Abstract
Diagnosis of histiocytosis can be difficult and one of the biggest challenges is to distinguish between reactive and neoplastic histiocytes on histology alone. Recently, OCT2 nuclear expression was reported in Rosai-Dorfman disease (RDD). Our purpose was to expand the testing of OCT2 on a broader variety of sporadic or H syndrome-related histiocytoses. Cases of histiocytoses were retrieved from the files of Ambroise Paré Pathology Department. All slides and molecular analyses were reviewed, and staining was completed with immunohistochemistry for OCT2. A total of 156 samples from different localizations were tested. Among sporadic cases, 52 patients had RDD, and 10 patients had mixed histiocytosis combining RDD with Erdheim Chester disease (ECD, n = 8), Langerhans cell histiocytosis (LCH, n = 2) or juvenile xanthogranuloma (JXG, n = 1). All these patients were positive for OCT2 in RDD characteristic histiocytes. Twenty-three patients had ECD and all but two (91% − 21/23) were negative for OCT2. By contrast, OCT2 was positive in 11/27 (41%) LCH and 6/16 (38%) JXG. Among the 10 samples of H syndrome-associated histiocytosis, 3 had typical RDD histology, 6 had unclassified histiocytosis, and one had mixed RDD-LCH; all were positive for OCT2. On 16 samples of granulomatous lymphadenitis, OCT2 was negative in epithelioid histiocytes. Our study shows that OCT2 has a sensitivity of 100% for RDD cases and mixed histiocytoses with an RDD component. It is negative in 92% of ECD but expressed in at least 38% of LCH, JXG, and C group histiocytoses. Finally, OCT2 is positive in all H syndrome-related histiocytoses, independent of their histology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Histiocytoses are characterized by tissue infiltration by cells of macrophage or dendritic cell lineages. They include several diseases, the most frequent of which being Langerhans-cell histiocytosis (LCH), Erdheim-Chester disease (ECD), Rosai-Dorfman-Destombes disease (RDD), and juvenile xanthogranuloma (JXG) [1]. Most of histiocytoses are considered as neoplasms, because they harbor somatic clonal alterations of an oncogene activating the MAP-kinase pathway [2]. Diagnosis is based on histological and molecular examination of biopsy samples correlated with clinical and radiological features. Immunohistochemistry plays an important role in the diagnosis of histiocytosis, and the most frequently used immunomarkers (CD163, CD68, CD1a, CD207, and S100) do not to distinguish between reactive and neoplastic histiocytes. Similarly, it is sometimes difficult to distinguish between different components within the same sample in cases of mixed histiocytosis.
The large majority of histiocytoses are sporadic, but a few cases are associated with H syndrome. H syndrome is an inherited condition due to mutation within SLC29A3 gene, with frequent occurrence of a spectrum of clinical symptoms, namely hyperpigmentation, hypertrichosis, hearing loss, hepatosplenomegaly, heart anomalies, hypogonadism, low height, hallux valgus, and hyperglycemia associated with insulin-dependent diabetes [3].
OCT2 is a nuclear marker originally identified as a B-cell marker. It is a transcription factor expressed in all stages of B cell development from B precursor cells to plasma cells [4]. Its role is important in immunoglobulin promoter transactivation and OCT2 is involved in the proliferation and differentiation of B cells. Besides B cell, it has been described also in neuronal cells [5]. Recently, the expression of OCT2 has also been described in histiocytoses, particularly in RDD [6, 7]. However, the expression of this marker is not limited to RDD. Indeed, in the original study, it was also expressed in a minority of LCH, and we recently showed its expression in some cases of ALK-positive histiocytosis [6,7,8]. Similarly, it was also described as positive in rare cases of histiocytic sarcomas [9].
Our aim was to determine the specificity and the sensitivity of OCT2 in RDD by expanding its testing on a broader variety of histiocytoses, including mixed histiocytoses and histiocytoses associated with H syndrome.
