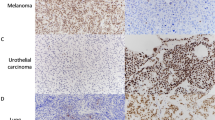
Wnt9b exhibited a nuclear staining pattern in metastatic breast carcinoma. No cytoplasmic or membranous immunoreactivity was observed (Fig. 1). Weak nonspecific staining was noted in the granulocytes and histiocytes.
Expression of Wnt9b and GATA3 in cytology specimens. a-c (200x): Metastatic breast cancer (ER-positive, HER2-negative) from the FNA biopsy of station 7 lymph node. Hematoxylin & Eosin-stained cell block a, demonstrates nuclear staining when stained with Wnt9b b, and GATA3 c. d-f (200x): Metastatic urothelial carcinoma of the bladder from pleural fluid cytology. Hematoxylin & Eosin-stained cell block d, stains negative for Wnt9b e, and positive for GATA3 f
Of the 96 breast cancer cases, 55 showed moderate (28, 29.2%) or strong (27, 28.1%) Wnt9b nuclear staining. Thirty (31.3%) showed weak staining, and 11 (11.5%) had no staining (Table 1). Over half (56, 58.3%) of the breast tumors expressed Wnt9b in 67% or more of the tumor cells, and 11.5% (11) expressed Wnt9b in 34% to 66% of the tumor cells (Table 2). Based on the Allred scoring system, 81.3% (78/96) of breast cancers were positive for Wnt9b. In comparison, 82.3% (79/96) of breast cancers were positive for GATA3 (Table 3).
The Nottingham grading of primary breast tumors was available in 44 of 96 cases. Among 44 cases, nine were Wnt9b-negative, four being Grade 2 and five being Grade 3. All 44 except one Grade 3 tumor were positive for GATA3. Of 63 tumors with known primary histologic types, 82.7% (43/52) and 94.2% (49/52) of the invasive ductal carcinomas were positive for Wnt9b and GATA3, respectively. For the invasive tumor with ductal and lobular features, 60% (3/5) and 100% (5/5) were positive for Wnt9b and GATA3, respectively. One of six invasive lobular carcinoma samples was negative for Wnt9b, and all six were positive for GATA3 (Table 3).
In 69 ER-positive tumors, 87% (60/69) were Wnt9b positive as opposed to 97.1% (67/69) for GATA3. Twelve of 18 (66.7%) ER-negative tumors were positive for Wnt9b, and 13 of 18 (72.2%) were positive for GATA3. Only nine tumors were HER2-positive. In these nine tumors, five were positive for Wnt9b and six were positive for GATA3. 81.2% (59/69) and 95.7% (66/69) of HER2-negative tumors were positive for Wnt9b and GATA3, respectively. Of 13 triple-negative tumors, ten were positive for both Wnt9b and GATA3 (76.9%) (Table 3).
The positive rates of GATA3 were higher than Wnt9b in metastases to bone/spine, chest wall, cerebrospinal fluid, supradiaphragmatic organs, and soft tissue. In contrast, Wnt9b and GATA3 had the same positive rates in ascites, liver, and non-axillary lymph node metastases (Table 3).
Expression of Wnt9b in non-breast metastatic cancer
The comparative expression of Wnt9b and GATA3 has been presented in Table 4 and Supplemental Table 1. Wnt9b and GATA3 were negative in most non-breast cancers. Wnt9b had a significantly better specificity; 115 of 123 cases were negative for Wnt9b (specificity of 93.5%), and 83 of 118 were negative for GATA3 (specificity of 70.3%) (Table 4), p < 0.0001 when compared using Fisher’s exact test. Six cases (three urothelial carcinomas, one lung adenocarcinoma, one colon adenocarcinoma, and one pancreatic adenocarcinoma) showed weak cytoplasmic Wnt9b staining. However, they did not have any nuclear staining and were considered Wnt9b-negative.
