Abstract
In the modern era of precision medicine, a number of class II immunohistochemistry (IHC) biomarkers are routinely tested in pathologic laboratories to select cancer patients who may be candidates for hormonal, targeted, and immune therapies. Pre-analytical factors, including tissue processing, are critical components of biomarker testing and require validation to ensure reliable results. In this study, we aimed to study the impact of tissue processing on biomarkers (including ER, PR, HER2, mismatch repair (MMR) proteins, BRAF V600E, and PD-L1) in a large prospective cohort of 109 tumors. We found that ER and MMR were not impacted; PR, HER2, and BRAF V600E were minimally affected; and PD-L1 regardless of the antibody clone was strongly influenced by a combination of tissue processing procedures and intratumoral heterogeneity. Our findings suggest that validation of pre-analytical parameters, such as tissue processing, is important for certain class II biomarkers, in particular PD-L1 IHC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the era of precision medicine, an expanding list of immunohistochemical (IHC) markers have become class II biomarkers, i.e., they provide prognostic and predictive information to select eligible patients who may benefit from hormonal, targeted, and/or immune therapies [1, 2]. These IHC biomarkers are increasingly and routinely tested in pathology laboratories worldwide.
In order to achieve high inter-laboratory concordance, a number of guidelines have been developed in recent years aiming to standardize the essential pre-analytical, analytical, and post-analytical components of IHC [3,4,5,6]. Any change in these critical components requires full re-validation of the IHC protocol [3]. For any modification in the pre-analytical phase, the validation set implicates prospective procurement of paired tissue samples to allow comparison of the IHC results across various pre-analytical conditions.
Recent advances in targeted therapies and immuno-oncology as well as the approval of companion or complementary IHC biomarkers have placed biomarker testing and interpretation under scrutiny. An example of such a biomarker is programmed death-ligand 1 (PD-L1) IHC as multiple commercial antibody clones exist and the positive criteria vary significantly depending on antibody clone and cancer type [7]. Additionally, it has been shown that PD-L1 expression is subject to tumor heterogeneity, staining inconsistency among different antibody clones, and inter- and intra-observer variability [7,8,9,10,11]. To date, the impact of pre-analytical factors on PD-L1 expression has been underexplored [12].
In this study, we aimed to assess the effect of tissue processing on the immunoexpression of several commonly used class II biomarkers including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), mismatch repair proteins (MMR), and PD-L1 using a large prospectively collected cohort of 109 tumors of various types. Two commonly available tissue processors and four different processing protocols were compared to determine the effects of tissue processing on IHC assessed using categorical, semiquantitative, and quantitative measurements.
Material and methods
Tissue procurement and processing
The study was approved by the institutional research ethics board. Formalin fixed tissue from 109 tumor resections was prospectively collected and processed using two tissue processors: Pathos delta (Milestone Medical, Kalamazoo, MI, USA) and Leica ASP330S (Leica Biosystems Inc., Concord, Ontario, Canada). The following four tissue protocols were tested: protocol 1 (P1)—rapid protocol for biopsies on Pathos, P2—routine overnight processing protocol on Pathos, P3—protocol for fat-rich tissue on Pathos, and P4—for fat-rich tissue on Leica. Detailed protocols are provided in Table 1. For breast cases, the cold ischemic time was less than 1 h and fixation time was 24–96 h (as for the remaining cases, while these times were not specifically recorded, most cases in our lab are processed in similar manner). The 109 consecutive tumor resections were procured including colorectal carcinomas (n = 28), breast carcinomas (n = 22), renal tumors (n = 21), head and neck squamous cell carcinomas (n = 13), melanomas (n = 11), bladder urothelial carcinomas (n = 11), endometrial carcinoma (n = 1), Merkel cell carcinoma (n = 1), and papillary thyroid carcinoma (n = 1). The procured tissue was size appropriate for each protocol, i.e., for P1 tissue size mimicked that obtained by a core biopsy of 1–3 mm in diameter, for the remaining protocols—tissue size was up to a nickel coin size and 2–3-mm thick depending on tumor availability.
IHC: staining and interpretation
Following processing, tissue microarrays (TMA) were created using triplicate 1-mm cores to account for tumor heterogeneity. Sequential sections from each block were stained with the following class II IHC markers: PD-L1 clones SP263, SP142 and 22C3, ER, PR, HER2, MMR proteins (MLH1, PMS2, MSH2 and MSH6), and BRAF V600E. Details of the antibodies utilized are summarized in Table 2.