Materials and methods
Cases were retrieved from the files of the Pathology Laboratory of Ambroise-Paré Hospital, which is in charge of the central histological review and molecular analyses of the French Histiocytosis Network. Patients signed informed consent for inclusion in prospective cohorts Gene Histio (until 2021) or Target Histio (since 2021), aiming to correlate phenotype and somatic genetic alterations with clinical data and evolution (NCT04437381). Clinical data were obtained from the databases of the corresponding studies. The diagnosis of different types of histiocytosis was established, based on the international criteria for the diagnosis of histiocytosis [10]. Diagnosis of H syndrome was based on the detection of symptoms of the spectrum and confirmed in all cases by detection of biallelic SLC29A3 mutations after informed consent of patients for genetic analysis and counseling [11].
Only patients with available adequate FFPE (formalin-fixed and paraffin-embedded) blocks were included. For all cases, hematoxylin & eosin (H&E) and immunohistochemistry (IHC) slides were reviewed, as well as molecular results. Depending on each case, IHC with different histiocyte markers was performed: CD163 (10D6, Leica), phosphoERK (ERK 1/2, Ozyme, 4370S), S100 (polyclonal, Dako), CD1a (O10, Dako, Glostrup, Denmark), CD207 (Novocastra, monoclonal antibody), and ALK (1A4). OCT2 (ABCAM, Ab178679) was performed on all cases. In cases of suspicion of B cell lymphoma, PAX5 (Dako) was performed. We also tested OCT2 on needle biopsies or surgical samples of granulomatous lymphadenitis. Immunohistochemistry was performed using Bond Max (Leica Biosystem, Newcastle, UK) with BOND Polymer Refine Detection Leica. FFPE sections were deparaffinized with BOND Dewax solution. Antigen retrieval was performed with citrate buffer retrieval solution at 95 °C, followed by 10 cycles of washing in BOND WASH solution. Tissue sections were then treated with 3% hydrogen peroxide, then incubated for monoclonal antibodies to OCT2 (ABCAM, Ab178679) at a 1/100 dilution for 15 min. For the detection of bound primary antibodies, a polymer detection kit with diaminobenzidine (DAB) chromogen was used.
For the interpretation of OCT2, reactive B lymphocytes served as internal positive control. The expression of OCT2 was interpreted separately by two pathologists (IAU and JFE) and difficult cases were reviewed together. The intensity of staining of histiocytes (weak/strong) was compared to reactive small lymphocytes of the same section.
Concerning molecular tests, the detection of somatic mutations was performed on DNA or RNA extracted from areas infiltrated with the highest percentage of histiocytes. The BRAFV600E mutation was detected by real-time PCR, pyrosequencing, or/and digital PCR. Cases without a BRAFV600E mutation were screened for other mutations in genes of the MAP kinase signaling pathway by targeted next-generation sequencing (NGS) as previously described (see Supplementary Fig. 1) [8, 12, 13].
Results
A total number of 156 samples (from 153 patients) of different types of histiocytoses were analyzed. The median age at the time of diagnosis was 41 years old (ranging between 2 days and 84 years old). The female/male ratio was 1:1.9. Nine of these patients had an H syndrome-related histiocytosis.
Sporadic histiocytoses
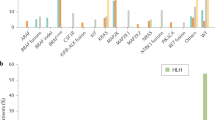
Samples of 52 patients with RDD were analyzed. Biopsies were from various tissues or organs, the most frequent of which were skin (n = 14), lymph node (n = 14), soft tissue and subcutis (n = 5), and bone (n = 4). Characteristic cells were large histiocytes with large nucleus and nucleoli, and abundant cytoplasm with emperipolesis. S100 was positive in 50 cases and, in the other two cases, it was either negative or positive only in a minority of histiocytes. In all cases characteristic RDD cells showed a nuclear positivity with OCT2 (Fig. 1). These cells were easy to distinguish from reactive B lymphocytes based on their morphology (Fig. 2). The positivity was strong in all but one case.
