Abstract
Urachal carcinoma (UrC) is an exceedingly rare neoplasm that develops from the urachus, an embryologic remnant of the urogenital sinus and allantois. The most commonly encountered histologic subtype is adenocarcinoma. The aim of this study is to characterize a series of UrC by morphology, immunohistochemistry, and molecular analysis. We retrospectively investigated seven cases of UrCs and assessed patient symptoms, imaging, histologic features, immunohistochemical profile, molecular characteristics, pathologic stages, and type of treatment. Immunostaining for CK7, CK20, Muc-2, CDX2, GATA3, β-catenin, and CK34βE12 was carried out on each neoplasm and on seven non-neoplastic urachal remnants as the control group. Additionally, a mutational analysis was performed using the QIAact Actionable Insights Tumor Panel Kit, which analyzes KRAS, NRAS, KIT, BRAF, PDGFRA, ALK, EGFR, ERBB2, PIK3CA, ERBB3, ESR1, and RAF1. Our cohort comprised five females and two males with a mean age of 64 years. UrCs consisted of two mucinous cystadenocarcinomas and five invasive, non-cystic adenocarcinomas. Carcinoma antigen expression profile was positive for CK20 and negative for CK34βE12 and GATA3 in all cases. Five of seven cases stained positively for Muc-2 and CDX2. On the contrary, non-neoplastic urachal remnants were immunoreactive for CK34βE12, CK7, and GATA3. Mutational analysis gave a positive result in four out of seven (57.1%) cases. All four positive tumors showed RAS mutation and one an additional mutation in PIK3CA. Urachal tumors exhibit peculiar morphologic, immunohistochemical, and molecular features. Due to the advanced stage at presentation, individualized treatment should be undertaken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Urachal carcinoma (UrC) is an uncommon neoplasm with poor prognosis which develops in the urachus, an embryologic remnant of the urogenital sinus and allantois [1]. The annual incidence of UrC accounts for 0.01% of all cancers in adults and has been estimated to comprise 0.17–0.34% of all bladder cancers [2]. Histologically, UrC results from malignant transformation of the columnar metaplasia of the transitional cell lining of the urachus [3,4,5]. Ultrasonography (US) is the gold standard for diagnosing urachal malignancy [6]. Despite the availability of other imaging techniques, such as computerized tomography (CT) or magnetic resonance imaging (MRI), accuracy of preoperative diagnosis remains poor [7]. Histologically, UrC may present mucinous, enteric, signet ring cell, and not otherwise specified features, although admixture of these morphologies can occur [8]. The biologic and clinical significance of these histologic subtypes, however, remains uncertain [9]. Given the rarity of UrC, several differential diagnoses should be considered [10]. Somatic mutations in the KRAS oncogene are described in many solid cancers, the detection of which is essential for better molecular characterization of primary tumors and targeting therapy [11]. Accurate staging of UrC is critical because of its propensity to become rapidly invasive [5]. Several types of stage classifications have been suggested, but those most often employed are the Sheldon and Mayo Staging Systems [9]. Management strategies rely on partial or radical cystectomy with complete removal of the urachal remnant and umbilicus. Lymph node dissection is not required unless lymph node involvement is confirmed by preoperative examination [12, 13]. Robotic surgery adds benefits to the conventional laparoscopic approach and is therefore a valid method for removal of abdominal wall masses [14]. There is currently no data to support the role of neoadjuvant or adjuvant chemotherapy for urachal carcinoma, although it is recommended for unresectable and/or metastatic disease. Individualized treatment is encouraged and a combination of radiotherapy and/or chemotherapy regimens can be adopted [15]. The aim of the study is to present a series of UrCs, detailing their prominent morphologic, immunohistochemical, and molecular features.