The listed biomarkers were scored in the following tumors: PD-L1—all tumors and all clones, ER/PR/HER2—breast carcinoma only, MMR—colorectal and endometrial carcinoma only, BRAF V600E—melanoma and papillary thyroid carcinoma only. IHC results from all 3 cores from each tumor were scored and averaged using the following scoring algorithms.
PD-L1 immunopositivity for urothelial, breast, and head and neck squamous cell carcinomas was determined using available algorithms as described in Table 3. These thresholds were established according to clinical responses to the associated immune checkpoint inhibitors in various clinical trials for urothelial carcinoma [13,14,15,16], head and neck squamous cell carcinomas [17,18,19,20,21], and breast carcinoma [22, 23]. In brief, the combined positive score (CPS) was defined as the number of PD-L1 positive tumor cells (TC) and immune cells (IC) divided by total number of TC × 100. PD-L1 22C3 was considered to be positive if CPS ≥ 10% in urothelial carcinoma or ≥ 1% in breast or head and neck squamous cell carcinomas; PD-L1 SP142 was deemed positive if IC ≥ 5% in urothelial and head and neck squamous cell carcinomas, or IC ≥ 1% in breast carcinoma; whereas SP263 was determined to be positive when ≥ 25% TCs or ICs were stained in urothelial carcinoma or ≥ 25% of TCs were stained in head and neck squamous cell carcinoma. The threshold of PD-L1 positivity for SP263 clone in breast carcinoma has yet to be determined. Additionally, we applied a universal semi-quantitative scoring system using a six-tiered cut-off for positive TC%, positive IC%, and CPS for all tumors: (0) < 1% TC/IC or CPS < 1; (1) 1–4.9% TC/IC or 1–4.9 CPS; (2) 5–9.9% TC/IC or CPS 5–9.9; (3) 10–24.9% TC/IC or CPS 10–24.9; (4) 25–49.9% TC/IC or CPS 25–49.9; and (5) ≥ 50% TC/IC or CPS ≥ 50.
For ER, PR, and HER2, scoring was performed according to the latest American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [24, 25]. In brief, for ER and PR, 1% cut off was used, and for HER2, the cut off for positivity was > 10% of tumor cells with strong complete membranous staining. The percentage of positive tumors cells was additionally recorded for ER and PR.
BRAF V600E was considered positive if moderate to strong granular cytoplasmic staining was seen in virtually all tumor cells. Mismatch repair deficiency was defined as absence of staining of MMR in virtually all tumor nuclei with an acceptable internal control. BRAF V600E mutation status in melanoma and HER2 amplification status in breast carcinoma were confirmed from the patient record.
Statistical analyses
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, USA). Fleiss’ kappa analyses were performed to determine the concordance among different platforms for each class II biomarker. p values less than 0.05 were considered to be statistically significant.
Results
PD-L1
The percent PD-L1 immunopositivity and concordance using clinical algorithms for urothelial, breast, and head and neck squamous cell carcinomas are shown in Fig. 1. The percent of PD-L1 positivity in urothelial carcinoma was 9–27% using SP263, 18–27% using 22C3, and 0–18% using SP142 clone. No urothelial carcinoma case was universally positive across all clones and platforms, whereas 4 (36%) were consistently negative. For breast carcinoma, PD-L1 immunopositivity was seen in 48–62% of cases using 22C3 and 24–40% using SP142. For head and neck squamous cell carcinoma, the positive rate was 0–31% for SP263, 54–85% for 22C3, and 8–23% for SP142. The number of cases that was universally positive and negative was 0 and 1 (8%), respectively.
There was substantial agreement among platforms using SP263 clone in urothelial carcinoma (kappa = 0.614), moderate agreement using 22C3 in breast carcinoma (kappa = 0.402), and fair agreement using SP142 in breast carcinoma (kappa =0.392), as well as using SP263, SP142, and 22C3 in head and neck squamous cell carcinoma (kappa = 0.357, 0.347, and 0.261 respectively). The kappa values across platform for 22C3 and SP142 in urothelial carcinoma did not reach significance (p > 0.05).
