Abstract
Oncogenic activation of the mitogen-activated protein kinase (MAPK) pathway due to KRAS or BRAF gain-of-function mutation is frequently found in ovarian serous borderline tumor (SBT) and their extraovarian implants. We investigated mutational status of KRAS and BRAF of the primary ovarian SBTs that had a high stage presentation in correlation with clinical outcome. Among 39 consecutive primary SBTs with either invasive implants (20 cases) or non-invasive implants (19 cases), KRAS and BRAF mutational analysis was informative in 34 cases. Sixteen cases (47%) harbored a KRAS mutation, while 5 cases (15%) had a BRAF V600E mutation. High-stage disease (IIIC) was seen in 31% (5/16) of patients with a KRAS mutation and 39% (7/18) of patients without a KRAS mutation (p = 0.64). KRAS mutations were present in 9/16 (56%) tumors with invasive implants/LGSC versus 7/18 (39%) tumors with non-invasive implants (p = 0.31). BRAF mutation was seen in 5 cases with non-invasive implants. Tumor recurrence was seen in 31% (5/16) of patients with a KRAS mutation, compared to 6% (1/18) of patients without a KRAS mutation (p = 0.04). A KRAS mutation predicted an adverse disease-free survival (31% survival at 160 months) compared to those with wild-type KRAS (94% at 160 months; log-rank test, p = 0.037; HR 4.47). In conclusion, KRAS mutation in primary ovarian SBTs is significantly associated with a worse disease-free survival, independent of the high tumor stage or histological subtypes of extraovarian implant. KRAS mutation testing of primary ovarian SBT may servce as a useful biomarker for tumor recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Serous borderline tumor (SBT) of the ovary is a low-grade epithelial neoplasm affecting primarily reproductive-age women [1, 2]. While patients with stage I SBT have an excellent prognosis, 10 to 20% of SBTs present with extrauterine involvement in the form of tumor implants at the time of initial surgery [3,4,5]. A subset of patients will develop tumor recurrence, and in up to 7% of SBTs, the tumor progresses to low-grade serous carcinoma (LGSC) over time [6, 7]. Established risk factors for the development of subsequent carcinoma include micropapillary/cribriform histology, advanced stage, bilaterality, ovarian surface involvement, and residual disease after surgery [5, 8,9,10,11,12,13]. While implants are, by definition, non-invasive, the term “invasive implant” remains as a qualifier for the diagnosis of extra-ovarian LGSC in the setting of a primary SBT [7, 12].
In recent decades, studies have established that abnormal activation of the mitogen-activated protein kinase (MAPK) pathway is important for the pathogenesis of SBT. Two key components of the pathway, KRAS and BRAF, are frequently mutated in these tumors [14,15,16,17,18]. Other less frequent MAPK activation pathways have also been implicated [19, 20]. In recent studies of the extraovarian implants of SBT, KRAS mutation in implants of either invasive or noninvasive type ware found as a worse prognostic indicator for tumor recurrence and disease-specific survival. BRAF V600E mutation, however, may portend a lower risk for progression to carcinoma [13, 14, 21, 22]. Only limited data suggested that KRAS mutation in primary ovary SBTs is similarly associated with an unfavorable prognosis [23]. In this study, we examined the presence of KRAS and BRAF mutations in the primary tumors of ovarian SBTs of patients with at least stage IIA disease in correlation with clinical outcome.
