Abstract
Intestinal-type adenocarcinoma (ITAC) of sinonasal tract is a rare malignant tumor with strong morphological, immunophenotypical, and molecular similarities to colorectal adenocarcinoma (CRC). Tumor budding (TB) is a well-established adverse prognostic marker in CRC and some head and neck tumors, with features of epithelial-mesenchymal transition (EMT). The aim of this study was to assess TB in ITAC and to evaluate its possible association with EMT markers in this setting. We selected 32 surgically resected specimens of non-mucinous/non-signet ring ITAC and evaluated them for TB according to the international recommendations developed for CRC. The expression of the EMT markers E-cadherin, ZEB1, ZEB2, SLUG, and SNAIL was evaluated by immunohistochemistry (IHC). Results were stratified using clinical and follow-up data (2/32 patients had metastatic disease and 4/32 died of disease). We observed TB in 13/32 (40.6%) ITAC cases including the 7 patients with relapse (p = 0.0005) and the 4 patients dead of disease (p = 0.02). Lymphovascular invasion was associated with TB (p = 0.008). Absence of TB was associated with low ZEB2 expression (p = 0.003). No other association with EMT markers emerged. Occupational exposure to wood and leather dust was not related to the presence of TB. TB interobserver concordance was substantial (proportion of agreement = 87%; Cohen’s kappa = 0.73). This work suggests that TB is associated with a worse prognosis in ITAC, but our findings do not seem to support the involvement of EMT in this specific setting. Further larger studies are needed to address this point.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal-type adenocarcinoma (ITAC) of the sinonasal tract is an uncommon neoplasm with an incidence of less than 1 case/100,000/year, variation depending on the examined population [1, 2]. Men are more affected than women, due to the occupational exposure to well-known etiological factors such as wood and leather dust, and other chemical agents, which increase the risk of hundreds of times than in non-exposed population [2, 3]. ITAC resembles colorectal adenocarcinoma (CRC) morphologically, immunophenotypically, and in part molecularly [1, 4, 5].
Tumor budding (TB) is a morphological feature, associated to adverse prognosis in many tumor types [6,7,8] and most of the experience with TB is derived from studies in gastrointestinal tract [7, 9,10,11,12,13]. In CRC, TB is usually associated with high tumor grade; advanced stage, lymphovascular invasion (LVI); nodal and distant metastasis; locoregional and distant recurrence; and worse overall, disease-free and recurrence-free survival [9,10,11,12]. The prognostic value of TB is conserved independently from the method applied for its assessment [10, 11, 13]. In CRC, the International Tumor Budding Consensus Conference (ITBCC) proposed a definition of TB, along with an assessment and scoring method [14], which has been recently validated [15]. This agreement is essential to compare study results and implement TB assessment in routine diagnostics [15]. In head and neck region, TB is an adverse prognostic marker in oral [16], tongue [17], nasopharyngeal [18], hypopharyngeal [19], laryngeal [20], and cutaneous [21] squamous cell carcinoma (SCC), but several definitions of TB have been applied [8].
Epithelial-mesenchymal transition (EMT) is the reversible biologic process that allows epithelial polarized cells to undergo multiple changes, which result in mesenchymal cell phenotype. During this process, epithelial cells lose their polarity and cell-cell adhesion, and gain migratory and invasive properties, resistance to anoikis/apoptosis [22, 23], and increase production of extracellular matrix component [24]. EMT is involved in development, wound healing (inflammation and fibrosis), and cancer progression and metastases [24]. Given the above mentioned properties, it is reasonable that TB might present complete or partial EMT [9, 25], as reported in CRC [13, 26], oral SCC [16], and many others [7, 27].
The aim of this retrospective study was to assess TB and to explore its role as a clinicopathologic prognosticator in a series of ITAC and evaluate its possible association with EMT markers.