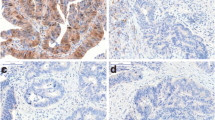
The results using a universal six-tiered cutoff are shown in Fig. 2. Overall, there was a fair agreement among the four platforms tested using this 6-tiered approach regardless of antibody clones and/or scoring methods (TC%, IC%, or CPS). The kappa values ranged from 0.248 to 0.354. Figure 3 illustrates the differences in PD-L1 expression between protocols.
Comparison of the differences in PD-L1 expression between protocols. First column, head and neck squamous cell carcinoma (HNSCC) case 5, clone 22C3 (negative, CPS < 1, with protocol 4, positive, CPS ≥ 1%, with the remaining protocols). Second column, breast carcinoma case 14, clone SP142 (positive, IC ≥ 1%, with protocols 1 and 3, negative, IC < 1%, with 2 and 4). Column 3, urothelial carcinoma case 4, clone SP263 (positive, TC or IC ≥ 25%, with protocol 2, negative, TC or IC < 25%, with the remaining protocols). Scale bar: 200 microns
ER, PR, and HER2 in breast carcinoma
The performance of ER, PR, and HER2 IHC is shown in Fig. 4. There was perfect concordance for ER (kappa = 1.000) with a frequency of ER immunopositivity of 77%. All positive cases showed diffuse ER positivity in 100% of tumor cells.
Comparison of the performance of other class II biomarkers across four procession platforms. NA, not available; PTC, papillary thyroid carcinoma. The numbers in ER and PR represent the actual percentage of positive tumor cells. The positive percentage for BRAF V600E is calculated for melanoma cases only
There was a substantial agreement of PR immunostaining across the platforms (kappa = 0.695). The rate of PR immunopositivity ranged from 55 to 62%. Four breast cancers (18%) showed discrepant PR results, in which the percentage of PR positivity ranged from 0 to 40%.
The concordance of HER2 IHC was substantial (kappa = 0.787). Two (10%) and 14 (64%) cases were interpreted as HER2 positive and negative respectively across all four platforms. Six cases were interpreted as HER2 equivocal in at least one of the platforms tested. Protocol P4 resulted in more equivocal cases (27%) compared with the other 3 protocols. The two cases that were equivocal across all four platforms were subjected to HER2 amplification testing by FISH, one of which showed HER2 amplification whereas the other was not HER2 amplified.
BRAF V600E
BRAF V600E IHC was evaluated in 1 papillary thyroid carcinoma and 11 melanomas (Fig. 4). There was near perfect concordance (kappa =0.925). Three melanomas (27%) and the papillary thyroid carcinoma were consistently positive for BRAF V600E, whereas 7 melanomas (64%) were negative across platforms. There was one case of melanoma showing BRAF V600E positivity using protocol P3 (which was the correct protocol for tissue type) and was interpreted as equivocal with weak granular cytoplasmic staining using the other three protocols. This case together with the 3 BRAF V600E-positive melanoma cases was shown to contain BRAF V600E mutation per chart review.
MMR
A perfect concordance with a kappa value of 1.000 was achieved for all MMR markers in colorectal (n = 24) and endometrial carcinoma (n = 1, Fig. 4). The rate of MMR deficiency was 8% (2/25) for MLH1, 20% (5/25) for PMS2, and 0% for MSH2 and MSH6.
Discussion
Among the class II biomarkers tested, we found that ER and MMR IHC were not impacted by processing; PR, HER2, and BRAF V600E were minimally affected by processing with strong correlation among platforms, whereas PD-L1 (regardless of antibody clones used) was strongly influenced by processing protocols.
It is known that the interpretation of HER2 and PD-L1 IHC is influenced by both intratumoral heterogeneity [7, 11, 26,27,28] and interobserver variability [9, 11, 29, 30]. Therefore, the different results across the platforms observed in PD-L1 and HER2 may be in part a result of intratumoral heterogeneity given the TMA cores were sampled from different areas of each tumor. On the other hand, as we used serial sections from the same TMA block from each processing platform to perform a panel of IHC, the performance of PD-L1 antibody should not be affected by intratumoral heterogeneity. Additionally, the IHC of various clones and platforms were interpreted and scored by the same pathologist to avoid the impact of interobserver variability. Other pre-analytical parameters, e.g., fixation and IHC protocol, remained the same. Therefore, it is reasonable to conclude that the difference in staining and interpretation across platforms is attributable to the variable tissue processing.