Materials and methods
Study case selection and histological review
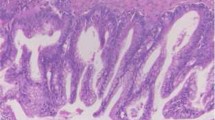
Consecutive cases of high stage SBTs were retrieved from pathology archives at a single institution accessioned between 1990 and 2020. Primary ovarian SBTs and their extraovarian lesions were histologically reviewed (AH and PH), and their extraovarian lesions were reclassified according to the 5th Edition 2020 WHO criteria [24] as either invasive implants/low-grade serous carcinoma or non-invasive implants. Briefly, tumor implants were assigned as invasive based on one or more of the following: destructive growth pattern at low magnification, presence of micropapillary architecture, and tumor cell nests surrounded by retraction artifact in dense fibrotic stroma (Fig. 1). A tumor implant without the aforementioned morphologic qualifiers was classified as non-invasive (Fig. 2), of either epithelial type (hierarchically branching papillae and detached clusters of cells without stromal response) or desmoplastic type (single cells or clusters of cells embedded in reactive-appearing or desmoplastic stroma). Patient demographics and clinical follow-up data were collected by medical record review. The study was performed under research protocols approved by the Institutional Review Board.
Representative ovarian serous borderline tumors (A, C) and extraovarian invasive/LGSC implants (B, D) from patients #4 (A, B) and #12 (C, D) from Table 1. Note the presence of retraction artifact, in which solid nests and some micropapillae are densely packed together within clear, lacunar-like spaces
Representative ovarian serous borderline tumors (A, C) and extraovarian non-invasive implants (B, D) from patients #22 (A, B) and #7 (C, D) from Table 1. A micropapillary pattern is defined as a 5 mm or greater area of small papillae with no fibrovascular cores that are at least 5 times as long as they are wide, often arising from a central, thicker papilla (C). Desmoplastic-type implants (B) show clusters of cells embedded within reactive-appearing desmoplastic stroma which predominates over the epithelial component. Epithelial-type implants (D) contain small to medium sized papillae, have detached clusters of cells not associated with stroma, and are within epithelium-lined spaces
KRAS and BRAF mutational analysis
Formalin-fixed, paraffin-embedded tumor blocks were selected from the primary ovarian borderline tumors. One hematoxylin and eosin-stained slide (H&E) and additional unstained sections were created. Once confirmed by H&E slide review, the corresponding target tumor tissue from the unstained slides were microdissected into a microcentrifuge tube. DNA was extracted by hydrothermal pressure method of simultaneous deparaffinization and lysis of formalin-fixed paraffin-embedded tissue followed by conventional column purification to obtain high quality DNA [25].
KRAS and BRAF mutation analysis by the highly sensitive single strand conformation polymorphism (SSCP) technique was performed according to previously described methods [26]. Briefly, 5–20 ng of extracted DNA was amplified using PCR primers flanking the mutational hotspot of exon 2 of the KRAS gene (forward primer: 5′-GACTGAATATAAACTTGTGG-3′ and reverse primer: 5′-CTGTATCAAAGAATGGTCCT-3′) and BRAF V600E mutation (forward primer: 5′-CTCTT CATAATGCTTGCTCTGATAGG-3′ and reverse primer: 5′-TAGTAACTCAGCAGCATCTCAGG-3′). The reaction was performed in a 50-μl solution containing 1 × PCR buffer, 0.1-mM dNTP, 1.5-mM MgCl2, and 2.5 units of AmpliTaq Gold DNA polymerase. PCR started with initial denaturation at 95 °C for 8 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55 °C for 1 min and synthesis at 72 °C for 2 min, with a final extension at 72 °C for 10 min (ABI Veriti Thermal Cycler, Applied Biosystem, Foster City, CA, USA). The PCR product was analyzed in duplicate by SSCP using MDE non-denaturing gel. Electrophoresis was carried out on ice for 2 h 45 min at 325 V. The SSCP gel was then stained with a SYBR Gold (Molecular Probes, Invitrogen, Norwalk, CT, USA) 1:10 000 in TE buffer added for 20 min and imaged by a Biorad GelDoc UV System (BioRad, Hercules, CA, USA). The presence of KRAS or BRAF mutations were determined by comparing the SSCP banding patterns with those of known KRAS mutation or BRAF V600E mutation positive controls (Fig. 3A and B). Cases with indeterminant KRAS mutation status by SSCP were evaluated by a Sanger sequencing analysis of DNA purified from the abnormal SSCP gel bands (Fig. 3C). Briefly, a mixture of deoxynucleoside triphosphates (dNTPs) and fluorescently labeled dideoxynucleoside triphosphates (ddNTPs) were utilized to generate nested fragments by chain termination during the synthesis of complimentary DNA [27].