Expression of OCT2 in sporadic and H syndrome-related histiocytoses. RDD, Rosai-Dorfman disease; ECD, Erdheim-Chester disease; JXG, juvenile xanthogranuloma; LCH, Langerhans-cell histiocytosis. *8 samples corresponded to double components on the same biopsy sample, while 3 samples were patients with only RDD histology, but ECD clinical manifestation. **including one case of mixed ECD-LCH
A1 Cutaneous localization of Rosai-Dorfman disease with emperipolesis lesions in mononucleated or binucleated histiocytes with vesicular nuclei (HES, 400x). A2 Histiocytes are OCT2 + with a strong staining (OCT2, 400x) and S100 partially negative (inset—S100, 400x). B1 Cutaneous localization of RDD with classic emperipolesis lesions (HES, 400x). B2 Neoplastic histiocytes are OCT2 + with nuclei bigger than the small B lymphocytes in the background (OCT2, 400x). C1 Biopsy sample from parotid gland, mixed ECD-RDD. At the left there is the ECD component with foamy histiocytes; at the right, there is the RDD component (HES, 200x). C2 ECD component at the left is OCT2-, while the RDD histiocytes were OCT2 + (OCT2, 200x). D1 Peritoneal localization of xanthogranuloma with Touton giant cells (HES, 200x). D2 OCT2 is strongly positive in neoplastic histiocytes and in giant cells (OCT2, 200x). E1 Skin localization of LCH with histiocytes with irregular nuclei with prominent nuclear grooves (HES, 400x) (E2) Histiocytes are OCT2 + (OCT2, 400x). F1 Atypical histiocytic infiltration in the skin of a patient with H syndrome with proven SLC29A3 mutation (HES, 400x). F2 Histiocytes were OCT2 + (OCT2, 400x)
Eight samples of mixed histiocytosis combined a component of typical RDD histology, with another component corresponding to ECD (n = 5), LCH (n = 2), or JXG (n = 1). Samples with mixed ECD-RDD were perirenal, bone, and parotid gland biopsies. One patient had two biopsies: skin and nasal cavity. Female:male ratio was 1:6. In all cases of mixed ECD-RDD histiocytosis with double components on biopsy samples (5/5), OCT2 was strongly positive in the RDD component and negative in the ECD component (Fig. 2). One of these cases was S100- in both components. Three other cases had a clinical presentation of ECD (perirenal and periaortic infiltration, and pleural and pericardial effusion) with a RDD morphology on the perirenal, pleural, or testicular biopsy sample. In these cases, OCT2 was positive. Mixed ECD-RDD cases harbored MAP2K1 (n = 2) or KRAS (n = 2) mutations. In other cases, either there was no mutation detected, or no molecular test was performed. The two cases of mixed RDD-LCH (lymph nodes), and the case of RDD-JXG (skin) showed a strong positivity of OCT2 in the RDD component.
Biopsies of the 23 patients with ECD were from various tissues or organs, the most frequent including perirenal and retroperitoneal (n = 7), skin (n = 5), bone (n = 3), mesentery and peritoneum (n = 3), pleura (n = 3) samples. One of these ECD patients also had skin LCH involvement which was OCT2 + . One patient had two biopsies: bone and perirenal. Histiocytes were negative for OCT2 in all cases, except the mixed ECD-LCH and another case with peritoneal biopsy, which showed a weak and heterogeneous expression of OCT2. In this latter case, a mutation of MAP2K1 was detected (see Supplementary table).
Biopsies in 27 patients with LCH were mostly from bone (n = 16) and skin (n = 7) lesions. 40% (11/27) of cases showed immunoreactivity for OCT2, either strong (4/11) or weak and heterogenous (7/11) (Figs. 1 and 2). Intraepidermal dendritic cells on skin samples were negative for OCT2. The four cases with strong positivity for OCT2 harbored the following mutations: BRAF c.1799 T > A (n = 2), BRAF c.1458_1472del (n = 1), and MAP2K1 c.171G > C (n = 1) (see Supplementary table). The seven cases with weak positivity for OCT2 were mutated as follows: BRAF c.1799 T > A (n = 4), BRAF c.1511_1517 + 2dup (n = 1), MAP2K1 c.173_187del (n = 1), and one case did not show any mutation on NGS. The 14 cases negative for OCT2 showed the following molecular results: 6 cases with BRAF c.1799 T > A, 3 cases with other BRAF mutations (c.1455_1469del, c.1457_1471del, and c.1457_1477delinsCACCGT), three cases MAP2K1 mutated (c.159_176del, c.306_311del, and c.313_318del) and two cases wild type but tested only for BRAF V600E with digital PCR.