Materials and methods
Patients and specimen characteristics
This study was carried out on the formalin-fixed and paraffin-embedded (FFPE) specimens of ITAC (diagnosed between January 2007 and October 2018), retrieved from the archives of the Surgical Pathology and Cytopathology Unit of the University of Padova with the following inclusion criteria: surgically resected samples, CDX2 and CK20 positive ITAC, availability of exposure settings, and follow-up data. Exclusion criteria: bioptic material, and pure mucinous or signet ring cell morphology. From our previously published cohort of ITAC [28], 32 patients resulted eligible. All the cases have been reviewed and diagnosis confirmed by two pathologists (VM and RC), following the 4th World Health Organization Classification of Head and Neck Tumors [1]. Moreover, both Barnes and Kleinsasser and Schroeder morphological classifications were applied [29, 30]. The study was performed according to the 1964 Helsinki declaration and its later amendments; it also adheres to the REporting recommendations for tumor MARKer prognostic studies (REMARK) guidelines [31].
Tumor budding evaluation
We applied the ITBCC recommendations developed for CRC, which define tumor budding (TB) as single cells or cell clusters of up to four tumor cells at the invasive front of the tumor (peritumoral TB) or within the tumor mass (intratumoral TB), counted on hematoxylin and eosin–stained (H&E) slides [14, 15]. For this purpose, all H&E slides of each case were reviewed independently by three pathologists (VM, RC, and FG), blinded to stage and outcome. The hotspot method was applied: all the fields along the invasive front were scanned at × 100 magnification before counting buds in the microscopic field with the greatest number of tumor buds (hotspot) at a × 200 magnification. The number of tumor buds was assessed in a field measuring 0.785 mm2 (the objective magnification of our Leica microscopes was normalized as previously described [14]). The absolute count of buds has been registered for each case, with patients then classified with low (0–4 buds), intermediate (5–9 buds), or high-grade budding (≥ 10 buds). In a minority of cases, we assessed intratumoral TB instead of peritumoral TB, due to the material’s fragmentation or the impossibility of invasive margin evaluation for sure. As recently reported, ITB and PTB are correlated when ITB is present [13]. To strengthen our analysis, we excluded from the TB evaluation the tiny fragments, as well as the margins of the larger fragments.
Immunohistochemical analysis
Four-micrometer-thick FFPE sections from each selected sample were cut to perform immunohistochemistry (IHC). Staining was done automatically (BondmaX, Leica Biosystems, Newcastle Upon Tyne, UK), as described elsewhere [32], using the Bond Polymer Refine Detection kit (Leica), with the antibodies listed in Table 1. Sections were then counterstained with hematoxylin. E-cadherin was considered positive when membranous immunoreaction was observed in neoplastic cells, whereas positive ZEB1, ZEB2, SNAIL, and SLUG reactions were defined by the presence of nuclear immunostaining. Appropriate positive and negative controls were used. Immunostaining for each marker was dichotomized as positive or negative. The slides were blindly assessed by two pathologists (VM and RC). Discrepancies were resolved via consensus (VM, RC, and AF).
Statistical analysis
SPSS (version 20.0, IBM SPSS Statistics, Chicago, IL, USA) and the free-software R (http://www.r-project.org/) programs were used for statistical analyses. The level of significance was set at p < 0.05. Percentages and means and standard deviations were used to summarize categorical and continuous variables, respectively. For categorical data (histopathological features, tumor budding, and IHC markers), the association between variables was tested using the Pearson chi-square and the Fisher exact test, as appropriate. Interobserver agreement between the three pathologists was measured with Cohen’s kappa statistic. Disease-free survival (DFS) was calculated from the date of surgical treatment to the first observed relapse, whereas overall survival (OS) was defined as the time from the diagnosis to death of disease. DFS and OS analyses were performed according to the Kaplan–Meier method and survival curves were compared using the log-rank test.