It has been shown that pre-analytical variables, e.g., cold ischemic time and fixation time, have a significant impact on the performance and interpretation of biomarkers in breast cancer [31,32,33]. Therefore, the current ASCO/CAP guidelines mandate documentation and standardization of these parameters when handling a breast cancer specimen [24, 25]. Little is known about the influence of tissue processing on biomarker IHC. Sujoy et al. compared ER immunostain between conventional and rapid processing assays using semi-quantitative Q scores, and found the ER results to be identical [34]. Bulte et al. showed that accelerated tissue processing had no significant impact on HER2 status [35]. In the current study, we evaluated both the categorical classification based on ASCO/CAP guidelines and the actual percentage of ER and PR expression. Consistent with what has been previously reported, we found that the processing platform has minimal if any impact on ER, PR, and HER2 interpretation. Together, these results show that rapid protocol and protocols designed for fatty tissue are suitable for biomarker evaluation in BC.
In the current study, we also evaluated the performance of other class II biomarkers. Overall, processing appeared to have no or minimal impact on BRAF V600E in melanoma and PTC, as well as MMR in colorectal and endometrial carcinoma.
The biomarker that appeared most impacted by tissue processing was PD-L1. The variation of PD-L1 staining was observed across all tumor types tested using either the clinical algorithms or a semiquantitative scoring scheme. Several recent studies have shown the impact of preanalytical variables on PD-L1 expression. For example, the type of decalcification agent impacts PD-L1 results for 22C3 clone, but not E1L3N clone [36]. Delayed fixation has been shown to decrease PD-L1 expression in the study by Van Seijen et al. [37], but to have no apparent impact on PD-L1 IHC in the study by Forest et al. [36]. Prolonged fixation does not appear to affect PD-L1 results [37]. In this study, we reported that processing protocol and platform have a significant impact on PD-L1 IHC. Therefore, validation and standardization of preanalytical variables, including tissue processing, should be considered in PD-L1 testing in a clinical laboratory.
Conclusions
Aside from PD-L1, other class II IHC biomarkers, e.g., ER, PR, HER2, MMR, and BRAF V600E, show perfect or high concordance of read out using different tissue processors and processing protocols. However, for PD-L1, the staining and interpretation are strongly influenced by a combination of tissue processing procedures and intratumoral heterogeneity. Optimization and validation of pre-analytical processes including processing protocols are essential for correct PD-L1 biomarker interpretation.
References
Torlakovic EE, Riddell R, Banerjee D, El-Zimaity H, Pilavdzic D, Dawe P, Magliocco A, Barnes P, Berendt R, Cook D, Gilks B, Williams G, Perez-Ordonez B, Wehrli B, Swanson PE, Otis CN, Nielsen S, Vyberg M, Butany J (2010) Canadian Association of Pathologists-Association canadienne des pathologistes National Standards Committee/Immunohistochemistry: best practice recommendations for standardization of immunohistochemistry tests. Am J Clin Pathol 133(3):354–365. https://doi.org/10.1309/ajcpdyz1xmf4hjwk
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R (2020) Review of precision cancer medicine: evolution of the treatment paradigm. Cancer Treat Rev 86:102019. https://doi.org/10.1016/j.ctrv.2020.102019
Torlakovic EE, Cheung CC, D’Arrigo C, Dietel M, Francis GD, Gilks CB, Hall JA, Hornick JL, Ibrahim M, Marchetti A, Miller K, van Krieken JH, Nielsen S, Swanson PE, Vyberg M, Zhou X, Taylor CR (2017) Evolution of quality assurance for clinical immunohistochemistry in the era of precision medicine. Part 3: Technical validation of immunohistochemistry (IHC) assays in clinical IHC laboratories. Appl Immunohistochem Mol Morphol: AIMM 25(3):151–159. https://doi.org/10.1097/pai.0000000000000470