The remaining cases of L group histiocytosis (n = 6) were mostly represented by skin (n = 2) biopsies in patients where neither histology nor clinic/radiology allowed to classify them as LCH or ECD. 5/6 cases showed a strong positivity for OCT2 and 3 out of these 5 cases exhibited a MAP2K1 mutation on NGS, while two were BRAF mutated. The OCT2-negative case harbored both BRAF and KRAS mutation.
Biopsies of JXG were skin and soft tissue samples (n = 16). JXG were positive for OCT2 in 38% (6/16) of cases (1/6 with strong positive staining, 5/6 with weak and heterogeneous staining) (Figs. 1 and 2). Molecular findings in these cases were as follows: 2/6 cases OCT2 + were BRAF mutated (c.1799 T > A and c.1780G > A), while 3/6 did not show any mutation on NGS or RNA sequencing. In one case, the RNA quantity was insufficient. Cases which were OCT2- showed the following molecular results: five cases were non-mutated with NGS or RNA sequencing, two cases had NTRK fusions, and one case was KRAS mutated (c.35G > C). In two cases no molecular test was performed.
Three cases of reticulohistiocytosis, one case of hereditary progressive mucinous histiocytosis, one case of progressive nodular histiocytosis, and 3 cases of C-group NOS histiocytosis were tested for OCT2. It was positive in reticulohistiocytosis (3/3), but negative in progressive nodular histiocytosis (n = 1) and hereditary progressive mucinous histiocytosis (n = 1). OCT2 was negative in 2/3 cases of C group NOS histiocytosis.
Two cases of histiocytosis NOS were included in the study (one bone, one skin biopsy). Histological features did not allow to categorize the cases into one specific group, but the clinical presentation allowed the diagnosis of histiocytosis, although DNA and RNA histiocytosis-dedicated NGS did not reveal any mutation. One case was OCT2- and the other one was OCT2 + .
H syndrome-related histiocytosis
Among the 9 patients with H syndrome-related histiocytosis, 4 had typical RDD histology (parotid gland, nasal, and lymph node samples), one of which exhibited an LCH additional component. By contrast, the 5 other patients had biopsies with histiocyte infiltration, whose histology was not suggestive of RDD. Samples of these latter cases were from the skin (n = 4), soft tissue (n = 1), and pleura (n = 1) and had a diffuse low-density infiltration with normal-sized mononucleated histiocytes without emperipolesis and were thus considered as unclassified histiocytosis. One of these patients had two different biopsies (pleura and skin). All the 10 analyzed samples were positive for OCT2 (Fig. 2). The patient with mixed RDD-LCH showed a positivity of OCT2 in the RDD component and a negativity of OCT2 in the LCH component.
Granulomatous lymphadenitis
On the 16 cases of granulomatous lymphadenitis, epithelioid histiocytes of granulomas were OCT2-, while the B lymphocytes serving as internal control were positive (see Supplementary Fig. 2).
Discussion
RDD is defined by histology, with infiltration of large histiocytes with large clear nucleus centered by a large nucleolus, with abundant cytoplasm and frequent emperipolesis [1, 10]. Frequently, neoplastic histiocytes are in minority and they can be masked by an abundant background infiltration containing reactive histiocytes as well as numerous lymphocytes and plasma cells. Emperipolesis can also be sometimes difficult to spot. Thus, patients without lymph node involvement frequently undergo multiple biopsies before RDD diagnosis is achieved. Neoplastic histiocytes in RDD are CD163 + and S100 + . S100 must show both a nuclear and cytoplasmic staining in order to be considered positive in neoplastic histiocytes. However, sometimes, the interpretation of both CD163 and S100 can be difficult due to a cytoplasmic uneven staining. The sharp nuclear staining of OCT2 exclusively in neoplastic histiocytes is easier to interpret and it proved to be extremely useful to make a diagnosis of RDD. It is also of major interest to achieve definitive diagnosis of RDD of the rare S100 negative cases (Fig. 2).