Material and methods
We retrospectively studied seven cases of UrC treated at Central Hospital of Bolzano between 2007 and 2017. For each case, we analyzed patient data, presenting symptoms, imaging methods, gross appearance, histology, pathologic staging, immunohistochemical and molecular profile, and type of treatment. All patients had undergone ultrasound evaluation (US), axial CT scan, and MRI. Surgery consisted of en bloc excisions of the urachal lesion, urachal ligament, and bladder dome in three cases, while cystectomy was carried out in four cases, three of which involved associated multi-organ resection. All cases were histologically evaluated on hematoxylin and eosin (H&E)-stained slides and reviewed by an experienced uropathologist to confirm diagnosis. The immunohistochemical panel included CK7, CK20, Muc-2, CDX2, GATA3, β-catenin, and CK34βE12 and was applied both to neoplasm and urachal remnants. A complete list of antibodies performed is given in Table 1. Immunostaining was performed using the Leica Bond III System (Leica, Newcastle, UK). According to Gopalan et al., inclusion criteria were: location of the tumor in the dome/anterior bladder wall, epicenter of carcinoma in the bladder wall, absence of widespread cystitis cystica/glandularis beyond the dome/anterior bladder wall, absence of a known primary elsewhere [10]. All cases were staged according to the Sheldon staging system for UrC reported in the WHO 2016 classification.
Molecular characterization
Sample and DNA isolation
Molecular analysis of the tumors was performed following the manufacturer’s instructions (Qiagen, Hilden, Germany). Formalin-fixed paraffin embedded (FFPE) material was used to prepare DNA samples. Tissue sections 5-μm thick were employed for DNA extraction using the GeneRead FFPE DNA Kit (Qiagen, Hilden, Germany) and DNA concentration was measured using the Nanodrop System (Thermo Fisher Scientific, MA, USA) and the QIAexpert (Qiagen, Hilden, Germany).
GeneReader sample preparation and sequencing
Overall, l16 ng of DNA was used as a template to generate libraries for sequencing. Libraries were prepared using the QIAGEN Library Kit v2.0 and the QIAact Actionable Insights Tumor Panel Kit (Qiagen, Hilden, Germany), which amplified 330 amplicons, covering 16.7 kb, containing 773 unique variant positions in 12 genes (KRAS, NRAS, KIT, BRAF, PDGFRA, ALK, EGFR, ERBB2, PIK3CA, ERBB3, ESR1, and RAF1). The libraries were then quantified by QIAexel (Qiagen, Hilden, Germany). Individual libraries were pooled prior to emulsion PCR and bead enrichment steps that were carried out by applying an automated protocol with the GeneRead Clonal Amp Q Kit (Qiagen, Hilden, Germany). Following bead enrichment, the pooled libraries were sequenced on the GeneReader platform (Qiagen, Hilden, Germany).
GeneReader data processing
QIAGEN Clinical Insights (QCI™) Analyze Software (Qiagen, Hilden, Germany) was employed to read data to the hg19 reference genome sequence, call sequence variants, and generate an interactive report for visualization of the sequencing results with a summary of the data. QCI Analyze software reports a set of high and low confidence variants based on the coverage of variant positions. Having analytically confirmed whether or not the variants listed are valid, data is uploaded to QCI Interpret software for clinical interpretation. A report for each sample is then created, based on detected variants and content, with a summary of findings and direct links to indicate the source.
Results
Our series consisted of five females and two males, ranging in age from 56 to 74 years (mean age 64 years). Symptoms and signs at diagnosis were hematuria and dysuria in five out of seven patients; a palpable mass right above the hypogastrium was detected in three patients. On ultrasound examination, a heterogeneous mass, mixed hypo- and hyperechoic in appearance, was seen in four cases. In the remaining three cases, a small hyperechoic cyst 1–2 cm in greatest diameter was found within the urachal ligament, close to or in contact with the bladder dome. CT and MRI were performed on all patients, revealing neoplastic involvement of adjacent organs, such as the colon and abdominal wall, in three cases.
Histologic analysis showed two mucinous cystadenocarcinomas and five invasive, non-cystic adenocarcinomas (Fig. 1). Sheldon stages at presentation were II (one patient), III (three patients), and IV (three patients). Immunostaining was positive for CK20 and negative for CK34βE12 and GATA3 in all cases, while Muc-2 and CDX2 were positive in five out of seven tumors. Non-neoplastic urachal remnants were reactive for CK7, GATA3, and CK34βE12. In our series, immunohistochemical nuclear staining of β-catenin was not detected, as we found only strong cytoplasmic and membranous positivity. Immunohistochemical findings are summarized in Tables 2 and 3.