Representative SSCP gels demonstrate positive BRAF V600E mutation (A) in the primary tumor of patient #28, wild-type status of patient #10, and appropriate positive and negative controls; and detection of KRAS exon 2, codon 12 mutation (GTT) (B) of the primary tumor from patient #7; wild-type KRAS (GGT) pattern from patient #23, and additional wild-type and common KRAS mutation controls (GTT, AGT, GAT, GCT, and TGT). All patient samples are run in duplicate for quality control. Representative Sanger sequencing of KRAS exon 2 (C) shows codons 11–14 with GGT to GTT mutation (G12V) from patient #20
Statistical analysis
Statistical analyses were performed using the two-tailed Student’s t-test for differences in the means of continuous variables and the Pearson chi-squared test for categorical variables. Statistical significance was determined by setting the level of p < 0.05 (alpha = 0.05) as significant. Follow-up time for disease-free survival calculation was measured in months from the day of initial surgery to the date of recurrence, defined as the date of surgical removal of tissue diagnosed as recurrent serous disease (SBT or LGSC), or date last known to be alive with or without disease. Recurrence was defined as tissue diagnosis of recurrent serous disease (either SBT or LGSC). The date of detection of tumor recurrence was defined as surgery date of the recurrent tumor. The Kaplan–Meier statistic method was used to generate a p-value using the Cox-Mantel log-rank test. The Mantel–Haenszel test was utilized to calculate relative risk in tests for which the Cox-Mantel log-rank test would fail (i.e., no recurrence within a subgroup).
Results
Clinicopathological characteristics of the study cohorts
A total of 39 cases of high stage SBT and follow-up data were included (Table 1). Patient age ranged from 26 to 79 years (mean 50.5, median 51). Laterality was unknown in 2 patients who had total hysterectomies with bilateral salpingo-oophorectomies at outside institutions. Bilateral ovarian SBTs were seen in 31 patients (84%), and unilateral tumors were seen in 6 patients. A micropapillary/cribriform pattern was seen in 6 primary ovarian tumors, and microinvasion was seen in 3 SBTs. Invasive implants/LGSC were present in 20 patients, and non-invasive implants were seen in 19 patients (Table 1). Two patients had both invasive and non-invasive implants. Pelvic lymph node involvement was seen in 14 patients (36%), and pelvic endosalpingiosis was seen in 15 patients (38%).
KRAS and BRAF mutational analysis
KRAS and BRAF mutation analysis was informative in 34 of 39 cases (Table 2). KRAS mutation was detected in 47% (16/34) of primary SBTs, while 5 tumors harbored BRAF V600E mutations (15%). KRAS and BRAF mutations were mutually exclusive. Clinicopathological features were comparable between patients with KRAS mutation and those without KRAS mutation, including patient age, presence of bilateral tumor, types of extraovarian implants, and tumor stage at presentation (Table 3). Notably, KRAS mutation correlated significantly with the presence of pelvic endosalpingiosis: 63% (10/16) of cases with KRAS mutation versus 22% (4/18) of cases without KRAS mutation (p = 0.017).
Correlation of implant types and disease progression
Of the 39 patients with follow-up data (ranging from 8 to 440 months), 8 patients experienced disease recurrence, 8 died of their disease, and 5 died of an unrelated cause. No statistically significant difference in terms of patient age or follow-up time was identified between patients with non-invasive implants and invasive/LGSC implants (Table 3). Similarly, no statistically significant difference in age was found between women with a KRAS mutation detected and women with wild-type KRAS tumor status.