There were three cases positive for both markers, including one Mullerian adenocarcinoma, one lung squamous cell carcinoma, and one pancreatic adenocarcinoma. Thirty-two cases were positive for GATA3 but negative for Wnt9b. These included twenty-six cases of urothelial carcinomas, four adenocarcinomas of the lung, one soft tissue leiomyosarcoma, and one esophageal adenocarcinoma. Five cases were negative for GATA3 but positive for Wnt9b, including one endometrioid adenocarcinoma, one ovarian serous carcinoma, one esophageal adenocarcinoma, one lung squamous cell carcinoma, and one adenoid cystic carcinoma (Table 4).
Urothelial carcinoma had the highest rate of GATA3 positivity among all non-breast cancer types: twenty-six (92.9%) of twenty-eight urothelial carcinomas were positive, while all twenty-eight were negative for Wnt9b. GATA3 also showed a higher positivity rate than Wnt9b in lung cancers (9.1% vs. 3.5%). Four out of thirty-nine lung adenocarcinomas were positive for GATA3, while all were negative for Wnt9b. Wnt9b was positive in three of eight gynecological tumors, while GATA3 was positive in one (Table 4). A comparison of Wnt9b and GATA3 expression in breast and non-breast tumors is summarized in Supplemental Table 2.
Comparison of Wnt9b performance in formalin-fixed versus CytoLyt®-fixed cell blocks
For breast and non-breast cases combined, Fisher’s exact test was used to determine if there was a significant association between the fixation agent used (formalin vs. CytoLyt®) and the Wnt9b accuracy. Overall, our results showed a 90.41% accuracy when formalin was used and an 86.62% accuracy for samples fixed in CytoLyt®. There was no statistically significant difference between these accuracies (p-value = 0.5111).
Discussion
De novo metastatic breast cancer comprises approximately 6% of diagnoses in women in the U.S. [36]. Also, nearly 30% of women initially diagnosed with early-stage disease will ultimately develop metastasis [37]. When dealing with metastatic cancers of unknown primary, the recommended approach is to use a panel of immunohistochemical markers, including several organ-specific markers in conjunction with the cytomorphologic findings and clinical history. However, there are still challenges due to the wide range of sensitivity and specificity of the immunohistochemical markers and their aberrant loss or expression in advanced or treated tumors [38]. Cytologic specimens pose a more significant challenge due to their small sample size, lack of histomorphology, and a broad spectrum of preanalytical factors such as collection media, preservatives, fixatives, processing, and cell block techniques [39].
GCDFP-15 has a higher specificity but lower sensitivity as a breast marker [40]. GCDFP-15 expression is usually seen in hormone receptor-positive, HER2-positive, and apocrine tumor types, whereas it is negative in most triple-negative breast cancers [41]. Mammaglobin also has a variably low sensitivity [42], and both these markers can show reactivity in various non-breast tumors [38].
GATA3 is superior to the two former markers and has shown a high positivity rate of 96% in non-triple-negative breast carcinomas [43]. Although GATA3 significantly improves the diagnosis of triple-negative breast cancers compared to GCDFP15 and Mammaglobin, it still has a low sensitivity (44–66%) [44]. GATA3 expression has been reported in various non-breast tumor types [18,19,20,21]. Particularly, GATA3 is a sensitive marker of urothelial origin and, therefore, cannot be used for differentiating breast and urothelial primaries [45].
SRY-related HMG-box 10 (SOX10), historically a marker of tumors of neural crest origin, can also be positive in breast basal-like, triple-negative, and metaplastic carcinomas and, therefore, can be a helpful addition to the panel when dealing with hormone receptor and HER2-negative tumors [46, 47]. However, one should remember its expression in various salivary gland tumors [48].
TRPS1, another GATA-binding transcription factor, is a new sensitive and specific marker for breast cancer with sensitivity ranging from 86 to 98% [22,23,24,25,26]. Yoon et al. showed TRPS1 expression in 100% of triple-negative primary and metastatic invasive lobular carcinomas, 99% of triple-negative primary and metastatic invasive breast carcinomas of no special type, and 95% of metaplastic breast carcinomas [49]. In a recent study of TRPS1 in cytology specimens, a similar high expression in all three breast cancer types was noted. More importantly, TRPS-1 showed positivity in 100% of triple-negative breast cases compared with GATA-3 (76%) [50].