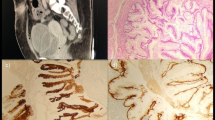
Cystectomy specimen and gross appearance of UrC. The neoplasm is located in the bladder dome and exhibits an intramural growth (a). Histological features consist of papillary fronds lined by atypical columnar cells, and mucin is visible at low magnification (100×). There is no evidence of glandular cystitis in the adjacent bladder dome urothelium (b)
Four out of the seven (57%) patients demonstrated RAS mutation (one patient NRAS p.Q61R (c.182A > G), one patient KRAS p.Q61L (c.182_183delAAinsTC), and two patients KRAS p.G12D (c.35G > A)). One patient showed an additional mutation in PIK3CA p.Q546K (c.1636C > A).
Discussion
UrC is an extremely rare neoplasm with poor prognosis which develops in the urachus, an embryologic remnant of the urogenital sinus and allantois. Involution usually occurs before birth and the urachus appears as a median umbilical ligament [1]. If regression is incomplete, the urachus may persist and give rise to various abnormalities, including malignances [16]. Urachal remnants can be seen in infants and during childhood, as thin-walled, internally homogenous cysts, containing straw-yellow umbilical fluid [17].
In approximately 30% of the general population, the urachal remnant may show tubular or cystic structures consisting of mucosa, connective tissue, and smooth muscle [18]. Histologically, UrC is known to arise following malignant transformation of the columnar metaplasia of urachal transitional epithelium. Cancer may also originate from enteric rests during embryologic development [3]. The urachus comprises intramucosal, intramuscular, and supravesical segments. It contains three distinct tissue layers: an epithelial canal lined with urothelium, submucosal connective tissue and an outer layer of smooth muscle. Urachal neoplasms can occur in any of these layers, along the urachal remnant from the umbilicus to the bladder dome and can be epithelial or mesenchymal [19, 20]. Begg et al. focused on the clinical importance of the urachus, stating that all apical tumors of the bladder should be considered urachal until proven otherwise. Since the urachus may extend as far as the anterior bladder wall, tumors located in the anterior wall may also be of urachal origin [19]. Several aspects of UrC pathogenesis are not fully understood [1].
Current knowledge of this disease mainly derives from case reports or small series [5, 21]. US is the first choice imaging modality for diagnosis of urachal malignancies. Nevertheless, the accuracy of preoperative diagnosis is poor [7], and differentiation between organized urachal abscess and carcinoma remains challenging [22]. CT and MRI are helpful in defining tumor staging, as well as in preoperative surgical planning. However, in cases of complicated cystic or contrast-enhancing lesions, it can be difficult to distinguish infected urachal remnants from urachal neoplasms [23]. In our series, a palpable mass was the presenting symptom in three patients with high-stage disease.
Since treatment options for advanced and metastatic UrC are limited, data suggests that early excision remains the recommended treatment for a suspicious urachal mass [20]. The urachus shares the same embryologic origin as the colon, consequently the large majority of urachal neoplasms are adenocarcinomas. They express tumor markers associated with gastrointestinal malignancies, including the carcinoembryonic antigens (CEA), CA 125 and CA 19.9 [24, 25]. UrC has a tendency for early peritoneal spread and may metastasize to the bones, lung, and liver. This may be due to delayed diagnosis, as urachal neoplasms are often asymptomatic [26, 27]. Pseudomyxoma peritonei arising from UrC has also been reported [27].
Glandular tumors are the most common neoplasms of the urachus and may exhibit mucinous, enteric, or signet ring cell features. Mixtures of these morphologies can occur, and histologic classification follows percentage cut-offs [28]. Non-cystic invasive adenocarcinoma accounts for 50% of tumors classified as mucinous. Although this subtyping is helpful in differential diagnosis, the clinical significance is still uncertain. All seven cases in our series were adenocarcinomas, two mucinous invasive cystadenocarcinomas and five non-cystic invasive adenocarcinomas not otherwise specified (NOS), of which four displayed enteric morphology, and one signet ring features.