H syndrome is an inherited condition caused by biallelic mutations in SLC29A3 gene, leading to various child-onset abnormalities, including deformities, auto-inflammatory disorders, and histiocytosis. There are overlapping features between H syndrome and familial Rosai-Dorfman disease, and histopathological analysis in skin lesions in H syndrome may show similar features with Rosai-Dorfman disease [14, 15]. Histiocytosis in H syndrome mainly affects the skin but it can involve other tissues such as nasal cavities. Some of the biopsies can also show other features combining variable proportion of fibrosis and small histiocytes that do not have the aspect of RDD [16]. We show in the present study that these atypical histiocyte infiltrations also express OCT2 (Fig. 2).
In pure Erdheim-Chester disease, OCT2 appeared to be negative in 91% of cases. Some patients with typical ECD, also have lesions with RDD histology. These cases are frequently males and harbor a MAP2K1 mutation [17]. In these cases, OCT2 can be positive. In all the mixed ECD-RDD cases that we tested, the RDD component was positive for OCT2.
The exact reason why OCT2 is expressed in both monocyte-macrophage and B-cell lineage is unknown. It was originally named by its ability of binding an octamer sequence in the promoter and enhancer region of the immunoglobulin gene [18]. OCT2 mRNA is transcripted from one single gene. Then, through a process of alternative splicing, the mRNA is able to generate multiple different protein variants, some with activating function and others with inhibitory function on transcription. It has been proven that in B lymphocytes, the OCT2 isoform (OCT2.1) has an activating effect on transcription due to a possession of C-terminal domain. On the other hand, in neuronal cells, the OCT2 isoforms (OCT2.4, OCT2.5) lack the C-terminal domain which has an inhibitory effect on transcription [19, 20]. Unfortunately, there is no data available indicating which isoform the Abcam antibody stains.
In skin lesions of LCH, the most important differential diagnosis is CD1a dendritic cell hyperplasia mimicking LCH (prurigo, arthropod bite reaction, spongiotic dermatitis, erythematous-squamous diseases, interface dermatitis) [21]. OCT2 expression might help in distinguishing between these two entities since it can be expressed in neoplastic histiocytes in LCH and it is negative in dendritic CD1a + cells. However, its testing should be further expanded on these inflammatory conditions.
As we noticed in our study, there was no correlation between molecular status and expression of OCT2 in LCH and JXG. Cases mutated for BRAF and MAP2K1 were present in OCT2 + cases (weak or strong staining) and in OCT2- as well (See Supplementary table).
Conclusion
OCT2 can be used as a nuclear marker for the diagnosis of Rosai-Dorfman disease with a sensitivity of 100% and a specificity of 74%. It can also be used to distinguish between different components in mixed histiocytoses with Rosai-Dorfman component since OCT2 is expressed in the RDD component.
Its expression can also be helpful to exclude ECD, in cases where differential diagnosis between ECD and other histiocytoses is difficult. Interestingly, OCT2 could direct the diagnosis towards H syndrome in cases of child-onset auto-inflammatory features or deformities accompanying atypical histiocyte tissue infiltration that do not exhibit RDD morphology. The expression of OCT2 is not rare in LCH and JXG and cannot be used to differentiate these entities from RDD. Finally, when a B-cell neoplasm is suspected, markers of B-cell such as PAX5 should be performed.