Results
Clinical and pathological features of the ITAC cases
Clinical and pathological characteristics of the patients are detailed in Table 2. The mean age was 67.7 ± 12.8 years (range 42–88) and the male to female ratio was 7:1. Most of the patients (81%) had a positive history of occupational exposures to wood and/or leather dust. The right (47%) and the left side (44%) had a similar frequency, while bilateral tumors (9%) were a minority; histologic subtypes were 2 papillary type or papillary tubular cylinder cell I (PTCC-I) (6.2%), 19 colonic type (PTCC-II) (59.4%), 3 solid type (PTCC-III) (9.4%), and 8 mixed (transitional) type (25%). LVI and perineural invasion were assessed in 28 of 32 cases (4 were excluded due to the material’s fragmentation). LVI was observed in 3 of 28 cases (11%), while perineural invasion was never observed. T-staging was T1 in 3 cases (9.4%), T2 in 16 cases (50.0%), T3 in 11 cases (34.4%), and T4 in 2 cases (6.2%). Distant metastases were present in two patients, with latero-cervical nodes localization (N2b) and multiple lung lesions (M1), respectively. All the patients received transnasal endoscopic surgery (TES). Eight patients (25%) had positive surgical margins. Data concerning post-operative therapy were available for all except 4 patients: 28 patients (100%) received adjuvant radiotherapy, 4 of which also adjuvant chemotherapy. During the follow-up period (range 12–94 months), we observed 7 local recurrences (22%) and 4 deaths of disease (12.5%).
Patients with TB have a worse prognosis
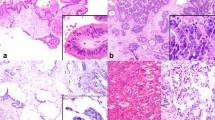
TB was present in 13 of 32 ITAC (40.6%) (an example of TB is shown in Fig. 1). The mean number of buds was 3 (range 1–11). Low-grade TB was present in the majority of cases (8 of 13; 61.5%), while intermediate (3 of 13; 23.1%) and high-grade (2 of 13; 15.4%) TB were less frequent. Due to the low case number, we analyzed together low-grade and high-grade TB cases. LVI was positively associated with TB (p = 0.008). All patients with relapse (p = 0.0005) and all patients dead of disease (p = 0.02) had TB. TB seemed independent from age, sex, stage of disease, tumor subtype, tumor grading, and occupational exposure to wood and/or leather dust. Association between clinical-pathological variables and TB is detailed in Table 3. Interobserver agreement between the three pathologists was substantial (proportion of agreement = 87%; Cohen’s kappa = 0.73) [33].
Median DFS was 19 versus 31 months in positive and negative cases for TB, respectively (p = 0.0002) (Fig. 2a). Median OS was 30 versus 31 months in positive and negative cases for TB, respectively (p = 0.013) (Fig. 2b).
TB and EMT markers
The expression of the EMT markers (Fig. 3) in relation to presence or absence of TB is presented in Table 4. Absence of TB was associated with low ZEB2 expression (p = 0.003). No other association emerged between TB and E-cadherin, ZEB1, SLUG, and SNAIL.
Discussion
This is the first study that explored TB in a series of ITAC. We documented that TB is frequent in these tumors (40% of our cases). The finding is not surprising, due to the wide description of the phenomenon mainly in gastrointestinal tract (i.e., CRC, esophageal SCC, and adenocarcinoma of the pancreas) and head and neck (oral, nasopharyngeal, hypopharyngeal, laryngeal and cutaneous SCC) [7, 10, 16,17,18,19,20,21, 23, 27]. Anyway, to the best of our knowledge, this is the first study which explored TB in the sinonasal tract.
The interobserver agreement was substantial, suggesting that the result is reliable, even dealing with fragmented material. From a clinical standpoint, TES is now the surgical approach of choice for most sinonasal tumors, so we expect to continue to receive these specimens in the next future [34]. Such fragmentation could affect the quantity of analyzed tissue but not the quality of the assessment. In any case, we ensured the quality of TB evaluation excluding the tiny fragments and the margins of the larger ones.
