Abstract
SOX10 immunohistochemistry is used to identify tumors of neural crest origin, including melanocytic neoplasms. SOX10 expression has also been identified in myoepithelial cells of the breast and in a subset of invasive mammary carcinomas. In order to characterize SOX10 expression in ductal carcinomas of the breast, the aim of this study was to characterize the SOX10 in invasive ductal carcinomas according to molecular subtype, DCIS, and benign breast tissue. Forty cases of invasive ductal carcinoma of the breast were retrieved, with ten cases with immunohistochemical profile compatible with luminal A-like, luminal B-HER2-positive, non-luminal HER2-positive, and triple-negative subtypes. Whole tissue sections from each case were stained with SOX10. Six (60%) of ten triple-negative tumors were SOX10+ compared with 1 (3%) of 30 carcinomas of other molecular subtypes. All but one of the positive tumors showed at least moderate expression in at least 40% of tumor cells. All seven cases SOX10+ carcinomas were grade 3 tumors. Of the 13 cases with DCIS available for assessment, one (8%) showed positive SOX10 expression (a case associated with triple-negative carcinoma). Twenty-two cases contained normal breast tissue that showed SOX10 expression in both myoepithelial and luminal cells, predominantly patchy with variable intensity. SOX10 showed incomplete myoepithelial staining compared to other myoepithelial markers. In conclusion, SOX10 IHC cannot reliably differentiate between high-grade triple-negative carcinomas, melanomas, and myoepithelial tumors in the breast. SOX10 is not as robust a myoepithelial marker compared with other established markers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
SOX10 is a DNA-binding transcription factor involved in the development of neural crest cells, including melanocytes and Schwann cells [1,2,3,4]. While SOX10 immunohistochemistry (IHC) is often used to help identify tumors of neural crest origin, such as melanocytic neoplasms and peripheral nerve sheath tumors [1, 5,6,7,8,9], SOX10 expression is not entirely specific. For example, SOX10 immunohistochemical expression is also present in normal and neoplastic tissue of salivary glands and sweat glands [6, 10,11,12,13].
In the breast, SOX10 immunohistochemical expression is present in both myoepithelial and luminal cells [6, 7, 11, 13]. A proportion of invasive ductal carcinomas and metaplastic carcinomas of the breast is also positive for SOX10 [6, 7, 11, 13,14,15,16]. Thus, understanding the specificity and features of SOX10 immunohistochemistry in the breast, including its expression in invasive ductal carcinoma of the breast, would be of diagnostic utility. The aim of the study is thus to characterize the SOX10 immunohistochemical patterns of invasive ductal carcinomas, ductal carcinoma in situ (DCIS), and benign breast tissue.
Methods
Case selection
This study was approved by the Research Ethics Board at the British Columbia Cancer Agency. Archival tissue from 40 cases of invasive ductal carcinomas, no special type, of the breast that were biopsied or resected in 2016 was included in the study. Special subtypes of carcinomas, including metaplastic carcinoma, adenoid cystic carcinoma, and carcinoma with apocrine differentiation, were excluded. Ten cases of invasive ductal carcinoma from each of four molecular classes defined by immunohistochemical surrogates based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal factor receptor 2 (HER2) status were retrieved [17]. Luminal A-like tumors were ER-positive, PR-positive, and HER2-negative; luminal B-HER2-positive tumors (triple-positive tumors) were ER-positive, PR-positive, and HER2-positive; non-luminal HER2-positive tumors were ER-negative, PR-negative, and HER2-positive; triple-negative (basal-like) tumors were ER-negative, PR-negative, and HER2-negative. ER and PR status were based on IHC and considered positive when at least 1% of tumor cells showed nuclear staining [18]. A tumor was considered HER2-positive when it showed an IHC 3+ pattern or a positive result based on fluorescence in situ hybridization (FISH) as outlined in the 2013 ASCO/CAP guidelines [19].
The pathologic features of each tumor including tumor grade, primary tumor (pT) category, and regional lymph nodes (pN) category were reviewed when available.