Literature data on the immunohistochemical profile of UrC is limited. In urachal adenocarcinoma subtypes, expression levels of CDX2, nuclear β-catenin, claudin-18, and Reg IV may be useful in distinguishing between mucinous, enteric, signet ring cell or not otherwise specified histotypes. CDX2 is frequently expressed in urachal adenocarcinomas, and staining can be diffused even in non-enteric subtypes. All urachal adenocarcinomas show membrane-cytoplasmic β-catenin staining [9]. Although nuclear localization of β-catenin may occur in urachal adenocarcinoma, diffuse nuclear positivity contradicts this diagnosis. The novel gastrointestinal tract markers claudin-18 and Reg IV are both expressed in urachal adenocarcinomas. An immunohistochemical panel including β-catenin and CK7 is valuable in differentiating urachal adenocarcinoma of enteric morphology from colonic adenocarcinoma. The various morphologic presentations of urachal adenocarcinomas have relatively similar or overlapping immunophenotypes [29]. In our series, the carcinoma antigen expression profile was invariably positive for CK20 and negative for CK34βE12 and GATA3. Nuclear staining for β-catenin was not detected in any of the cases, as all cases showed only strong cytoplasmic and membranous positivity. In five of the seven cases, Muc-2 and CDX2 were positive (Table 2, Fig. 2). Non-neoplastic urachal remnants were strongly and diffusely positive for CK34βE12, CK7, and GATA3, which indicate urothelial features (Table 3).
Due to the rarity of UrC, differential diagnosis must be considered, and it is worth noting that immunostains do not unequivocally discriminate urachal from colorectal carcinoma. However, diffuse positivity for CK34βE12 would support a diagnosis of urachal carcinoma, while diffuse nuclear immunoreactivity for β-catenin would argue against it [10]. The most challenging differential diagnosis is bladder adenocarcinoma, which accounts for 0.5–2% of all malignant bladder tumors [30]. For the diagnosis of UrC, we considered the following criteria: tumor in the dome/anterior bladder wall, epicenter of carcinoma in the bladder wall, absence of widespread cystitis cystica/glandularis beyond the dome/anterior bladder wall, no evidence of a primary neoplasm elsewhere [10]. In accordance with Mostofi et al. [31], if the mucosa adjacent to the tumor shows polypoid formation, von Brunn’s nests, and glandular or mucinous metaplasia, the tumor is probably a primary vesical adenocarcinoma. Additional evidence favoring this diagnosis is the presence of areas of transitional and squamous cell carcinoma alongside the adenocarcinoma. If the adenocarcinoma is intramural, the tissue is from an area of the bladder other than the dome or the anterior wall and the mucosa is ulcerated; then, the tumor is probably of urachal origin [31]. Since the urachus can be the site of numerous inflammatory and reactive processes and consequently undergo frequent cystic changes, an antibody panel capable of clearly differentiating an epithelium with reactive atypical changes from neoplastic cells would be extremely useful. The immunophenotype we detected was noticeably different in the control and neoplastic cases. These features can be exploited, particularly when only scarce or fragmented material is available and morphology alone is inconclusive.
Literature provides mutational hot-spots of selected genes. By means of pyrosequencing in a multicenter study, Módos et al. found 10 out of 22 patients with KRAS mutations (27%) followed by BRAF (18%) and NRAS (5%) [16]. Using next generation sequencing (NGS; Generead System, Qiagen), we were able to confirm these findings and frequency of mutational events, as a result of which, our series can be considered an external validation cohort. We also found an additional, so far unreported mutation in the oncogene PIK3CA p.Q546K occurring in association with the well-documented and more frequent RAS p.G12D mutation. Activation of the PI3K/AMT/MTOR pathway, although not so widespread, may provide new insight into the molecular landscape of UrC and add to the understanding of UrC carcinogenesis which remains widely unknown [16]. The molecular mutations of UrC, however, seem to be unique, implying that clinical decision-making for UrC cannot be simply adopted from urothelial or colorectal practice [16]. In our study, four of the seven cases were RAS mutated (57%) and one (14%) case, as previously mentioned, showed an additional mutation for PIK3CA. This suggests that RAS mutation analysis may be helpful in diagnosing UrC.