We found that the presence of TB in ITAC was associated with LVI (p = 0.008), recurrence (p = 0.0005), and death of disease (p = 0.02), which is similar to the role of TB in CRC [35, 36]. Survival analyses showed also a reduced DFS (p = 0.0002) and OS (p = 0.013) in those patients with tumors positive for TB. Our results showed also that TB seems to be independent from stage of disease (similarly to CRC), tumor subtype, and tumor grading in ITAC. Overall, these findings are in line with the original thought that TB corresponds to the initial phase of tumor invasion and is related to adverse prognosis [37,38,39,40] and also with the growing more recent evidences (as reviewed in [9, 12]). In our cohort, the presence of TB was independent from age and sex, which is in line with the majority of the studies in other settings, such as CRC, oral, laryngeal, and hypopharyngeal SCC [13, 16, 41]. In most of the cases, ITAC is an occupational-related tumor, and no relationship between TB and occupational exposure to wood and/or leather dust emerged.
The majority of positive tumors had a low-grade budding (61.5%), with a mean number of buds of 3. The small sample size impairs the possibility to achieve reliable conclusion about risk stratification, as for CRC in which intermediate- and high-grade TB categories are related to a high risk for nodal metastasis in pT1, while in advanced stages, high-grade TB is a risk factor for recurrence and shorter survival [14]. As a numerical variable, TB is expected to be more informative in risk stratification on a continuous scale than cut-offs, which instead are more practical in routine diagnostics [14]. Moreover, relevant cut-offs vary according to the clinical-pathological context and the tumor type [8, 14]. For example, while in CRC, the validated range of buds are 0–4, 5–9, and ≥ 10 [14, 15], the majority of studies in head and neck SCC used a cut-off point of 5 buds to discriminate low-risk versus high-risk cases [8]. Larger studies are required to address the role that the number of buds could have in ITAC patients.
While TB can be considered the morphological expression of EMT, the actual dominant opinion is that TB features can be part of the EMT spectrum, which can be complete or only partial [9]. We analyzed E-cadherin, as key cell-cell adhesion molecule and caretaker of the epithelial phenotype, and the main EMT-inducing transcription factors (ZEB1, ZEB2, SNAIL, and SLUG), which expression usually promotes the acquisition of mesenchymal properties. These are transcription repressors of E-cadherin and trigger the switch to mesenchymal state. Our findings showed that in ITAC, TB is poorly or not related to EMT, since almost all the cases maintained full E-cadherin expression and did not gain the expression of EMT-inducing transcription factors. Anyway, this is not the first time that TB was found unrelated to EMT [42].
The main strengths of this study are the homogeneous features (as previously detailed [28]) of the cases of this rare tumor and the application of an internationally validated scoring method to assess TB [15]. Evidences suggest that the prognostic role of TB is independent from the assessment method [9,10,11,12,13], but the use of a method which has international consensus allows comparison between studies.
The present study has other limitations. First, this is a single-center cohort study. Although we have demonstrated the prognostic role of TB in ITAC patients, further validation across multiple centers is required to establish the consistency of our result. Second, the relatively small sample size is a limitation, although ITAC is a rare tumor. Third, we had only one patient with lymph node metastases and one with distant spread of disease. Therefore, we did not have the chance to explore the role of TB as potential predictor of metastases, which is well-established in several tumors, such as CRC and esophageal SCC [10, 43,44,45,46] but also oral, nasopharyngeal, hypopharyngeal, and laryngeal cancers (as reviewed in [8]).
Conclusions
Our study documents that TB is associated with lymphovascular invasion, recurrence, death of disease, reduced DFS and OS in patients with ITAC, suggesting that TB is an adverse prognostic factor. No association between the presence of TB and the expression of EMT markers was found. These results encourage further larger studies with the main aims to deepen these findings and explore the potential benefit of TB assessment as a clinical routine in ITAC cases, similarly to colorectal adenocarcinoma.