Torlakovic EE, Cheung CC, D’Arrigo C, Dietel M, Francis GD, Gilks CB, Hall JA, Hornick JL, Ibrahim M, Marchetti A, Miller K, van Krieken JH, Nielsen S, Swanson PE, Vyberg M, Zhou X, Taylor CR (2017) Evolution of quality assurance for clinical immunohistochemistry in the era of precision medicine - Part 2: Immunohistochemistry test performance characteristics. Appl Immunohistochem Mol Morphol: AIMM 25(2):79–85. https://doi.org/10.1097/pai.0000000000000444
Cheung CC, D’Arrigo C, Dietel M, Francis GD, Fulton R, Gilks CB, Hall JA, Hornick JL, Ibrahim M, Marchetti A, Miller K, van Krieken JH, Nielsen S, Swanson PE, Taylor CR, Vyberg M, Zhou X, Torlakovic EE (2017) Evolution of quality assurance for clinical immunohistochemistry in the era of precision medicine: Part 4: Tissue tools for quality assurance in immunohistochemistry. Appl Immunohistochem Mol Morphol: AIMM 25(4):227–230. https://doi.org/10.1097/pai.0000000000000469
Cheung CC, D’Arrigo C, Dietel M, Francis GD, Gilks CB, Hall JA, Hornick JL, Ibrahim M, Marchetti A, Miller K, van Krieken JH, Nielsen S, Swanson PE, Taylor CR, Vyberg M, Zhou X, Torlakovic EE (2017) Evolution of quality assurance for clinical immunohistochemistry in the era of precision medicine: Part 1: Fit-for-purpose approach to classification of clinical immunohistochemistry biomarkers. Appl Immunohistochem Mol Morphol: AIMM 25(1):4–11. https://doi.org/10.1097/pai.0000000000000451
Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, Dietel M, Longshore JW, Lopez-Rios F, Penault-Llorca F, Viale G, Wotherspoon AC, Kerr KM, Tsao MS (2017) Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol Off J Am Soc Clin Oncol 35(34):3867–3876. https://doi.org/10.1200/jco.2017.74.7642
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B, Mistry A, Kalamegham R, Averbuch S, Novotny J, Rubin E, Emancipator K, McCaffery I, Williams JA, Walker J, Longshore J, Tsao MS, Kerr KM (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12(2):208–222. https://doi.org/10.1016/j.jtho.2016.11.2228
Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, Bubendorf L, Chirieac L, Chen G, Chou TY, Chung JH, Dacic S, Lantuejoul S, Mino-Kenudson M, Moreira AL, Nicholson AG, Noguchi M, Pelosi G, Poleri C, Russell PA, Sauter J, Thunnissen E, Wistuba I, Yu H, Wynes MW, Pintilie M, Yatabe Y, Hirsch FR (2018) PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 13(9):1302–1311. https://doi.org/10.1016/j.jtho.2018.05.013
Hodgson A, Slodkowska E, Jungbluth A, Liu SK, Vesprini D, Enepekides D, Higgins K, Katabi N, Xu B, Downes MR (2018) PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol In press 42:1059–1066
Wang C, Hahn E, Slodkowska E, Eskander A, Enepekides D, Higgins K, Vesprini D, Liu SK, Downes MR, Xu B (2018) Reproducibility of PD-L1 immunohistochemistry interpretation across various types of genitourinary and head/neck carcinomas, antibody clones, and tissue types. Hum Pathol 82:131–139. https://doi.org/10.1016/j.humpath.2018.07.024
Lantuejoul S, Sound-Tsao M, Cooper WA, Girard N, Hirsch FR, Roden AC, Lopez-Rios F, Jain D, Chou TY, Motoi N, Kerr KM, Yatabe Y, Brambilla E, Longshore J, Papotti M, Sholl LM, Thunnissen E, Rekhtman N, Borczuk A, Bubendorf L, Minami Y, Beasley MB, Botling J, Chen G, Chung JH, Dacic S, Hwang D, Lin D, Moreira A, Nicholson AG, Noguchi M, Pelosi G, Poleri C, Travis W, Yoshida A, Daigneault JB, Wistuba II, Mino-Kenudson M (2020) PD-L1 testing for lung cancer in 2019: perspective from the IASLC Pathology Committee. J Thorac Oncol 15(4):499–519. https://doi.org/10.1016/j.jtho.2019.12.107
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicenter, phase 2 trial. Lancet (London, England) 387(10031):1909–1920. https://doi.org/10.1016/s0140-6736(16)00561-4
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, S-l T, Shen X, Boyd Z, Hegde PS, Chen DS, Vogelzang NJ (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515(7528):558–562. https://doi.org/10.1038/nature13904