Having recorded the gross, histologic, immunohistochemical, and molecular characteristics of our cases, we focused on defining disease stage, for which we applied the Sheldon staging system following the WHO 2016 recommendations [2]. The Sheldon staging system does not account for the fact that urachal tumors can occur along the urachus, from the umbilicus to the bladder, or that these tumors are actually extra-vesical with a tendency to invade the bladder. When the tumor is located close to the umbilicus, the abdominal wall is usually involved, whereas if it is close to the bladder, invasion of the bladder is common [32]. According to the Sheldon staging system, our patients were classified as stages II (one patient), III (three patients), and IV (three patients). Several alternative staging approaches to the traditional Sheldon system have been proposed to provide better tumor distribution across stages. However, the prognostic utility of stage substratification is yet to be validated [9].
Survival data on UrC is sparse due to its low prevalence. This entity remains a diagnostic challenge because of its rarity and lack of specific symptoms [5]. The most common symptoms are mucinuria, hematuria, abdominal pain, urinary frequency, and urinary tract infections [14, 33,34,35]. Most patients present with advanced disease and one third are unresectable at diagnosis [36].
Prognosis of UrC depends on tumor stage. Prognostic factors for impaired survival are lymph node metastasis, tumor growth in the abdominal wall, peritoneum and/or adjacent organs, distant metastasis, and macroscopic residual tumor [5]. The most common sites of metastasis are lung, liver, and bone [37]. All urachal mucinous neoplasms, regardless of tumor type, tend to behave in an aggressive manner that includes the development of pseudomyxoma peritonei [38]. Pseudomyxoma peritonei should be considered in the differential diagnosis of abdominal and retroperitoneal tumors, especially in the case of abdominal cystic-solid masses [39].
The cases analyzed in the present study are all from the same study center, the Central Hospital of Bolzano, South Tyrol, Italy. The incidence of urachal carcinoma is 1 per 1 million per year, depending on the geographical region [40]. Over 10 years, our region, with 560,000 inhabitants, has experienced exactly the above-mentioned incidence. Analysis of additional cases would be of great interest. Unfortunately, our histopathologic casistic limited our access to further cases, also on account of the strict selection criteria used according to Gopalan et al. [10]. Our intent was to provide a comprehensive picture of this pathology in our region in order to better frame and contextualize the disease in our geographical area. To achieve our goal, we scrutinized this rare disease by means of a meticulous approach comprising imaging methods, surgical resection strategies, and characterization of the gross, morphologic, immunohistochemical, and molecular profile.
Due to its infrequency, there are no guidelines for the management of this tumor [18]. Surgery is the mainstay of treatment, and long-term survival following surgical intervention is seen in a significant number of patients [41, 42]. Lymph node dissection is not required unless nodal involvement is confirmed by preoperative examination [12, 13]. Robotic surgery can add to the benefits of the conventional laparoscopic approach and can therefore be used in the treatment of abdominal wall masses [14]. Postoperatively, there is no data to support chemotherapy in the adjuvant setting, leaving the option for unresectable and/or metastatic disease. Individualized therapies are encouraged with radiotherapy and/or chemotherapy regimens [15]. A modified combination of 5-fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) appears efficacious for metastatic UrC [42]. Treatment with sunitinib may cause partial necrosis of the tumor paralleled by symptom improvement [18]. Anecdotal response to systemic therapies utilized in colorectal cancer and to drugs targeting the epidermal growth factor receptor has been reported [43]. Adjuvant regimens including a combination of cisplatin and 5-fluorouracil have shown partial tumor regression in small cohorts with late metastatic or recurrent disease [21]. Alternatively, patients with peritoneal spread might benefit from HIPEC (hyperthermic intraperitoneal chemotherapy) [44].
Conclusions
UrC is a rare neoplasm with poor prognosis, often presenting at an advanced stage. An early diagnosis is often difficult due to the lack of specific signs and symptoms. US is the first choice for diagnosis of urachal malignancies; nevertheless, the accuracy of preoperative diagnosis remains poor. Although morphology may not be relevant, UrC shows anatomic, immunohistochemical, and molecular features that can lead to the final diagnosis. Besides management strategies that rely on surgical resection with recommendations encouraging en bloc excision including the umbilicus, individualized therapy could be taken into consideration on a case-by-case basis.