References
El-Naggar A, Chan J, Grandis J et al (2017) WHO classification of head and neck tumours. IARC, Lyon
Kılıç S, Samarrai R, Kılıç SS, Mikhael M, Baredes S, Eloy JA (2018) Incidence and survival of sinonasal adenocarcinoma by site and histologic subtype. Acta Otolaryngol 138:415–421. https://doi.org/10.1080/00016489.2017.1401229
Binazzi A, Corfiati M, Di Marzio D et al (2018) Sinonasal cancer in the Italian national surveillance system: epidemiology, occupation, and public health implications. Am J Ind Med 61:239–250. https://doi.org/10.1002/ajim.22789
Franchi A, Massi D, Palomba A, Biancalani M, Santucci M (2004) CDX-2, cytokeratin 7 and cytokeratin 20 immunohistochemical expression in the differential diagnosis of primary adenocarcinomas of the sinonasal tract. Virchows Arch 445:63–67. https://doi.org/10.1007/s00428-004-1030-4
Franchi A, Innocenti DRD, Palomba A, Miligi L, Paiar F, Franzese C, Santucci M (2014) Low prevalence of K-RAS, EGF-R and BRAF mutations in sinonasal adenocarcinomas. Implications for anti-EGFR treatments. Pathol Oncol Res 20:571–579. https://doi.org/10.1007/s12253-013-9730-1
Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I (2018) Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer 118:577–586. https://doi.org/10.1038/bjc.2017.425
Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A (2013) Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer 49:1032–1039. https://doi.org/10.1016/j.ejca.2012.10.022
Mäkitie AA, Almangush A, Rodrigo JP, Ferlito A, Leivo I (2019) Hallmarks of cancer: tumor budding as a sign of invasion and metastasis in head and neck cancer. Head Neck 41:3712–3718. https://doi.org/10.1002/hed.25872
Grigore A, Jolly M, Jia D et al (2016) Tumor budding: the name is EMT. Partial EMT. J Clin Med 5:51. https://doi.org/10.3390/jcm5050051
Cappellesso R, Luchini C, Veronese N, Lo Mele M, Rosa-Rizzotto E, Guido E, de Lazzari F, Pilati P, Farinati F, Realdon S, Solmi M, Fassan M, Rugge M (2017) Tumor budding as a risk factor for nodal metastasis in pT1 colorectal cancers: a meta-analysis. Hum Pathol 65:62–70. https://doi.org/10.1016/j.humpath.2017.04.013
Petrelli F, Pezzica E, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Corti D, Barni S (2015) Tumour budding and survival in stage II colorectal cancer: a systematic review and pooled analysis. J Gastrointest Cancer 46:212–218. https://doi.org/10.1007/s12029-015-9716-1
Maffeis V, Nicolè L, Cappellesso R (2019) RAS, cellular plasticity, and tumor budding in colorectal cancer. Front Oncol 9:1255. https://doi.org/10.3389/fonc.2019.01255
Trinh A, Lädrach C, Dawson HE, ten Hoorn S, Kuppen PJK, Reimers MS, Koopman M, Punt CJA, Lugli A, Vermeulen L, Zlobec I (2018) Tumour budding is associated with the mesenchymal colon cancer subtype and RAS/RAF mutations: a study of 1320 colorectal cancers with Consensus Molecular Subgroup (CMS) data. Br J Cancer 119:1244–1251. https://doi.org/10.1038/s41416-018-0230-7
Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, el Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P (2017) Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol 30:1299–1311. https://doi.org/10.1038/modpathol.2017.46
Dawson H, Galuppini F, Träger P, Berger MD, Studer P, Brügger L, Zlobec I, Inderbitzin D, Lugli A (2019) Validation of the International Tumor Budding Consensus Conference 2016 recommendations on tumor budding in stage I-IV colorectal cancer. Hum Pathol 85:145–151. https://doi.org/10.1016/j.humpath.2018.10.023
Hong K-O, Oh K-Y, Shin W-J, Yoon HJ, Lee JI, Hong SD (2018) Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum Pathol 80:123–129. https://doi.org/10.1016/j.humpath.2018.06.012