Immunohistochemistry
Whole tissue sections of invasive ductal carcinoma and, when available, DCIS and benign breast ducts and lobules were stained for SOX10. Whole sections were obtained from the most representative block containing the tumor. IHC for SOX10 was performed using a mouse monoclonal antibody (Biocare Medical, clone BC34, Pacheco, CA) on Ventana Benchmark XT (Ventana Medical Systems, AZ, USA) at 1:200 dilution. Slides were treated with CC1 for 56 min, incubated with the primary antibody at room temperature for 32 min, and then detected using the Optiview DAB detection system. Melanoma was used as a positive control for SOX10.
SOX10 staining within invasive ductal carcinoma and DCIS was scored based on the proportion of tumor cells showing nuclear staining and intensity of staining (none, weak, moderate, and strong). A tumor was considered to be SOX10-positive when at least 1% of tumor nuclei showed nuclear staining. SOX10 staining was also assessed with benign breast tissue when available. The entire whole section was examined, and all ducts were evaluated for SOX10 staining.
Results
Invasive ductal carcinoma
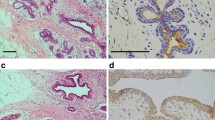
Whole tissue sections of 40 cases of invasive ductal carcinoma were stained with SOX10, including material from 16 resections and 24 core needle biopsies. Ten cases from each of the four molecular classes as determined by immunohistochemical surrogates (luminal A-like, luminal B-HER2-positive (triple positive), non-luminal HER2-positive, and triple-negative (basal-like) tumors) were included in the study. Six (60%) of 10 triple-negative carcinomas were SOX10-positive (Fig. 1a, b). In comparison, only 1 (3%) of 30 carcinomas from the other molecular classes was SOX10-positive, which was a case of non-luminal HER2-positive carcinoma.
Six (86%) of the seven SOX10-positive tumors showed at least 40% of tumor cells with moderate or strong nuclear staining (Table 1). One case of triple-negative carcinoma showed weak nuclear staining in 10% of cells. All seven SOX10-positive carcinomas were grade 3 tumors (Table 2).
Ductal carcinoma in situ
DCIS was available for evaluation in 13 cases. Of these cases, only 1 (8%) case of DCIS was SOX10-positive (Fig. 1c). Eighty percent of tumor nuclei showed strong intensity staining. This case of SOX10-positive DCIS was associated with a triple-negative invasive carcinoma that was SOX10 positive.
Benign breast tissue
All 22 cases with normal breast tissue available for assessment showed SOX10 nuclear expression in both myoepithelial and luminal cells of benign ducts and lobules, usually of patchy positivity and variable intensity (Fig. 2a, b). Occasionally, more uniform and strong staining was seen in foci of adenosis. In eight cases, foci of usual ductal hyperplasia (UDH) were also positive for SOX10. In two cases, UDH colonized by DCIS was positive for SOX10 (Fig. 3a–d).
SOX10 expression in UDH and myoepithelial cells. a DCIS with residual UDH, particularly at the periphery of the duct. Infiltrating glands of invasive ductal carcinoma are present around the DCIS as well. b The CK5/6 highlights the remnant of UDH left within the duct. c With the ER stain, the neoplastic cells of the DCIS are strongly and diffusely positive. The surrounding invasive ductal carcinoma is also strongly and diffusely positive. d SOX10 immunohistochemical stain. The UDH is positive for SOX10, but the larger neoplastic cells of the DCIS are negative. Myoepithelial cells around the DCIS are also SOX10 positive, showing a patchy and variably weak to moderate intensity. e On p63, the myoepithelial cells around the DCIS show strong nuclear staining. f The SMMHC shows strong cytoplasmic staining in the myoepithelial cells surrounding the DCIS. Both the p63 and SMMHC stains show loss of myoepithelial cells around invasive ductal carcinoma in the stroma surrounding the DCIS
The expression of SOX10 in myoepithelial cells surrounding DCIS was compared with other myoepithelial markers. SOX10 showed a more patchy, incomplete, and weaker staining pattern (Fig. 3d) compared with p63, SMMHC (Fig. 3e and f), CK5/6 (not shown), and CK14 (not shown).