We have recently identified a new marker of breast origin, Wnt9b, which was found to be as sensitive as GATA3, but with better specificity in surgical specimens [34]. A subsequent study by Shaker et al. expanded their cohort to include triple-negative breast cancers and metaplastic carcinomas. The study showed above 90% Wnt9b expression in breast carcinomas and non-metaplastic triple-negative breast carcinomas and 80% expression in metaplastic carcinomas. Wnt9b expression in metaplastic carcinomas was significantly higher than GATA3 and SOX10 but slightly lower than TRPS1 [51].
To our knowledge, this is the first study that examines the sensitivity and specificity of Wnt9b in cytologic specimens for the diagnosis of metastatic breast carcinoma. We excluded the metastatic breast cancers from the ipsilateral axillary lymph nodes in our series and included only the distant metastases, as immunohistochemistry is not routinely used on malignant axillary lymph node FNAs if the concurrent core needle biopsy of the ipsilateral breast shows invasive breast cancer unless there is a suspicion of another primary. Wnt9b demonstrated a slightly better specificity than GATA3 (93.5% vs. 70.3%), albeit with a lower sensitivity (81.3% vs. 92.7%). In practice, using a panel of immunohistochemical markers is generally recommended. Therefore, combining these two markers may increase diagnostic accuracy in cytology specimens.
One of the advantages of using Wnt9b as a breast marker is its absence of expression in urothelial carcinoma as opposed to GATA3, which is also a urothelial marker [34]. The current series demonstrated the same finding: twenty-eight urothelial carcinomas from the bladder, ureter, and kidney were all negative for Wnt9b, while twenty-six were GATA3 positive.
Among over 50 cases of lung cancer, two squamous cell carcinomas were positive for Wnt9b, while four adenocarcinomas and one squamous cell carcinoma were positive for GATA3. GATA3 is known to be expressed in squamous cell carcinomas of the head and neck and lung origins [52, 53]. Similar to GATA3, Wnt9b was expressed in two of the ten lung squamous cell carcinomas. In our previous study on tumor resections, Wnt9b was negative in all 26 lung cancers [34]. The discrepancy could be related to our small sample size or different tissue preparation.
The expression of Wnt9b (37.5%) and GATA3 (12.5%) in metastatic gynecological cancers was surprisingly high. In our previous study, Wnt9b was negative in all 47 gynecological cancer resection microarrays [34]. On the other hand, GATA3 was positive in only 7.0% of 89 endometrial adenocarcinomas. Further studies in this regard may elucidate the reason for this discrepancy.
The cytology specimens differ from core needle biopsies and surgical resections in sample cellularity and lack of histologic data. Also, there is a high number of metastatic tumors that present as cytologic samples. Therefore, the use of immunohistochemistry is necessary in such instances. However, the cytologic samples also differ in tissue acquirement, fixation, and processing methods. Therefore, it is crucial to validate any new immunohistochemical marker on these specimens. Our study compared Wnt9b performance in 75 formalin-fixed versus 144 cytolyt®-fixed cell blocks. We found a 90.41% accuracy when formalin was used and an 86.62% accuracy for samples fixed in CytoLyt® with no statistically significant difference.
One of the limitations of our study is the low number of some non-breast tumor categories. Also, we only included 13 triple-negative breast cancers with a 76.9% positive rate for Wnt9b. A recent paper demonstrated a Wnt9b positivity rate of 97% in 34 triple-negative breast cancers [52]. This discrepancy may be due to different tissue processing and fixation (cytology), low cellularity of tumor cells in the cell blocks, our lower number of cases, or loss of Wnt9b expression in metastatic tumors. We did not have any metaplastic breast carcinomas in our cohort. Wnt9b showed a good sensitivity of 80% in the same study [38]. Finally, we did not compare Wnt9b performance with the other new breast markers TRPS1.