Of the 39 patients, 20 had invasive/LGSC implants, whereas 21 had only non-invasive implants. Two patients had both invasive and non-invasive implants (Table 1). High-stage disease (IIIC) was seen in 70% (14/20) of patients with invasive/LGSC implants, whereas only 16% (3/19) with only non-invasive implants had high stage disease. When stratified by implant type, recurrence occurred in 40% (8/20) of patients with invasive/LGSC implants, compared to 0% (0/19) of patients with only non-invasive implants (p = 0.002) (Table 3). The rate of disease-free survival at 160 months was 34% in patients with invasive/LGSC implants (95% confidence interval 0–56.9%), compared to 100% disease-free survival in patients with non-invasive implants. Patients with invasive/LGSC implants had a worse disease-free survival (log-rank test, p-value = 0.003; Mantel–Haenszel hazard ratio 8.49) than those with non-invasive implants (Fig. 4).
A Disease-free survival represented graphically by Kaplan–Meier survival curve compared by the log-rank test (alpha = 0.05). A Patients with KRAS mutations in the primary tumor had a worse disease-free survival (n = 34, log-rank test, p-value = 0.037, hazard ratio 4.47) than those with wild-type KRAS. B Patients with invasive/LGSC implants had a worse disease-free survival (n = 39, log-rank test, p-value = 0.003; Mantel–Haenszel hazard ratio 8.49) than those with only non-invasive implants
Prognostic correlation with KRAS and BRAF mutation status
Among the 34 cases with informative KRAS and BRAF mutational analysis and clinical follow-up data, stage IIC disease or above was seen in 75% (12/16) of patients with KRAS mutation and 72% (13/18) of the patients without KRAS mutation (p = 0.85). Similarly, the presence of high-stage disease (IIIC) at presentation was seen in 31% (5/16) of patients with KRAS mutations and 39% (7/18) of patients without KRAS mutations (p = 0.64). KRAS mutations were present in 56% (9/16) of tumors with invasive implants/LGSC, compared to 39% (7/18) of tumors with non-invasive implants (p = 0.311). Tumor recurrence was seen in 31% (5/16) of patients with KRAS mutations, compared to 6% (1/18) of patients without KRAS mutations (p = 0.04). Independent of the tumor stage and the histologic subtypes of implants, KRAS mutation in the primary tumors predicted a worse disease-free survival (31% at 160 months) compared to those with wild-type KRAS in the primary ovarian tumor (94% at 160 months; log-rank test, p = 0.037; HR 4.47). BRAF mutation was only seen in 5 cases with non-invasive implants.
Discussion
The two most prevalent genes involved in the pathogenesis of ovarian low-grade serous tumors (SBT and LGSC) are KRAS and BRAF [15, 16]. Mutations in the two genes result in abnormal gain of function, leading to uncontrolled activation of the MAPK signaling pathway. Close to 50% of SBTs and LGSCs harbor KRAS or BRAF mutations [15, 17, 28]. KRAS and BRAF mutations are mutually exclusive in the vast majority of cases [15, 29]. In the current study of at least stage IIA ovarian SBTs, KRAS mutation was identified in the primary tumors of 16/34 patients (47%), while BRAF V600E mutation was identified in 5/34 patients (15%), consistent with the existing literature.