Elseragy A, Salo T, Coletta RD, Kowalski LP, Haglund C, Nieminen P, Mäkitie AA, Leivo I, Almangush A (2019) A proposal to revise the histopathologic grading system of early oral tongue cancer incorporating tumor budding. Am J Surg Pathol 43:703–709. https://doi.org/10.1097/PAS.0000000000001241
Luo W-R, Gao F, Li S-Y, Yao K-T (2012) Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology 61:1072–1081. https://doi.org/10.1111/j.1365-2559.2012.04350.x
Imai T, Ito S, Oikawa T, Asada Y, Matsumoto K, Miyazaki T, Yamazaki T, Satoh I, Noguchi T, Matsuura K (2019) Risk factors for cervical lymph node metastasis in endoscopically resected superficial hypopharyngeal cancers. Auris Nasus Larynx 46:424–430. https://doi.org/10.1016/j.anl.2018.09.005
Sarioglu S, Acara C, Akman FC et al (2010) Tumor budding as a prognostic marker in laryngeal carcinoma. Pathol Res Pract 206:88–92. https://doi.org/10.1016/j.prp.2009.09.006
Gonzalez-Guerrero M, Martínez-Camblor P, Vivanco B et al (2017) The adverse prognostic effect of tumor budding on the evolution of cutaneous head and neck squamous cell carcinoma. J Am Acad Dermatol 76:1139–1145. https://doi.org/10.1016/j.jaad.2017.01.015
Smit MA, Geiger TR, Song J-Y, Gitelman I, Peeper DS (2009) A Twist-Snail axis critical for TrkB-induced epithelial-mesenchymal transition-like transformation, anoikis resistance, and metastasis. Mol Cell Biol 29:3722–3737. https://doi.org/10.1128/MCB.01164-08
Dawson H, Grundmann S, Koelzer VH, Galván JA, Kirsch R, Karamitopoulou E, Lugli A, Inderbitzin D, Zlobec I (2015) Tyrosine kinase receptor B (TrkB) expression in colorectal cancers highlights anoikis resistance as a survival mechanism of tumour budding cells. Histopathology 66:715–725. https://doi.org/10.1111/his.12603
Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119:1420–1428. https://doi.org/10.1172/JCI39104
Zlobec I, Lugli A (2018) Tumour budding in colorectal cancer: molecular rationale for clinical translation. Nat Rev Cancer 18:203–204. https://doi.org/10.1038/nrc.2018.1
Zlobec I, Lugli A (2010) Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: tumor budding as oncotarget. Oncotarget 1:651–661. https://doi.org/10.18632/oncotarget.199
Niwa Y, Yamada S, Koike M, Kanda M, Fujii T, Nakayama G, Sugimoto H, Nomoto S, Fujiwara M, Kodera Y (2014) Epithelial to mesenchymal transition correlates with tumor budding and predicts prognosis in esophageal squamous cell carcinoma. J Surg Oncol 110:764–769. https://doi.org/10.1002/jso.23694
Maffeis V, Cappellesso R, Zanon A et al (2019) HER2 status in sinonasal intestinal-type adenocarcinoma. Pathol Res Pract 215:152432. https://doi.org/10.1016/j.prp.2019.04.024
Barnes L (1986) Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol 10:192–202
Kleinsasser O, Schroeder HG (1988) Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol 245:1–15
Sauerbrei W, Taube SE, McShane LM, Cavenagh MM, Altman DG (2018) Reporting recommendations for tumor marker prognostic studies (REMARK): an abridged explanation and elaboration. J Natl Cancer Inst 110:803–811. https://doi.org/10.1093/jnci/djy088
Cappellesso R, Marioni G, Crescenzi M et al (2015) The prognostic role of the epithelial-mesenchymal transition markers E-cadherin and Slug in laryngeal squamous cell carcinoma. Histopathology 67:491–500. https://doi.org/10.1111/his.12668
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Rampinelli V, Ferrari M, Nicolai P (2018) Intestinal-type adenocarcinoma of the sinonasal tract: an update. Curr Opin Otolaryngol Head Neck Surg 26:115–121. https://doi.org/10.1097/MOO.0000000000000445