In summary, this is the first study examining the utility of Wnt9b in diagnosing metastatic breast carcinoma in cytology specimens. Wnt9b exhibited a nuclear staining pattern in metastatic breast carcinoma and no significant difference in accuracy in Formalin-fixed versus CytoLyt®-fixed specimens. While GATA3 showed a slightly higher sensitivity, Wnt9b had a significantly better specificity (93.5% vs. 70.3%). This, for the most part, is related to GATA3 expression in urothelial carcinomas, as all twenty-eight urothelial carcinomas in our cohort were negative for Wnt9b. Therefore, Wnt9b is superior to GATA3 if the differential diagnosis includes breast and urothelial primaries, and using these two markers in an immunohistochemical panel may increase diagnostic accuracy in cytology specimens. Further studies with a larger cohort are needed to evaluate the performance of Wnt9b for cytologic diagnosis of triple-negative and metaplastic breast cancers, various salivary gland, and skin adnexal tumors, and compare its function with the other new breast marker TRPS1.
References
American Society of Clinical Oncology (2023) Breast Cancer: statistics. Cancer. Net Editorial Board. Available from https://www.cancer.net/cancer-types/breast-cancer-metastatic/statistics
Pfeiffer RM, Webb-Vargas Y, Wheeler W, Gail MH (2018) Proportion of U.S. Trends in Breast Cancer Incidence Attributable to Long-term Changes in Risk Factor Distributions. Cancer Epidemiol Biomarkers Prev 27(10):1214–1222. https://doi.org/10.1158/1055-9965.EPI-18-0098
Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM (2020) Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health 8(8):e1027–e1037. https://doi.org/10.1016/S2214-109X(20)30215-1
Roskell DE, Buley ID (2004) Fine needle aspiration cytology in cancer diagnosis. BMJ 329(7460):244–245. https://doi.org/10.1136/bmj.329.7460.244
Kasraeian S, Allison DC, Ahlmann ER, Fedenko AN, Menendez LR (2010) A comparison of fine-needle aspiration, core biopsy, and surgical biopsy in the diagnosis of extremity soft tissue masses. Clin Orthop Relat Res 468(11):2992–3002. https://doi.org/10.1007/s11999-010-1401-x
Wang M, Kundu U, Gong Y (2020) Pitfalls of FNA diagnosis of thymic tumors. Cancer Cytopathol 128(1):57–67. https://doi.org/10.1002/cncy.22211
Paksoy N, Ozbek B (2018) Cytopathologist-performed and ultrasound-guided fine needle aspiration cytology enhances diagnostic accuracy and avoids pitfalls: An overview of 20 years of personal experience with a selection of didactic cases. Cytojournal 15:8. https://doi.org/10.4103/cytojournal.cytojournal_20_17. Published 2018 Mar 8
Viacava P, Naccarato AG, Bevilacqua G (1998) Spectrum of GCDFP-15 expression in human fetal and adult normal tissues. Virchows Arch 432(3):255–260. https://doi.org/10.1007/s004280050163
Gown AM, Fulton RS, Kandalaft PL (2016) Markers of metastatic carcinoma of breast origin. Histopathology 68(1):86–95. https://doi.org/10.1111/his.12877
Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT (1989) Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol 20(3):281–287. https://doi.org/10.1016/0046-8177(89)90137-8
Onuma K, Dabbs DJ, Bhargava R (2008) Mammaglobin expression in the female genital tract: immunohistochemical analysis in benign and neoplastic endocervix and endometrium. Int J Gynecol Pathol 27(3):418–425. https://doi.org/10.1097/PGP.0b013e31815d05ec
