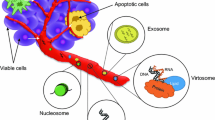
Abstract
The ability to detect cancer cells in the blood or in the bone marrow offers invaluable information which potentially impacts early diagnosis, monitoring of treatment, and prognosis. Accessing blood or other body fluids has the additional advantage of being less invasive than biopsy. Consequently, considerable effort has been invested in the last 20 years in optimizing assays which may identify malignant cells at these anatomic sites. Detection of nucleic acids has been applied as alternative approach in this context, first targeting single cancer-associated genes using PCR-based technology, and recently using assays which identify different DNA classes, as well as microRNAs and exosomes. The present review focuses on studies which applied these assays to the detection of cells or cellular components originating from gynecological cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The observation that certain populations of cancer cells carry the ability to leave the primary tumor, survive in the circulation, and metastasize in an organ-specific manner has been first reported by Stephen Paget, and subsequently supported by a large body of experimental and clinical data [1]. Although not all cells in the bloodstream are endowed with the ability to colonize distant organs, it appeared logical that their presence outside the primary organ would be a marker of more aggressive disease. Additionally, the detection of such cells in the peripheral circulation could potentially be used in the primary diagnosis of cancer or in early identification of relapse.
Analyses of peripheral blood for the detection of circulating tumor cells (CTCs) have most often been used in this setting [2, 3]. However, numerous studies, particularly of breast cancer, have focused on disseminated cancer cells (DTCs) in the bone marrow [4]. In these studies, tumor cells have been detected using immunohistochemistry (IHC) or flow cytometry (FCM) targeting epithelial/tumor-specific antigens or by PCR-based methods [2,3,4].
Recent scientific advances have uncovered the presence of cell-free DNA (cfDNA), and in particular circulating tumor DNA (ctDNA), as well as microRNAs (miRNAs) and long non-coding RNA (lncRNA), in body fluids, raising interest in the identification of sub-cellular markers in so-called liquid biopsies as tools in the diagnosis and monitoring of cancer, including prediction of therapeutic response and prognostication. An obvious advantage to this approach is the fact that it is less invasive than tissue biopsies, with superior accessibility compared to sampling deep-seated lesions, the latter often the case in metastatic disease. While blood has been the most frequently studied material, other body fluids, e.g. serous effusions and urine, may provide useful information guiding clinical management [5,6,7].
The present review focuses on studies of CTCs and DTCs in gynecological cancers. Selected, more recent studies dealing with the benefit of analyzing sub-cellular components in body fluids are additionally discussed.
CTC and DTC
Studies of CTC or DTC in gynecological cancers have predominantly focused on ovarian carcinoma (OC), though some studies of endometrial, cervical, and vulvar carcinoma have been published.
Early reports
The first modern-era studies analyzing the presence of CTCs in the blood were published more than 50 years ago, the majority in the 1960s, and many of these studies did not exclusively focus on gynecological cancer. In the absence of ancillary techniques, the diagnosis was based on morphology, resulting in potential misinterpretation. The potential use of fluorochromes was assessed by Turchetti and co-workers but was conceded to also label leukocytes [8].
Methodological factors, including hemolysis, filtration, and labeling, have likely contributed to the highly variable rate of CTC detection in these studies [reviewed in 8]. Terminology which differs from the current one, such as detection of “atypical cells” in patients with uterine leiomyoma or ovarian cyst, makes interpretation of these data further difficult [9]. In the largest study focusing exclusively on gynecological cancer, 474 blood samples from patients with endometrial carcinoma were assessed, of which 45 and 16 were highly suspicious and inconclusive, respectively. The authors judiciously concluded that a certain diagnosis of malignancy could not be made in these specimens [10]. Of note, cells diagnosed as carcinoma cells were reported to be present in the blood of patients with CIN3, though infrequently [11].
OC
The presence and clinical relevance of CTCs and/or DTCs in OC has been extensively investigated. A meta-analysis and a review of studies with clinical endpoint have both concluded that the presence of such cells is associated with poor outcome in this disease [12, 13]. However, data are more equivocal, and studies published to date are far from unanimous. While technical variations most likely play a central role in this result, inclusion criteria are no less important, particularly in view of our current understanding that the different OC histotypes are molecularly distinct diseases. Inclusion of borderline tumors and non-epithelial tumors in some of these studies further complicates attempts to compare them.
Following a long pause after the above-discussed studies in the 1960s, probably dictated by technical limitations, the first study ushering in modern technology in this area was published by Braun et al. in 2001 [14]. Fine needle aspirates from the bone marrow of 108 patients diagnosed with FIGO stage I–III OC of different histotype were assessed using the A45-B/B3 antibody, directed against heterodimers of cytokeratin (CK) 8/18 and 8/19, as well as a common CK epitope. The presence of tumor cells in the bone marrow, observed in 32/108 (30%) patients, was associated with the presence of extra-peritoneal metastases (19/32 patients, compared to 2/76 of bone marrow-negative cases), and was an independent marker of distant disease-free survival (DFS) and overall survival (OS) in multivariate analysis.
These results were not reproduced in a study published in 2002, in which blood specimens from 90 patients, of whom 73 additionally had bone marrow aspirates, were assessed. Tumors were of different histotype in this study as well, including four borderline tumors. Tumor cells, detected using a MOC31 antibody, were found in the bone marrow and blood in 15/73 (21%) and 11/90 (12%) of specimens, respectively. Patients with tumor cells in the peripheral blood all had tumor in the bone marrow. Detection of tumor cells in either of these compartments was unrelated to progression-free survival (PFS) or OS [15].