Rogers AC, Winter DC, Heeney A, Gibbons D, Lugli A, Puppa G, Sheahan K (2016) Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer 115:831–840. https://doi.org/10.1038/bjc.2016.274
Steinestel K, Lennerz JK, Eder S, Kraft K, Arndt A (2014) Invasion pattern and histologic features of tumor aggressiveness correlate with MMR protein expression, but are independent of activating KRAS and BRAF mutations in CRC. Virchows Arch 465:155–163. https://doi.org/10.1007/s00428-014-1604-8
Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M (1989) An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 63:539–543. https://doi.org/10.1002/1097-0142(19890201)63:3<539::AID-CNCR2820630323>3.0.CO;2-S
Hase K, Shatney C, Johnson D et al (1993) Prognostic value of tumor “budding” in patients with colorectal cancer. Dis Colon Rectum 36:627–635. https://doi.org/10.1007/BF02238588
Gabbert H, Wagner R, Moll R, Gerharz C-D (1985) Tumor dedifferentiation: an important step in tumor invasion. Clin Exp Metastasis 3:257–279. https://doi.org/10.1007/BF01585081
Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC (2002) Tumour ‘budding’ as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40:127–132. https://doi.org/10.1046/j.1365-2559.2002.01324.x
Boxberg M, Kuhn P-H, Reiser M, Erb A, Steiger K, Pickhard A, Straßen U, Koob I, Kolk A, Warth A, Jesinghaus M, Weichert W (2019) Tumor budding and cell nest size are highly prognostic in laryngeal and hypopharyngeal squamous cell carcinoma: further evidence for a unified histopathologic grading system for squamous cell carcinomas of the upper aerodigestive tract. Am J Surg Pathol 43:303–313. https://doi.org/10.1097/PAS.0000000000001178
Yamada N, Sugai T, Eizuka M, Tsuchida K, Sugimoto R, Mue Y, Suzuki M, Osakabe M, Uesugi N, Ishida K, Otsuka K, Matsumoto T (2017) Tumor budding at the invasive front of colorectal cancer may not be associated with the epithelial-mesenchymal transition. Hum Pathol 60:151–159. https://doi.org/10.1016/j.humpath.2016.10.007
Nakamura T, Mitomi H, Kanazawa H, Ohkura Y, Watanabe M (2008) Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum 51:568–572. https://doi.org/10.1007/s10350-008-9192-9
Beaton C, Twine CP, Williams GL, Radcliffe AG (2013) Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Color Dis 15:788–797. https://doi.org/10.1111/codi.12129
Pai RK, Cheng Y-W, Jakubowski MA, Shadrach BL, Plesec TP, Pai RK (2017) Colorectal carcinomas with submucosal invasion (pT1): analysis of histopathological and molecular factors predicting lymph node metastasis. Mod Pathol 30:113–122. https://doi.org/10.1038/modpathol.2016.166
Ito E, Ozawa S, Kijima H, Kazuno A, Nishi T, Chino O, Shimada H, Tanaka M, Inoue S, Inokuchi S, Makuuchi H (2012) New invasive patterns as a prognostic factor for superficial esophageal cancer. J Gastroenterol 47:1279–1289. https://doi.org/10.1007/s00535-012-0587-y
Acknowledgments
The preliminary results of this study were presented at the 31st European Congress of Pathology that was held in Nice, France, September 2019. The authors are grateful to Martina Boscolo Bielo for her contribution to statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was performed according to the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maffeis, V., Cappellesso, R., Galuppini, F. et al. Tumor budding is an adverse prognostic marker in intestinal-type sinonasal adenocarcinoma and seems to be unrelated to epithelial-mesenchymal transition. Virchows Arch 477, 241–248 (2020). https://doi.org/10.1007/s00428-020-02748-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02748-1