References
Emile JF, Cohen-Aubart F, Collin M et al (2021) Histiocytosis. Lancet 398(10295):157–170
Durham BH, Lopez Rodrigo E, Picarsic J et al (2019) Activating mutations in CSF1R and additional receptor tyrosine kinases in histiocytic neoplasms. Nat Med 25(12):1839–1842
Molho-Pessach V, Ramot Y, Camille F et al (2014) H syndrome: the first 79 patients. J Am Acad Dermatol 70(1):80–88
Yin L, Xu J, Li M et al (2016) Oct2 and Bob1 are sensitive and specific markers in lineage determination of B cell lymphomas with no expression of conventional B cell markers. Histopathology 69(5):775–783
Liu YZ, Dawson SJ, Latchman DS (1996) Alternative splicing of the Brn-3a and Brn-3b transcription factor RNAs is regulated in neuronal cells. J Mol Neurosci 7(1):77–85
Ravindran A, Goyal G, Go RS et al (2021) Rosai-Dorfman disease displays a unique monocyte-macrophage phenotype characterized by expression of OCT2. Am J Surg Pathol 45(1):35–44
Kiruthiga KG, Younes S, Natkunam Y (2022) Strong coexpression of transcription factors PU.1 and Oct-2 in Rosai-Dorfman disease. Am J Clin Pathol 158(6):672–677
Kemps PG, Picarsic J, Durham BH et al (2022) ALK-positive histiocytosis: a new clinicopathologic spectrum highlighting neurologic involvement and responses to ALK inhibition. Blood 139(2):256–280
Wang E, Hutchinson CB, Huang Q et al (2010) Histiocytic sarcoma arising in indolent small B-cell lymphoma: report of two cases with molecular/genetic evidence suggestive of a “transdifferentiation” during the clonal evolution. Leuk Lymphoma 51(5):802–812
Emile JF, Abla O, Fraitag S et al (2016) Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood 127(22):2672–2681
Morgan NV, Morris MR, Cangul H et al (2010) Mutations in SLC29A3, encoding an equilibrative nucleoside transporter ENT3, cause a familial histiocytosis syndrome (Faisalabad histiocytosis) and familial Rosai-Dorfman disease. PLoS Genet 6(2):e1000833
Emile JF, Diamond EL, Hélias-Rodzewicz Z et al (2014) Recurrent RAS and PIK3CA mutations in Erdheim-Chester disease. Blood 124(19):3016–3019
Melloul S, Hélias-Rodzewicz Z, Cohen-Aubart F et al (2019) Highly sensitive methods are required to detect mutations in histiocytoses. Haematologica 104(3):e97–e99
Bolze A, Abhyankar A, Grant AV et al (2012) A mild form of SLC29A3 disorder: a frameshift deletion leads to the paradoxical translation of an otherwise noncoding mRNA splice variant. PLoS One 7(1):e29708
Chouk H, Ben Rejeb M, Boussofara L et al (2021) Phenotypic intrafamilial variability including H syndrome and Rosai-Dorfman disease associated with the same c.1088G > A mutation in the SLC29A3 gene. Hum Genomics 15:63
Doviner V, Maly A, Ne’eman Z et al (2010) H syndrome: recently defined genodermatosis with distinct histologic features. A morphological, histochemical, immunohistochemical, and ultrastructural study of 10 cases. Am J Dermatopathol 32(2):118–28
Razanamahery J, Diamond EL, Cohen-Aubart F et al (2020) Erdheim-Chester disease with concomitant Rosai-Dorfman like lesions: a distinct entity mainly driven by MAP2K1. Haematologica 105(1):e5–e8
Latchman DS (1996) The Oct-2 transcription factor. Int J Biochem Cell Biol 28(10):1081–1083
Lillycrop KA, Latchman DS (1992) Alternative splicing of the Oct-2 transcription factor RNA is differentially regulated in neuronal cells and B cells and results in protein isoforms with opposite effects on the activity of octamer/TAATGARAT-containing promoters. J Biol Chem 267(35):24960–24965
Lillycrop KA, Estridge JK, Latchman DS (1993) The octamer binding protein Oct-2 inhibits transactivation of the herpes simplex virus immediate-early genes by the virion protein Vmw65. Virology 196(2):888–891
Fraitag S, Emile JF (2022) Cutaneous histiocytoses in children. Histopathology 80(1):196–215
Funding
This article was supported by the Programme de Recherche Translationnelle sur le Cancer from the French National Cancer Institute [PRT-K19-143] and by unrestricted grants from the non-profit association AREP.
IAU received support from the European Society of Pathology for this work.
Author information
Authors and Affiliations
Contributions
IAU and JFE wrote de manuscript. JFE obtained funding. All co-authors contributed to collection and analysis of data, and correction of the manuscript. All co-authors accepted the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The article is an original work. The study (NCT04437381) has been approved by the ethical committee CESREES #2814848bis. Patients signed informed consent for translational research. This work was presented in part during the 38th annual meeting of the Histiocyte Society (Stockholm, September 2022).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ungureanu, I.A., Cohen-Aubart, F., Héritier, S. et al. OCT2 expression in histiocytoses. Virchows Arch 483, 81–86 (2023). https://doi.org/10.1007/s00428-023-03508-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-023-03508-7