Discussion
In this study, SOX10 immunohistochemical expression was assessed in mammary invasive ductal carcinomas as stratified by four different molecular classes as determined by IHC surrogates. We showed that mammary triple-negative invasive ductal carcinomas are frequently positive for SOX10 on IHC, with 6 of 10 cases demonstrating expression. In comparison, tumors from other molecular classes are rarely positive, and in our study, only 1 of 30 cases, a non-luminal HER2-positive tumor, was positive. In all but one case, these SOX10 positive tumors showed at least moderate intensity nuclear staining in at least 40% of tumor nuclei. All SOX10 positive invasive ductal carcinomas were high-grade (grade 3) tumors.
The finding that triple-negative invasive ductal carcinomas are frequently positive for SOX10, while tumors from other molecular classes are rarely positive is consistent with previous studies. Studies assessing SOX10 immunohistochemical expression amongst a variety of neoplasms by tissue microarrays have previously reported that only a minority (12 to 17%) of invasive ductal carcinomas are SOX10-positive [6, 7, 13]. When SOX10 expression in invasive ductal carcinomas of the breast is stratified by molecular subtype and hormonal status, it is apparent that SOX10 expression is more common in triple negative invasive ductal carcinomas, being present in 38 to 71% of such cases [14, 15]. Most tumors showed diffuse staining. Although HER-2 status was not reported, Miettinen et al. reported that the majority (60%) of SOX10-positive invasive ductal carcinomas in their study were either ER-negative or had a low expression for ER [6]. In comparison, luminal and non-luminal HER2-positive, invasive ductal carcinomas are infrequently positive for SOX10, and in one study, only 1 (15%) of 7 non-luminal HER-positive and 1 (7%) of 14 luminal B invasive ductal carcinomas were SOX10-positive [20].
SOX10 expression in metastatic breast carcinoma also appears to be associated with a triple-negative phenotype. Nelson et al. reported that 3 (38%) triple-negative tumors were SOX10-positive compared with none of the 18 luminal or non-luminal HER2-positive cases [16]. In regard to SOX10 expression in other subtypes of mammary carcinoma, 46% of triple-negative metaplastic mammary carcinomas were SOX10 positive in one study [14], while invasive lobular carcinomas have thus far been reported to be SOX10 negative [6, 7, 16].
In regard to DCIS, we showed one (8%) case of DCIS that was SOX10-positive, which is consistent with a low rate of SOX10 positivity in DCIS previously reported by Cimino-Mathews et al. (1 of 24 cases) [14]. Notably, the SOX10-positive DCIS in our study was associated with a SOX10-positive triple-negative invasive carcinoma.
SOX10 is used as an immunohistochemical marker for melanomas and peripheral nerve sheath tumors, including schwannomas, neurofibromas, and a subset of malignant peripheral nerve sheath tumors [1, 5,6,7,8,9]. The majority of metastatic melanomas are SOX10 positive [6, 8]. SOX10 expression is also detected in some salivary gland neoplasms, particularly those with myoepithelial differentiation [10,11,12], myoepitheliomas and mixed tumors of the skin or soft tissue [6], some skin adnexal neoplasms [6], and gliomas and other tumors of the central nervous system [13, 21]. This reflects the cell of origin for these tumors, as SOX10 expression is detected in melanocytes, peripheral nerves, myoepithelial and luminal cells of salivary glands, sweat glands, and glial cells [1, 6, 12, 13, 21]. Basal cells of prostate glands [6] and bronchial glands [1] have also been reported to show SOX10 expression.