Limited studies have suggested that KRAS mutation in primary ovarian tumors is associated with unfavorable prognoses and disease progression to LGSC [23]. Focusing on KRAS and BRAF mutation status of the primary ovarian SBTs in this study, the outcome analysis found that KRAS mutation detectable in the primary ovarian SBTs is a significant prognostic indicator for tumor recurrence: SBTs harboring a KRAS mutation had a considerably higher recurrence rate than those without the mutation (31% vs 6%) and a significantly worse disease-free survival (31% vs 94% at 160 months, log-rank test, p-value 0.037, hazard ratio 4.47). Remarkably, such prognostic value is independent of the high tumor stage and histologic subtypes of the extraovarian implants (Table 3). It is worth noting that a recent study of 215 cases of low-grade serous carcinoma concluded that the tumors with MAP kinase pathway gene mutations had an improved overall survival comparing with those without the mutations of these genes [30]. This parodoxical findings may be explained by the major difference in the study cohorts where the majority of the cases were conventional low-grade serous carcinoma (175 cases) and only 39 cases were associated with ovarian serous borderline tumor. Moreover, the observed survival advantage was based on the total number of mutations of all MAP kindase genes including KRAS, NRAS, BRAF, etc.), and therefore, it is unclear the prognostic impact of individual gene mutations in this study as mutations of different MAP kinase genes may have different biological impact. For example, BRAF V600E mutation appears to be associated with SBTs and is uncommon in low-grade serous carcinoma including SBT with invasive implants. Previous studies showed 23–48% of serous borderline tumors carrying the BRAF mutation while the rate dropped to 0–33% in low-grade serous carcinomas [31]. Regarding BRAF mutation status in implants of SBT, one study found that 14 of 63 (22%) noninvasive implants and none of 7 invasive implants had BRAF mutation [29] and a similar finding was found in our previous study as well [21]. Similarly in another study, none of 23 recurrent low-grade serous carcinomas had BRAF mutation but 5 of 13 noncurrent cases had the mutation [23]. Our current investigation focused only on SBTs in correlation with KRAS or BRAF mutations and conventational low-grade serous carcinoma was not included. While we found KRAS mutation is signficantly assoicatedd with a worse diseae recurrence free survial, future long-term follow up studies are needed to ascertain if the presence of KRAS mutation in serous borderline tumors may eventually impact the the patient overall survival.
KRAS mutation in the primary SBT is not significantly correlated with the histological subtypes of implants: KRAS mutations were present in 9/16 (56%) tumors with invasive implants/LGSC versus 7/18 (39%) tumors with non-invasive implants (p = 0.311). The finding of KRAS mutation status in non-invasive implants may explain why non-invasive implants also carry a significant risk for subsequent development of LGSC, though lower than invasive/LGSC implants [7, 12]. Consistent with existing data [7, 12], high-stage disease (IIIC) was seen in 70% (14/20) of patients with invasive/LGSC implants, in contrast to 16% (3/19) with non-invasive implants. When the histologic subtype of extraovarian implants was considered, tumor recurrence occurred in 8 of 20 patients with invasive/LGSC implants (40%), compared to 0 of 19 patients with only non-invasive implants. Consistently, patients with invasive/LGSC implants had a worse disease-free survival with 34% survival at 160 months compared to 100% survival at 160 months in those with non-invasive implants (log-rank test, p = 0.003; Mantel–Haenszel hazard ratio 8.49).
KRAS mutation in primary ovarian SBTs correlates significantly with the presence of peritoneal endosalpingiosis in this study, present in 63% (10/16) of cases with KRAS mutation versus 22% (4/18) of cases without KRAS mutation (p = 0.017). While the overwhelming majority of studies support a theory of clonality between SBTs and implants and progression of SBTs to LGSC [13], the possibility of alternative mechanisms of concomitant pathogenesis may also occur. It is possible that some implants may arise not from an ovarian primary tumor but from endosalpingiosis. The documented higher frequency of endosalpingiosis in SBTs associated with subsequent carcinoma relative to those without subsequent carcinoma suggests that endosalpingiosis may be a direct precursor of primary peritoneal LGSC [32]. Future studies are required to ascertain the biological relationship between KRAS mutation of the primary ovarian SBT and peritoneal endosalpingiosis with respect to tumor recurrence or progressive transformation.
BRAF V600E mutation was seen in only 5 primary ovarian SBTs with only non-invasive extraovarian implants in this current study. BRAF mutations have been shown to be less prevalent in LGSC than SBTs [33]. Similarly, BRAF mutation is rare in advanced-stage LGSC [34]. In SBTs, BRAF mutation is more common in low-stage tumors and is associated with improved prognosis and lower frequencies of tumor recurrence [22, 23]. It has been proposed that BRAF mutation may have a prohibitive effect on tumor recurrence related to cellular senescence [35].