Powles T, O’Donnell PH, Massard C, Arkenau HT, Friedlander TW, Hoimes CJ, Lee JL, Ong M, Sridhar SS, Vogelzang NJ, Fishman MN, Zhang J, Srinivas S, Parikh J, Antal J, Jin X, Gupta AK, Ben Y, Hahn NM (2017) Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: updated results from a phase 1/2 open-label study. JAMA Oncol 3(9):e172411. https://doi.org/10.1001/jamaoncol.2017.2411
Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, O’Donnell PH, Drakaki A, Tan W, Kurland JF, Rebelatto MC, Jin X, Blake-Haskins JA, Gupta A, Segal NH (2016) Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol Off J Am Soc Clin Oncol 34(26):3119–3125. https://doi.org/10.1200/jco.2016.67.9761
Tahara M, Muro K, Hasegawa Y, Chung HC, Lin CC, Keam B, Takahashi K, Cheng JD, Bang YJ (2018) Pembrolizumab in Asia-Pacific patients with advanced head and neck squamous cell carcinoma: analyses from KEYNOTE-012. Cancer Sci 109(3):771–776. https://doi.org/10.1111/cas.13480
Cohen EEW, Soulieres D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet (London, England) 393(10167):156–167. https://doi.org/10.1016/s0140-6736(18)31999-8
De Meulenaere A, Vermassen T, Aspeslagh S, Huvenne W, Van Dorpe J, Ferdinande L, Rottey S (2017) Turning the tide: clinical utility of PD-L1 expression in squamous cell carcinoma of the head and neck. Oral Oncol 70:34–42. https://doi.org/10.1016/j.oraloncology.2017.05.002
Segal NH, Ou S-HI, Balmanoukian AS, Fury MG, Massarelli E, Brahmer JR, Weiss J, Schoffski P, Antonia SJ, Massard C, Zandberg DP, Khleif S, Li X, Rebelatto M, Steele K, Robbins PB, Blake-Haskins JA, Butler MO (2015) Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J Clin Oncol 33(15_suppl):3011–3011. https://doi.org/10.1200/jco.2015.33.15_suppl.3011
Colevas AD, Bahleda R, Braiteh F, Balmanoukian A, Brana I, Chau NG, Sarkar I, Molinero L, Grossman W, Kabbinavar F, Fasso M, O’Hear C, Powderly J (2018) Safety and clinical activity of atezolizumab in head and neck cancer: results from a phase I trial. Ann Oncol Off J Eur Soc Med Oncol 29(11):2247–2253. https://doi.org/10.1093/annonc/mdy411
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, Hui R, Curigliano G, Toppmeyer D, O’Shaughnessy J, Loi S, Paluch-Shimon S, Tan AR, Card D, Zhao J, Karantza V, Cortes J (2018) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase 2 KEYNOTE-086 study. Ann Oncol Off J Eur Soc Med Oncol 30:397–404. https://doi.org/10.1093/annonc/mdy517
Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang CW, Zhang W, Iizuka K, Foster PG, Molinero L, Funke R, Powderly J (2018) Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol 5:334–342. https://doi.org/10.1001/jamaoncol.2018.5152
Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, Hayes DF, Lakhani SR, Chavez-MacGregor M, Perlmutter J, Perou CM, Regan MM, Rimm DL, Symmans WF, Torlakovic EE, Varella L, Viale G, Weisberg TF, McShane LM, Wolff AC (2020) Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol Off J Am Soc Clin Oncol Jco1902309. https://doi.org/10.1200/jco.19.02309
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med 142(11):1364–1382. https://doi.org/10.5858/arpa.2018-0902-SA
Wolff AC, MEH H, Allison KH, Harvey BE, Mangu PB, JMS B, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol Off J Am Soc Clin Oncol 36(20):2105–2122. https://doi.org/10.1200/jco.2018.77.8738
Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, Cross JV, Dillon PM, Mills AM (2017) PD-L1 expression and intratumoral heterogeneity across breast cancer subtypes and stages: an assessment of 245 primary and 40 metastatic tumors. Am J Surg Pathol 41(3):334–342. https://doi.org/10.1097/pas.0000000000000780
Li M, Li A, Zhou S, Xu Y, Xiao Y, Bi R, Yang W (2018) Heterogeneity of PD-L1 expression in primary tumors and paired lymph node metastases of triple negative breast cancer. BMC Cancer 18(1):4. https://doi.org/10.1186/s12885-017-3916-y