The finding that SOX10 IHC is frequently positive in high-grade triple-negative invasive ductal carcinomas of the breast has significant implications for surgical pathology practices. Firstly, if the lineage of a high-grade tumor is in question on a biopsy or even resection of the breast, then SOX10 expression within the tumor does not necessarily provide evidence for melanoma or other tumors of neural crest origin. In such a scenario, other melanocytic markers for melanoma, namely HMB45 and melan-A, may be helpful [22, 23]. A note was made that S100 positivity, a sensitive marker for melanoma [22, 23], has been reported in a significant percentage of breast carcinomas [24, 25]. More specific markers for breast carcinomas are useful but have limited sensitivity. GATA3 is positive in 43 to 71% of triple negative invasive ductal carcinomas [20, 26,27,28,29], which is lower than in other breast carcinomas [26]. Triple negative carcinomas are frequently negative for GCDFP 15 and mammaglobin [15, 29, 30]. Therefore, based on this and other studies, SOX10 positivity can be used as an adjunct marker for triple negative carcinomas of the breast, including invasive ductal carcinomas and metaplastic carcinomas.
SOX10 has previously been described to be expressed in benign breast tissue in both myoepithelial cells and luminal cells [6]. This was confirmed in our study. The typical pattern observed was a heterogeneous expression characterized by patchy nuclear staining of variable intensity. Foci of UDH or adenosis may show increased expression. This would presumably be easy to recognize in daily practice, but care should be taken to not mistake such a pattern as evidence for a neoplastic process, especially in cases of SOX10-positive UDH being colonized by DCIS. Furthermore, SOX10 expression in the luminal and myoepithelial cells within breast tissue can act as an internal control for IHC. Nevertheless, SOX10 does not seem to be as robust a marker for intact myoepithelial layer around DCIS compared with other myoepithelial markers p63 (another nuclear stain), CK5/6 or CK14 [31].
The strength of this study is the characterization of SOX10 expression in both neoplastic and nonneoplastic breast tissue. Using whole tissue sections, we were able to assess for heterogeneous expression of SOX10 in benign breast tissue and to demonstrate SOX10 expression in UDH, including foci of DCIS.
The limitation of this study is the limited number of cases. Despite this, we were still able to demonstrate that a high proportion of triple-negative invasive ductal carcinomas are SOX10 positive on IHC, while tumors from other molecular subgroups as determined by IHC surrogates are only rarely positive for SOX10.
In conclusion, SOX10 positivity on its own cannot differentiate between high-grade triple-negative invasive ductal carcinoma of the breast, melanomas, and myoepithelial tumors. SOX10 expression on IHC seems to be rare in tumors that are ER and/or HER2 positive. Furthermore, SOX10 stains the luminal and myoepithelial cells of benign breast ducts and lobules, adenosis and usual ductal carcinoma but does not appear as robust a myoepithelial marker as other established markers.
References
Nonaka D, Chiriboga L, Rubin BP (2008) SOX10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 32(9):1291–1298. https://doi.org/10.1097/PAS.0b013e3181658c14
Fufa TD, Harris ML, Watkins-Chow DE, Levy D, Gorkin DU, Gildea DE, Song L, Safi A, Crawford GE, Sviderskaya EV, Bennett DC, McCallion AS, Loftus SK, Pavan WJ (2015) Genomic analysis reveals distinct mechanisms and functional classes of SOX10-regulated genes in melanocytes. Hum Mol Genet 24(19):5433–5450. https://doi.org/10.1093/hmg/ddv267
Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M (1998) SOX10, a novel transcriptional modulator in glial cells. J Neurosci 18(1):237–250