Sasaki E, Tsunoda N, Hatanaka Y, Mori N, Iwata H, Yatabe Y (2007) Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol 20(2):208–214. https://doi.org/10.1038/modpathol.3800731
Lamb CA, Vanzulli SI, Lanari C (2019) Hormone receptors in breast cancer: more than estrogen receptors Receptores hormonales en cáncer de mama: receptores de estrógenos y algo más. Medicina (B Aires) 79(Spec 6/1):540–545
Wei S, Said-Al-Naief N, Hameed O (2009) Estrogen and progesterone receptor expression is not always specific for mammary and gynecologic carcinomas: a tissue microarray and pooled literature review study. Appl Immunohistochem Mol Morphol 17(5):393–402. https://doi.org/10.1097/PAI.0b013e31819faa07
Wendroth SM, Mentrikoski MJ, Wick MR (2015) GATA3 expression in morphologic subtypes of breast carcinoma: a comparison with gross cystic disease fluid protein 15 and mammaglobin. Ann Diagn Pathol 19(1):6–9. https://doi.org/10.1016/j.anndiagpath.2014.12.001
Asch-Kendrick R, Cimino-Mathews A (2016) The role of GATA3 in breast carcinomas: a review. Hum Pathol 48:37–47. https://doi.org/10.1016/j.humpath.2015.09.035
Lu S, Yakirevich E, Wang LJ, Resnick MB, Wang Y (2019) Cytokeratin 7-negative and GATA binding protein 3-negative breast cancers: Clinicopathological features and prognostic significance. BMC Cancer 19(1):1085. https://doi.org/10.1186/s12885-019-6295-8. Published 2019 Nov 12
Liu H, Shi J, Wilkerson ML, Lin F (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138(1):57–64. https://doi.org/10.1309/AJCP5UAFMSA9ZQBZ
Zhao L, Antic T, Witten D et al (2013) Is GATA3 expression maintained in regional metastases?: a study of paired primary and metastatic urothelial carcinomas. Am J Surg Pathol 37(12):1876–1881. https://doi.org/10.1097/PAS.0b013e31829e2525
Mantilla JG, Antic T, Tretiakova M (2017) GATA3 as a valuable marker to distinguish clear cell papillary renal cell carcinomas from morphologic mimics. Hum Pathol 66:152–158. https://doi.org/10.1016/j.humpath.2017.06.016
So JS, Epstein JI (2013) GATA3 expression in paragangliomas: a pitfall potentially leading to misdiagnosis of urothelial carcinoma. Mod Pathol 26(10):1365–1370. https://doi.org/10.1038/modpathol.2013.76
Rohra P, Ding C, Yoon EC, Gan Q (2022) A pilot study: Comparison of TRPS1 and GATA3 immunoperoxidase staining using cytologic smears in entities reportedly positive for GATA3 [published online ahead of print, 2022 Jul 5]. Cancer Cytopathol https://doi.org/10.1002/cncy.22623
Ai D, Yao J, Yang F et al (2021) TRPS1: a highly sensitive and specific marker for breast carcinoma, especially for triple-negative breast cancer. Mod Pathol 34(4):710–719. https://doi.org/10.1038/s41379-020-00692-8
Yoon EC, Wang G, Parkinson B et al (2022) TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma. Hum Pathol 125:97–107. https://doi.org/10.1016/j.humpath.2022.04.006
Parkinson B, Chen W, Shen T, Parwani AV, Li Z (2022) TRPS1 Expression in Breast Carcinomas: Focusing on Metaplastic Breast Carcinomas. Am J Surg Pathol 46(3):415–423. https://doi.org/10.1097/PAS.0000000000001824
Allison KH, Hammond MEH, Dowsett M et al (2020) Estrogen and Progesterone Receptor Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Guideline Update. Arch Pathol Lab Med 144(5):545–563. https://doi.org/10.5858/arpa.2019-0904-SA