Brunnstrom H, Johansson A, Westbom-Fremer S, Backman M, Djureinovic D, Patthey A, Isaksson-Mettavainio M, Gulyas M, Micke P (2017) PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol 30(10):1411–1421. https://doi.org/10.1038/modpathol.2017.59
Cooper WA, Russell PA, Cherian M, Duhig EE, Godbolt D, Jessup PJ, Khoo C, Leslie C, Mahar A, Moffat DF, Sivasubramaniam V, Faure C, Reznichenko A, Grattan A, Fox SB (2017) Intra- and interobserver reproducibility assessment of PD-L1 biomarker in non-small cell lung cancer. Clin Cancer Res 23(16):4569–4577. https://doi.org/10.1158/1078-0432.ccr-17-0151
Pekmezci M, Szpaderska A, Osipo C, Ersahin C (2012) The effect of cold ischemia time and/or formalin fixation on estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 results in breast carcinoma. Pathol Res Int 2012:947041–947047. https://doi.org/10.1155/2012/947041
Li X, Deavers MT, Guo M, Liu P, Gong Y, Albarracin CT, Middleton LP, Huo L (2013) The effect of prolonged cold ischemia time on estrogen receptor immunohistochemistry in breast cancer. Mod Pathol 26(1):71–78. https://doi.org/10.1038/modpathol.2012.135
Neumeister VM, Anagnostou V, Siddiqui S, England AM, Zarrella ER, Vassilakopoulou M, Parisi F, Kluger Y, Hicks DG, Rimm DL (2012) Quantitative assessment of effect of preanalytic cold ischemic time on protein expression in breast cancer tissues. J Natl Cancer Inst 104(23):1815–1824. https://doi.org/10.1093/jnci/djs438
Sujoy V, Nadji M, Morales AR (2014) Brief formalin fixation and rapid tissue processing do not affect the sensitivity of ER immunohistochemistry of breast core biopsies. Am J Clin Pathol 141(4):522–526. https://doi.org/10.1309/ajcpo7z4sfiydsxn
Bulte JP, Halilovic A, Kalkman S, van Cleef PHJ, van Diest PJ, Strobbe LJA, de Wilt JHW, Bult P (2018) Assessment of HER2 status in breast cancer biopsies is not affected by accelerated tissue processing. Histopathology 73(1):81–89. https://doi.org/10.1111/his.13507
Forest F, Cote G, Laville D, Da Cruz V, Dal Col P, Camy F, Mobarki M, Clemenson A, Yvorel V, Peoc’h M (2019) Impact of delayed fixation and decalcification on PD-L1 expression: a comparison of two clones. Virchows Archiv Int J Pathol 475(6):693–699. https://doi.org/10.1007/s00428-019-02613-w
van Seijen M, Brcic L, Gonzales AN, Sansano I, Bendek M, Brcic I, Lissenberg-Witte B, Korkmaz HI, Geiger T, Kammler R, Stahel R, Thunnissen E (2019) Impact of delayed and prolonged fixation on the evaluation of immunohistochemical staining on lung carcinoma resection specimen. Virchows Archiv Int J Pathol 475(2):191–199. https://doi.org/10.1007/s00428-019-02595-9
Funding
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
Author information
Authors and Affiliations
Contributions
Bin Xu managed the database, scored PD-L1, performed statistics, and drafted the manuscript.
Samira Alminawi performed the immunohistochemistry and drafted Table 2.
Patrice Boulianne and Yan Ming Shang performed tissue processing and drafted Table 1.
Michelle R. Downes participated in the study design, scored PD-L1, and edited the manuscript.
Elzbieta Slodkowska designed the study, scored all immunohistochemistry, and edited the manuscript.
Corresponding author
Ethics declarations
The manuscript complied with the Ethical Standards.
Conflict of interest
Dr. Downes has been an advisory board member for Astra Zeneca and Hoffman La Roche and has received speaker’s honoraria from Astra Zeneca. All other authors have no disclosures. The SP263 antibody was purchased using a Sunnybrook Health Sciences Centre departmental Educational Grant courtesy of Astra Zeneca.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Quality in Pathology
Rights and permissions
About this article
Cite this article
Xu, B., Alminawi, S., Boulianne, P. et al. The impact of pre-analytical parameters on class II biomarkers by immunohistochemistry: concordance across four tissue processing protocols. Virchows Arch 478, 985–993 (2021). https://doi.org/10.1007/s00428-020-02960-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02960-z