Pusch C, Hustert E, Pfeifer D, Sudbeck P, Kist R, Roe B, Wang Z, Balling R, Blin N, Scherer G (1998) The SOX10/SOX10 gene from human and mouse: sequence, expression, and transactivation by the encoded HMG domain transcription factor. Hum Genet 103(2):115–123. https://doi.org/10.1007/s004390050793
Karamchandani JR, Nielsen TO, van de Rijn M, West RB (2012) SOX10 and S100 in the diagnosis of soft-tissue neoplasms. Appl Immunohistochem Mol Morphol 20(5):445–450. https://doi.org/10.1097/PAI.0b013e318244ff4b
Miettinen M, McCue PA, Sarlomo-Rikala M, Biernat W, Czapiewski P, Kopczynski J, Thompson LD, Lasota J, Wang Z, Fetsch JF (2015) SOX10--a marker for not only schwannian and melanocytic neoplasms but also myoepithelial cell tumors of soft tissue: a systematic analysis of 5134 tumors. Am J Surg Pathol 39(6):826–835. https://doi.org/10.1097/PAS.0000000000000398
Mohamed A, Gonzalez RS, Lawson D, Wang J, Cohen C (2013) SOX10 expression in malignant melanoma, carcinoma, and normal tissues. Appl Immunohistochem Mol Morphol 21(6):506–510. https://doi.org/10.1097/PAI.0b013e318279bc0a
Ordonez NG (2013) Value of SOX10 immunostaining in tumor diagnosis. Adv Anat Pathol 20(4):275–283. https://doi.org/10.1097/PAP.0b013e318297a9d0
Shin J, Vincent JG, Cuda JD, Xu H, Kang S, Kim J, Taube JM (2012) SOX10 is expressed in primary melanocytic neoplasms of various histologies but not in fibrohistiocytic proliferations and histiocytoses. J Am Acad Dermatol 67(4):717–726. https://doi.org/10.1016/j.jaad.2011.12.035
Hsieh MS, Lee YH, Chang YL (2016) SOX10-positive salivary gland tumors: a growing list, including mammary analogue secretory carcinoma of the salivary gland, sialoblastoma, low-grade salivary duct carcinoma, basal cell adenoma/adenocarcinoma, and a subgroup of mucoepidermoid carcinoma. Hum Pathol 56:134–142. https://doi.org/10.1016/j.humpath.2016.05.021
Ivanov SV, Panaccione A, Nonaka D, Prasad ML, Boyd KL, Brown B, Guo Y, Sewell A, Yarbrough WG (2013) Diagnostic SOX10 gene signatures in salivary adenoid cystic and breast basal-like carcinomas. Br J Cancer 109(2):444–451. https://doi.org/10.1038/bjc.2013.326
Ohtomo R, Mori T, Shibata S, Tsuta K, Maeshima AM, Akazawa C, Watabe Y, Honda K, Yamada T, Yoshimoto S, Asai M, Okano H, Kanai Y, Tsuda H (2013) SOX10 is a novel marker of acinus and intercalated duct differentiation in salivary gland tumors: a clue to the histogenesis for tumor diagnosis. Mod Pathol 26(8):1041–1050. https://doi.org/10.1038/modpathol.2013.54
Tacha D, Qi W, Ra S, Bremer R, Yu C, Chu J, Hoang L, Robbins B (2015) A newly developed mouse monoclonal SOX10 antibody is a highly sensitive and specific marker for malignant melanoma, including spindle cell and desmoplastic melanomas. Arch Pathol Lab Med 139(4):530–536. https://doi.org/10.5858/arpa.2014-0077-OA
Cimino-Mathews A, Subhawong AP, Elwood H, Warzecha HN, Sharma R, Park BH, Taube JM, Illei PB, Argani P (2013) Neural crest transcription factor Sox10 is preferentially expressed in triple-negative and metaplastic breast carcinomas. Hum Pathol 44(6):959–965. https://doi.org/10.1016/j.humpath.2012.09.005
Harbhajanka A, Chahar S, Miskimen K, Silverman P, Harris L, Williams N, Varadan V, Gilmore H (2018) Clinicopathological, immunohistochemical and molecular correlation of neural crest transcription factor SOX10 expression in triple negative breast carcinoma. Hum Pathol 80:163–169. https://doi.org/10.1016/j.humpath.2018.06.007
Nelson ER, Sharma R, Argani P, Cimino-Mathews A (2017) Utility of SOX10 labeling in metastatic breast carcinomas. Hum Pathol 67:205–210. https://doi.org/10.1016/j.humpath.2017.08.011
Tang P, Tse GM (2016) Immunohistochemical surrogates for molecular classification of breast carcinoma: a 2015 update. Arch Pathol Lab Med 140(8):806–814. https://doi.org/10.5858/arpa.2015-0133-RA
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134(6):907–922. https://doi.org/10.1043/1543-2165-134.6.907