Chen JQ, Litton J, Xiao L et al (2010) Quantitative immunohistochemical analysis and prognostic significance of TRPS-1, a new GATA transcription factor family member, in breast cancer. Horm Cancer 1(1):21–33. https://doi.org/10.1007/s12672-010-0008-8
Ghosh N, Hossain U, Mandal A, Sil PC (2019) The Wnt signaling pathway: a potential therapeutic target against cancer. Ann N Y Acad Sci 1443(1):54–74. https://doi.org/10.1111/nyas.14027
Zhong Z, Yu J, Virshup DM, Madan B (2020) Wnts and the hallmarks of cancer. Cancer Metastasis Rev 39(3):625–645. https://doi.org/10.1007/s10555-020-09887-6
Kirikoshi H, Sekihara H, Katoh M (2001) WNT10A and WNT6, clustered in human chromosome 2q35 region with head-to-tail manner, are strongly coexpressed in SW480 cells. Biochem Biophys Res Commun 283(4):798–805. https://doi.org/10.1006/bbrc.2001.4855
Yin P, Wang W, Zhang Z, Bai Y, Gao J, Zhao C (2018) Wnt signaling in human and mouse breast cancer: Focusing on Wnt ligands, receptors and antagonists. Cancer Sci 109(11):3368–3375. https://doi.org/10.1111/cas.13771
Echternkamp SE, Laster DB (1976) Plasma LH concentrations for prepubertal, postpubertal, anestrous and cyclic ewes of varying fecundity. J Anim Sci 42(2):444–447. https://doi.org/10.2527/jas1976.422444x
Goel S, Chin EN, Fakhraldeen SA, Berry SM, Beebe DJ, Alexander CM (2012) Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J Biol Chem 287(20):16454–16466. https://doi.org/10.1074/jbc.M112.362137
Lu S, Yakirevich E, Yang D, Xiao Y, Wang LJ, Wang Y (2021) Wnt Family Member 9b (Wnt9b) Is a New Sensitive and Specific Marker for Breast Cancer. Am J Surg Pathol 45(12):1633–1640. https://doi.org/10.1097/PAS.0000000000001784
Allred DC, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11(2):155–168
Surveillance, Epidemiology, and End Results Program, National Cancer Institute (2023) SEER*Explorer. Breast cancer- Stage distribution of SEER incidence cases, 2011–2020 by sex, all races/ethnicities, all ages
O’Shaughnessy J (2005) Extending survival with chemotherapy in metastatic breast cancer. Oncologist 10(Suppl 3):20–29
Ding Q, Huo L, Peng Y, Yoon EC, Li Z, Sahin AA (2022) Immunohistochemical Markers for Distinguishing Metastatic Breast Carcinoma from Other Common Malignancies: Update and Revisit. Semin Diagn Pathol 39(5):313–321. https://doi.org/10.1053/j.semdp.2022.04.002
Roy-Chowdhuri S (2020) Immunocytochemistry of cytology specimens for predictive biomarkers in lung cancer. Transl Lung Cancer Res 9(3):898–905. https://doi.org/10.21037/tlcr.2019.12.31
Wick MR, Lillemoe TJ, Copland GT, Swanson PE, Manivel JC, Kiang DT (1989) Gross cystic disease fluid protein-15 as a marker for breast cancer: immunohistochemical analysis of 690 human neoplasms and comparison with alpha-lactalbumin. Hum Pathol 20(3):281–287. https://doi.org/10.1016/0046-8177(89)90137-8
Darb-Esfahani S, von Minckwitz G, Denkert C et al (2014) Gross cystic disease fluid protein 15 (GCDFP-15) expression in breast cancer subtypes. BMC Cancer 14:546. https://doi.org/10.1186/1471-2407-14-546
Sasaki E, Tsunoda N, Hatanaka Y, Mori N, Iwata H, Yatabe Y (2007) Breast-specific expression of MGB1/mammaglobin: an examination of 480 tumors from various organs and clinicopathological analysis of MGB1-positive breast cancers. Mod Pathol 20(2):208–214. https://doi.org/10.1038/modpathol.3800731