References
Scabini S, Rimini E, Romairone E, Scordamaglia R, Vallarino L, Giasotto V, Ferro C, Ferrando V (2009) Urachal tumour: case report of a poorly understood carcinoma. World J Surg Oncol 7:82. https://doi.org/10.1186/1477-7819-7-82
Sheldon CA, Clayman RV, Gonzalez R, et al (1984) Malignant urachal lesions. J Urol 131(1): 1–8
Schnur J, Nguyen S, Divino C, Heimann T, Vidal C (2009) Coexisting rectal and urachal carcinoma: a case report. Am J Clin Oncol 32:220–221. https://doi.org/10.1097/01.coc.0000230170.25322.cd
Van Calsteren K, Van Mensel K, Joniau S et al (2006) Urachal carcinoma during pregnancy. Urology 67:1290.e19–1290.e21. https://doi.org/10.1016/j.urology.2005.12.041
Kamat AM (2013) Commentary on “the clinical epidemiology of urachal carcinoma: results of a large, population based study.” bruins HM, Visser O, Ploeg M, Hulsbergen-van de Kaa CA, Kiemeney LA, Witjes JA, Department of Urology, Radboud University medical Centre, Utrecht. Urol Oncol Semin Orig Investig 31:720
Nagasaki A, Handa N, Kawanami T (1991) Diagnosis of urachal anomalies in infancy and childhood by contrast fistulography, ultrasound and CT. Pediatr Radiol 21:321–323
Bi X, Wu Z, Han H, Zhou F (2017) Clinical comparison of patients with benign urachal masses versus urachal carcinomas. Chin J Cancer 36:2
Amin MB, Smith SC, Eble JN, Rao P, Choi WWL, Tamboli P, Young RH (2014) Glandular neoplasms of the urachus: a report of 55 cases emphasizing mucinous cystic tumors with proposed classification. Am J Surg Pathol 38:1033–1045. https://doi.org/10.1097/PAS.0000000000000250
Paner GP, Lopez-Beltran A, Sirohi D, Amin MB (2016) Updates in the pathologic diagnosis and classification of epithelial neoplasms of urachal origin. Adv Anat Pathol 23:71–83
Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, Olgac S (2009) Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 33:659–668. https://doi.org/10.1097/PAS.0b013e31819aa4ae
Darwanto A, Hein AM, Strauss S, Kong Y, Sheridan A, Richards D, Lader E, Ngowe M, Pelletier T, Adams D, Ricker A, Patel N, Kühne A, Hughes S, Shiffman D, Zimmermann D, te Kaat K, Rothmann T (2017) Use of the QIAGEN GeneReader NGS system for detection of KRAS mutations, validated by the QIAGEN Therascreen PCR kit and alternative NGS platform. BMC Cancer 17:358. https://doi.org/10.1186/s12885-017-3328-z
Chen D, Li Y, Yu Z et al (2014) Investigating urachal carcinoma for more than 15 years. Oncol Lett 8:2279–2283. https://doi.org/10.3892/ol.2014.2502
Martín LB, Valbuena L, Castañón LB (2015) Urachal adenocarcinoma of the bladder, our experience in 20 years. Arch Esp Urol 68:178–182
Kosanovic R, Romero RJ, Arad JK, Gallas M, Seetharamaiah R, Gonzalez AM (2014) Rare use of robotic surgery for removal of large urachal carcinoma. J Robot Surg 8:177–180. https://doi.org/10.1007/s11701-013-0415-2
Collins DC, Velázquez-Kennedy K, Deady S, Brady AP, Sweeney P, Power DG (2016) National incidence, management and survival of urachal carcinoma. Rare Tumors 8:97–101. https://doi.org/10.4081/rt.2016.6257
Módos O, Reis H, Niedworok C et al (2016) Mutations of KRAS, NRAS, BRAF, EGFR, and PIK3CA genes in urachal carcinoma – occurence and prognostic significance. Oncotarget 7:39293–39301. https://doi.org/10.18632/oncotarget.9828
Ormeci T, Kiremit MC, Erkurt B, Örmeci A (2015) An unusual long-term survey of a patient with widespread malignant urachal tumor, not given chemotherapy or radiotherapy. Case Rep Radiol 2015:183787–183784. https://doi.org/10.1155/2015/183787
Testa I, Verzoni E, Grassi P, Colecchia M, Panzone F, Procopio G (2014) Response to targeted therapy in urachal adenocarcinoma. Rare Tumors 6:124–127. https://doi.org/10.4081/rt.2014.5529
Begg C (1930) The Urachus : its anatomy , histology and development. J Anat 64:170–183