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF, American Society of Clinical Oncology, College of American Pathologists (2014) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256. https://doi.org/10.5858/arpa.2013-0953-SA
Cimino-Mathews A, Subhawong AP, Illei PB, Sharma R, Halushka MK, Vang R, Fetting JH, Park BH, Argani P (2013) GATA3 expression in breast carcinoma: utility in triple-negative, sarcomatoid, and metastatic carcinomas. Hum Pathol 44(7):1341–1349. https://doi.org/10.1016/j.humpath.2012.11.003
Bannykh SI, Stolt CC, Kim J, Perry A, Wegner M (2006) Oligodendroglial-specific transcriptional factor SOX10 is ubiquitously expressed in human gliomas. J Neuro-Oncol 76(2):115–127. https://doi.org/10.1007/s11060-005-5533-x
Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW (2008) Immunohistochemical characteristics of melanoma. J Cutan Pathol 35(5):433–444. https://doi.org/10.1111/j.1600-0560.2007.00891.x
Jing X, Michael CW, Theoharis CG (2013) The use of immunocytochemical study in the cytologic diagnosis of melanoma: evaluation of three antibodies. Diagn Cytopathol 41(2):126–130. https://doi.org/10.1002/dc.21791
Dwarakanath S, Lee AK, Delellis RA, Silverman ML, Frasca L, Wolfe HJ (1987) S-100 protein positivity in breast carcinomas: a potential pitfall in diagnostic immunohistochemistry. Hum Pathol 18(11):1144–1148
Stroup RM, Pinkus GS (1988) S-100 immunoreactivity in primary and metastatic carcinoma of the breast: a potential source of error in immunodiagnosis. Hum Pathol 19(8):949–953
Peng Y, Butt YM, Chen B, Zhang X, Tang P (2017) Update on immunohistochemical analysis in breast lesions. Arch Pathol Lab Med 141(8):1033–1051. https://doi.org/10.5858/arpa.2016-0482-RA
Krings G, Nystrom M, Mehdi I, Vohra P, Chen YY (2014) Diagnostic utility and sensitivities of GATA3 antibodies in triple-negative breast cancer. Hum Pathol 45(11):2225–2232. https://doi.org/10.1016/j.humpath.2014.06.022
Dang DN, Raj G, Sarode V, Molberg KH, Vadlamudi RK, Peng Y (2015) Significantly increased PELP1 protein expression in primary and metastatic triple-negative breast carcinoma: comparison with GATA3 expression and PELP1's potential role in triple-negative breast carcinoma. Hum Pathol 46(12):1829–1835. https://doi.org/10.1016/j.humpath.2015.07.023
Zombori T, Cserni G (2018) Immunohistochemical analysis of the expression of breast markers in basal-like breast carcinomas defined as triple negative cancers expressing keratin 5. Pathol Oncol Res 24(2):259–267. https://doi.org/10.1007/s12253-017-0246-y
Lewis GH, Subhawong AP, Nassar H, Vang R, Illei PB, Park BH, Argani P (2011) Relationship between molecular subtype of invasive breast carcinoma and expression of gross cystic disease fluid protein 15 and mammaglobin. Am J Clin Pathol 135(4):587–591. https://doi.org/10.1309/AJCPMFR6OA8ICHNH
Dewar R, Fadare O, Gilmore H, Gown AM (2011) Best practices in diagnostic immunohistochemistry: myoepithelial markers in breast pathology. Arch Pathol Lab Med 135(4):422–429. https://doi.org/10.1043/2010-0336-CP.1
Acknowledgments
The authors would like to thank Dr. Helga Klein-Parker for her contribution to the data collection of this study.
Contributions
Author KC, Author DI, and Author MH conceived and designed the study and wrote, edited, and reviewed the manuscript. Author KC and Author MH researched and analyzed data. All authors gave final approval for publication. Author MH takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript.
Funding
This study was funded by Department of Pathology & Laboratory Medicine of the University of British Columbia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Research Ethics Board at the British Columbia Cancer Agency.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiu, K., Ionescu, D.N. & Hayes, M. SOX10 expression in mammary invasive ductal carcinomas and benign breast tissue. Virchows Arch 474, 667–672 (2019). https://doi.org/10.1007/s00428-019-02557-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02557-1