Kandalaft PL, Simon RA, Isacson C, Gown AM (2016) Comparative Sensitivities and Specificities of Antibodies to Breast Markers GCDFP-15, Mammaglobin A, and Different Clones of Antibodies to GATA-3: A Study of 338 Tumors Using Whole Sections. Appl Immunohistochem Mol Morphol 24(9):609–614. https://doi.org/10.1097/PAI.0000000000000237
Krings G, Nystrom M, Mehdi I, Vohra P, Chen YY (2014) Diagnostic utility and sensitivities of GATA3 antibodies in triple-negative breast cancer. Hum Pathol 45(11):2225–2232. https://doi.org/10.1016/j.humpath.2014.06.022
Liu H, Shi J, Wilkerson ML, Lin F (2012) Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 138(1):57–64. https://doi.org/10.1309/AJCP5UAFMSA9ZQBZ
Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, Taube JM, Illei PB, Argani P (2013) Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol 44(6):959–65. https://doi.org/10.1016/j.humpath.2012.09.005
Nelson ER, Sharma R, Argani P, Cimino-Mathews A (2017) Utility of Sox10 labeling in metastatic breast carcinomas. Hum Pathol 67:205–210. https://doi.org/10.1016/j.humpath.2017.08.011
Ivanov S, Panaccione A, Nonaka D et al (2013) Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer 109:444–451. https://doi.org/10.1038/bjc.2013.326
Yoon EC, Wang G, Parkinson B, Huo L, Peng Y, Wang J, Salisbury T, Wu Y, Chen H, Albarracin CT, Resetkova E, Middleton LP, Krishnamurthy S, Gan Q, Sun H, Huang X, Shen T, Chen W, Parwani AV, Sahin AA, Li Z, Ding Q (2022) TRPS1, GATA3, and SOX10 expression in triple-negative breast carcinoma. Hum Pathol 125:97–107. https://doi.org/10.1016/j.humpath.2022.04.006
Abdelwahed M, Yurtsever N, Savant D, Karam P, Gimenez C, Das K, Sheikh-Fayyaz S, Khutti S (2022) Utility of TRPS-1 immunohistochemistry in diagnosis of metastatic breast carcinoma in cytology specimens. J Am Soc Cytopathol 11(6):345–351. https://doi.org/10.1016/j.jasc.2022.06.007
Shaker N, Shafi S, Parkinson B, Chen W, Parwani AV, Ding Q, Li Z (2022) Wnt Family Member 9b (Wnt9b) Is a Sensitive and Specific Marker for Triple-negative Breast Carcinoma Including Metaplastic Carcinoma. Am J Sur Pathol. 2022 Published Ahead-of-Print
Wang Y, Lu S, Amin A, Wang L (2021) Coexpress of GATA-3 and ER in Anorectal and Head and Neck Squamous Cell Carcinoma Mimicking Metastatic Breast Cancer. Appl Immunohistochem Mol Morphol 29(6):409–413. https://doi.org/10.1097/PAI.0000000000000887
Miettinen M, McCue PA, Sarlomo-Rikala M, Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W, Lasota J, Wang Z (2014) GATA3: a multispecific but potentially useful marker in surgical pathology: a systematic analysis of 2500 epithelial and nonepithelial tumors. Am J Surg Pathol 38(1):13–22. https://doi.org/10.1097/PAS.0b013e3182a0218f
Author information
Authors and Affiliations
Contributions
YB, SL and SM collected the data and wrote the manuscript. DY performed the immunohistochemistry on the slides. YB, SL, DY, YW, EY, SH, LP, and SM reviewed the manuscript.
Corresponding author
Ethics declarations
The principles outlined in the Declaration of Helsinki were followed. The study was approved by the Institutional Review Board of Lifespan Health System in Rhode Island.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baykara, Y., Lu, S., Yang, D. et al. Utility of Wnt family member 9b (Wnt9b) immunohistochemistry in the cytologic diagnosis of metastatic breast carcinoma. Virchows Arch (2023). https://doi.org/10.1007/s00428-023-03645-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00428-023-03645-z