Meeks JJ, Herr HW, Bernstein M, al-Ahmadie HA, Dalbagni G (2013) Preoperative accuracy of diagnostic evaluation of the urachal mass. J Urol 189:1260–1262. https://doi.org/10.1016/j.juro.2012.10.043
Siefker-Radtke AO, Gee J, Shen Y et al (2003) Multimodality management of urachal carcinoma: the M. D. Anderson Cancer center experience. J Urol 169:1295–1298. https://doi.org/10.1097/01.ju.0000054646.49381.01
Dong A, Zuo C, Wang Y, Lu J, Zhu H (2014) Organized urachal abscess mimicking urachal carcinoma on FDG PET/CT. Clin Nucl Med 39:71–73. https://doi.org/10.1097/RLU.0b013e31827a263e
Goldman IL, Caldamone AA, Gauderer M, Hampel N, Wesselhoeft CW, Elder JS (1988) Infected urachal cysts: a review of 10 cases. J Urol 140:375–378. https://doi.org/10.1016/S0022-5347(17)41612-0
Guarnaccia S, Pais V, Grous J, Spirito N (1991) Adenocarcinoma of the urachus associated with elevated levels of CA 125. J Urol 145:140–141. https://doi.org/10.1016/S0022-5347(17)38271-X
Behrendt MA, De Jong J, Van Rhijn BWG (2016) Urachal cancer: contemporary review of the pathological, surgical, and prognostic aspects of this rare disease. Minerva Urol Nefrol 68:172–184
Siefker-Radtke A (2006) Urachal carcinoma: surgical and chemotherapeutic options. Expert Rev Anticancer Ther 6:1715–1721
Niu HT, Dong P, Wang JN, Huang J, Zeng YX (2016) Analysis of treatment and prognosis in post-operative patients with urachal carcinoma. Zhonghua Yi Xue Za Zhi 96:1923–1925. https://doi.org/10.3760/oma.j.issn.0376-2491.2016.24.011
Bissonnette MLZ, Kocherginsky M, Tretiakova M, Jimenez RE, Barkan GA, Mehta V, Sirintrapun SJ, Steinberg GD, White KP, Stricker T, Paner GP (2013) The different morphologies of urachal adenocarcinoma do not discriminate genomically by micro-RNA expression profiling. Hum Pathol 44:1605–1611. https://doi.org/10.1016/j.humpath.2013.01.008
Paner GP, McKenney JK, Barkan GA et al (2011) Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: diagnostic implications and pitfalls. Am J Surg Pathol 35:787–798. https://doi.org/10.1097/PAS.0b013e3182189c11
Singh I, Prasad R (2013) Primary urachal mucinous adenocarcinoma of the urinary bladder. J Clin Diagn Res 7:911–913. https://doi.org/10.7860/JCDR/2013/5597.2973
HOWARD AH, BERGMAN RT (1948) Mucous adenocarcinoma of the urinary bladder. J Urol 59:455–460. https://doi.org/10.1016/S0022-5347(17)69397-2
Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA (2007) Predictors of survival from urachal cancer: a mayo clinic study of 49 cases. Cancer 110:2434–2440. https://doi.org/10.1002/cncr.23070
Mizusawa H, Oguchi T, Domen T, Koizumi K, Mimura Y, Saito T, Kato H (2014) Two cases of lower abdominal tumors difficult to differentiate from urachal tumors. Nihon Hinyokika Gakkai Zasshi 105:17–21. https://doi.org/10.5980/jpnjurol.105.17
Yoshida Y, Yamanaka K, Ueda N et al (2014) A case of urachal carcinoma with multiple lung metastases treated by TS-l/CDDP chemotherapy. Acta Urol Jpn 60:147–150
Nakamura K, Terada N, Kobayash T et al (2013) Pseudomyxoma peritonei arising from urachal carcinoma. Acta Urol Jpn 59:657–662
Behrendt MA, van Rhijn BWG (2016) Genetics and biological markers in urachal cancer. Transl Androl Urol 5:655–661. https://doi.org/10.21037/tau.2016.04.01
Kapoor R, Darasani N, Bansal A (2016) Rare presentation of urachal adenocarcinoma with skip metastasis to colon. Indian J Urol 32:244–246. https://doi.org/10.4103/0970-1591.185106
Prakash MR, Vijayalaxmi SV, Maitreyee R, Ranjit KP (2014) Complex mucinous cystadenoma of undetermined malignant potential of the urachus: a rare case with review of the literature. Malays J Pathol 36:145–148