In conclusion, KRAS mutation present in the primary ovarian serous borderline tumors is significantly associated with a greater risk of tumor recurrence and a shorter disease-free survival, independent of their high tumor stage at presentation and the histologic subtypes of extraovarian implant. Additional studies of larger cohorts with longer clinical follow up are important to solidify the value of KRAS mutation testing of primary ovarian serous borderline tumors as a prognostic biomarker.
References
Bell KA, Smith Sehdev AE, Kurman RJ (2001) Refined diagnostic criteria for implants associated with ovarian atypical proliferative serous tumors (borderline) and micropapillary serous carcinomas. Am J Surg Pathol 25:419–432
Ahn G, Folkins AK, McKenney JK et al (2016) Low-grade serous carcinoma of the ovary: clinicopathologic analysis of 52 invasive cases and identification of a possible noninvasive intermediate lesion. Am J Surg Pathol 40:1165–1176
Ayhan A, Guvendag Guven ES, Guven S et al (2005) Recurrence and prognostic factors in borderline ovarian tumors. Gynecol Oncol 98:439–445
Park JY, Kim DY, Kim JH et al (2009) Surgical management of borderline ovarian tumors: the role of fertility-sparing surgery. Gynecol Oncol 113:75–82
Hannibal CG, Vang R, Junge J et al (2014) A nationwide study of serous “borderline” ovarian tumors in Denmark 1978–2002: centralized pathology review and overall survival compared with the general population. Gynecol Oncol 134:267–273
Longacre TA, McKenney JK, Tazelaar HD et al (2005) Ovarian serous tumors of low malignant potential (borderline tumors): outcome-based study of 276 patients with long-term (> or =5-year) follow-up. Am J Surg Pathol 29:707–723
Vang R, Hannibal CG, Junge J et al (2017) Long-term behavior of serous borderline tumors subdivided into atypical proliferative tumors and noninvasive low-grade carcinomas: a population-based clinicopathologic study of 942 cases. Am J Surg Pathol 41:725–737
McKenney JK, Balzer BL, Longacre TA (2006) Lymph node involvement in ovarian serous tumors of low malignant potential (borderline tumors): pathology, prognosis, and proposed classification. Am J Surg Pathol 30:614–624
Seidman JD, Kurman RJ (2000) Ovarian serous borderline tumors: a critical review of the literature with emphasis on prognostic indicators. Hum Pathol 31:539–557
Prat J, De Nictolis M (2002) Serous borderline tumors of the ovary: a long-term follow-up study of 137 cases, including 18 with a micropapillary pattern and 20 with microinvasion. Am J Surg Pathol 26:1111–1128
Bell DA, Longacre TA, Prat J et al (2004) Serous borderline (low malignant potential, atypical proliferative) ovarian tumors: workshop perspectives. Hum Pathol 35:934–948
Hannibal CG, Vang R, Junge J et al (2017) A nationwide study of ovarian serous borderline tumors in Denmark 1978–2002. Risk of recurrence, and development of ovarian serous carcinoma. Gynecol Oncol 144:174–180
Chui MH, Xing D, Zeppernick F et al (2019) Clinicopathologic and molecular features of paired cases of metachronous ovarian serous borderline tumor and subsequent serous carcinoma. Am J Surg Pathol 43:1462–1472
Singer G, Oldt R 3rd, Cohen Y et al (2003) Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst 95:484–486
Ho CL, Kurman RJ, Dehari R et al (2004) Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res 64:6915–6918
Jones S, Wang TL, Kurman RJ et al (2012) Low-grade serous carcinomas of the ovary contain very few point mutations. J Pathol 226:413–420
Hunter SM, Anglesio MS, Ryland GL et al (2015) Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 6:37663–37677
Krzystyniak J, Ceppi L, Dizon DS et al (2016) Epithelial ovarian cancer: the molecular genetics of epithelial ovarian cancer. Ann Oncol 27(Suppl 1):i4–i10