Liang L, Zhou N, Xu H, Liu D, Lu Y, Li F, Guo J (2017) Urachal mucinous adenocarcinoma with pseudomyxoma peritonei: a case report. Medicine (Baltimore) 96:e7548. https://doi.org/10.1097/MD.0000000000007548
Bruins HM, Visser O, Ploeg M, Hulsbergen-van de Kaa CA, Kiemeney LALM, Witjes JA (2012) The clinical epidemiology of urachal carcinoma: results of a large, population based study. J Urol 188:1102–1107. https://doi.org/10.1016/j.juro.2012.06.020
Zong L, Chen P (2013) Surgical and chemotherapeutic experience regarding a urachal carcinoma with repeated relapse: case report and literature review. World J Surg Oncol 11(1):170. https://doi.org/10.1186/1477-7819-11-170
Yanagihara Y, Tanji N, Miura N, Shirato A, Nishimura K, Fukumoto T, Azuma K, Miyauchi Y, Kikugawa T, Yokoyama M (2013) Modified FOLFOX6 chemotherapy in patients with metastatic urachal cancer. Chemotherapy 59:402–406. https://doi.org/10.1159/000362400
Collazo-Lorduy A, Castillo-Martin M, Wang L, Patel V, Iyer G, Jordan E, al-Ahmadie H, Leonard I, Oh WK, Zhu J, McBride RB, Cordon-Cardo C, Solit DB, Sfakianos JP, Galsky MD (2016) Urachal carcinoma shares genomic alterations with colorectal carcinoma and may respond to epidermal growth factor inhibition. Eur Urol 70:771–775. https://doi.org/10.1016/j.eururo.2016.04.037
Krane LS, Kader AK, Levine EA (2012) Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for patients with peritoneal carcinomatosis secondary to urachal adenocarcinoma. J Surg Oncol 105:258–260. https://doi.org/10.1002/jso.22081
Funding
Internal Funding, Department of Pathology and Diagnostics, University and Hospital Trust of Verona, and Internal Funding, Department of Pathology, Central Hospital of Bolzano.
Author information
Authors and Affiliations
Contributions
Riva G.: study design; data collection; data interpretation; manuscript preparation; literature search; review and approval of the final manuscript. Mian C.: study design; data collection; molecular analysis; data interpretation; review and approval of the final manuscript. Luchini C.: review and approval of the final manuscript. Girolami I.: review and approval of the final manuscript. Ghimenton C.: review and approval of the final manuscript. Cima L.: review and approval of the final manuscript. Novelli L.: review and approval of the final manuscript. Hanspeter E.: data collection; data interpretation; review and approval of the final manuscript. Mazzoleni G.: data collection; data interpretation; review and approval of the final manuscript. Schwienbacher C.: molecular analysis; data interpretation; review and approval of the final manuscript. Pycha S.: review and approval of the final manuscript. D’Elia C.: review and approval of the final manuscript. Trenti E.: review and approval of the final manuscript. Pycha A.: data collection; review and approval of the final manuscript. Eccher A.: study design; data collection; data interpretation; review and approval of the final manuscript. Nesi G.: review and approval of the final manuscript. Brunelli M.: review and approval of the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
All the procedures for this study were in accordance with the ethical standards of local institution (authorization no 36-2018, Bolzano) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of interest
The authors declare that they have no conflict of interests.
Informed consent
informed consent was acquired from patients in order to perform all investigations and to allow use of colected results.
Rights and permissions
About this article
Cite this article
Riva, G., Mian, C., Luchini, C. et al. Urachal carcinoma: from gross specimen to morphologic, immunohistochemical, and molecular analysis. Virchows Arch 474, 13–20 (2019). https://doi.org/10.1007/s00428-018-2467-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2467-1