Anglesio MS, Arnold JM, George J et al (2008) Mutation of ERBB2 provides a novel alternative mechanism for the ubiquitous activation of RAS-MAPK in ovarian serous low malignant potential tumors. Mol Cancer Res 6:1678–1690
Xing D, Suryo Rahmanto Y, Zeppernick F et al (2017) Mutation of NRAS is a rare genetic event in ovarian low-grade serous carcinoma. Hum Pathol 68:87–91
Zuo T, Wong S, Buza N et al (2018) KRAS mutation of extraovarian implants of serous borderline tumor: prognostic indicator for adverse clinical outcome. Mod Pathol 31:350–357
Chui MH, Kjaer SK, Frederiksen K et al (2019) BRAF(V600E) -mutated ovarian serous borderline tumors are at relatively low risk for progression to serous carcinoma. Oncotarget 10:6870–6878
Tsang YT, Deavers MT, Sun CC et al (2013) KRAS (but not BRAF) mutations in ovarian serous borderline tumour are associated with recurrent low-grade serous carcinoma. J Pathol 231:449–456
WHO (2020) Classification of Tumours 5th Edition - Female Genital Tumours. (IARC Press, Lyon, France
Zhong H, Liu Y, Talmor M et al (2013) Deparaffinization and lysis by hydrothermal pressure (pressure cooking) coupled with chaotropic salt column purification: a rapid and efficient method of DNA extraction from formalin-fixed paraffin-embedded tissue. Diagn Mol Pathol 22:52–58
Dillon DA, Johnson CC, Topazian MD et al (2000) The utility of Ki-ras mutation analysis in the cytologic diagnosis of pancreatobiliary neoplasma. Cancer J 6:294–301
Metzker ML (2005) Emerging technologies in DNA sequencing. Genome Res 15:1767–1776
Mayr D, Hirschmann A, Löhrs U et al (2006) KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol 103:883–887
Ardighieri L, Zeppernick F, Hannibal CG et al (2014) Mutational analysis of BRAF and KRAS in ovarian serous borderline (atypical proliferative) tumours and associated peritoneal implants. J Pathol 232:16–22
Gershenson DM, Sun CC, Westin SN et al (2022) The genomic landscape of low-grade serous ovarian/peritoneal carcinoma and its impact on clinical outcomes. Gynecol Oncol 165:560–567
Malpica A, Wong KK (2016) The molecular pathology of ovarian serous borderline tumors. Ann Oncol 27(Suppl 1):i16–i19
Silva EG, Tornos C, Zhuang Z et al (1998) Tumor recurrence in stage I ovarian serous neoplasms of low malignant potential. Int J Gynecol Pathol 17:1–6
Grisham RN, Iyer G, Garg K et al (2013) BRAF mutation is associated with early stage disease and improved outcome in patients with low-grade serous ovarian cancer. Cancer 119:548–554
Wong KK, Tsang YT, Deavers MT et al (2010) BRAF mutation is rare in advanced-stage low-grade ovarian serous carcinomas. Am J Pathol 177:1611–1617
Zeppernick F, Ardighieri L, Hannibal CG et al (2014) BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol 38:1603–1611
Author information
Authors and Affiliations
Contributions
Dr. Austin McHenry conducted study case selection, data collection and analysis, and drafted the manuscript. Dr. Douglas Rottmann conducted initial study case selection and data collection. Dr. Natalia Buza conducted study data review and contributed to the writing of the manuscript. Dr. Pei Hui designed the study, conducted data review and analysis, and finilized the manucirpt.
Corresponding author
Ethics declarations
Ethics approval
This study has been performed in compliance with the institutional ethical standards.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McHenry, A., Rottmann, D.A., Buza, N. et al. KRAS mutation in primary ovarian serous borderline tumors correlates with tumor recurrence. Virchows Arch 483, 71–79 (2023). https://doi.org/10.1007/s00428-023-03564-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03564-z