Banys et al. analyzed the presence of DTCs in bone marrow aspirates from 112 OC patients with tumors of unspecified histology using the A45-B/B3 antibody. DTCs were found in 28/112 (25%) specimens, and their presence was associated with increased risk of relapse and shorter relapse-free survival, though it was unrelated to various clinicopathologic parameters or OS [16].
Subsequent studies focusing on OC have similarly generated mixed data. Judson and co-workers analyzed blood samples from 64 women with newly diagnosed or recurrent ovarian tumors, the latter consisting of various entities, including carcinomas, carcinosarcomas, borderline tumors, and one carcinoid tumor. CTCs were found in 12 cases (18.7%) using CK8/18 and EGFR antibodies, and their presence was unrelated to PFS or OS based on a relatively short follow-up period with a mean of 18.7 months [17].
Fan et al. analyzed a series of blood samples from 66 patients with OC of various histology, in which cells were identified as invasive CTCs (iCTCs) based on expression of epithelial markers (EpCAM, ESA, and pan-cytokeratin) and invasion of cell adhesion matrix. iCTCs were found in 43/66 (60.6%) of samples, and their presence or higher iCTC counts were directly associated with more advanced (FIGO III/IV) stage, disease recurrence, and shorter DFS, though not significantly with OS, with a median follow-up period of 18 months [18].
The same group published three more recent papers using the same assay for iCTC selection, in which cells were assessed using FCM [19,20,21]. The first study included blood samples from 129 patients, of whom 88 had OC and 41 benign abdominal diseases. Seventy-eight (88.6%) OC patients had > 5 iCTC/1 mL blood, defined as positive finding, and the latter was significantly related to more advanced disease, and to shorter PFS and OS. iCTC counts outperformed CA 125 in identifying patients with OC in general, as well as those with high-risk disease [19]. In the second study, iCTC, labeled by antibodies against CD44 and seprase, were reported to be more sensitive than CA 125 in monitoring disease progression [20], whereas in the third one, the feasibility of using this assay for ex vivo testing of chemoresistance was demonstrated [21].
He et al. developed an assay based on FCM and in vivo imaging using an antibody against folate receptor, which was able to detect rare CTCs in the peripheral blood in a murine model [22]. Application of the folate receptor antibody to 20 blood specimens from patients with tumors of various histologies led to detection of CTCs in 18 specimens [23].
Poveda and co-workers studied a cohort of 216 patients with OC, defined as “papillary/serous” or “other” who participated in a phase III study comparing treatment with pegylated liposomal doxorubicin (PLD) with trabectedin to PLD alone in relapsed disease. CTCs in the peripheral blood were isolated using the FDA-approved CellSearch system. Patients with ≥ 2 CTCs prior to therapy (n = 31; 1.4%) had significantly higher risk of progression or death in univariate analysis, though this association was not significant in multivariate analysis [24].
Liu et al. used the same platform and same cutoff (CellSearch system, ≥ 2 CTCs) as Poveda in analysis of the clinical relevance of CTCs in a series of 78 patients, including 30 newly diagnosed ones and 48 with recurrent disease. Histology was specified for all patients, among whom 57 had serous carcinoma. CTC counts were comparable in newly diagnosed and recurrent tumors. No differences in clinicopathologic parameters, PFS or OS were observed between patients with ≥ 2 CTCs in the blood and those with CTC-negative specimens [25].
In another study of 214 patients with different epithelial malignancies, including 14 with OC, recruited for phase I clinical trials, early variations in CTC counts, measured using the CellSearch system, were not useful in predicting radiologic response based on RECIST criteria [26].
Lee et al. analyzed blood samples from 54 patients, including 24 with newly diagnosed OC and 30 with recurrent disease. Tumors were of various histotypes. CTCs were identified as EpCAM- and DAPI-positive cells negative for CD45. The presence of CTC clusters was significantly associated with platinum resistance, whereas detection of ≥ 3 CTCs was associated with worse PFS, though unrelated to OS [27].
Detection of CTCs based on a multi-parameter assay has been the subject of several studies. Aktas et al. analyzed the presence of CTCs in a series of 156 blood specimens, 70 obtained at diagnosis and 86 after chemotherapy, from 122 patients with tumors of different histotype, of which 95 were serous, 8 mucinous and 18 designated as “other histotypes” [28]. CTCs were detected using the AdnaTest BreastCancer which identifies the EpCAM, MUC1, and HER2 transcripts, combined with detection of CA 125. The presence of DTCs was additionally analyzed in bone marrow aspirates by IHC using the A45-B/B3 antibody. CTCs were found in 19 and 27% of pre-operative and post-chemotherapy specimens, respectively. DTCs were found in 33/95 (35%) analyzed specimens. The presence of DTCs could be assessed at the same time point before surgery in 79 cases, with 59% concordance observed with the presence of CTCs, whereas similar analysis for 43 post-chemotherapy cases showed 56% concordance. Detection of CTCs in preoperative and post-chemotherapy specimens was associated with shorter OS and was unrelated to DFS or clinicopathologic parameters.
In a more recent study, the same group combined the AdnaTest Ovarian Cancer, detecting EpCAM, MUC1, and CA 125 transcripts, with detection of the DNA repair molecule ERCC1 in analysis of paired blood specimens obtained pre- and post-chemotherapy from 65 OC patients. As in the previous study, tumors were diagnosed as serous (n = 52), mucinous (n = 9) or “other” (n = 4) and a three-tier grading system, rather than the WHO 2014 guidelines, was applied. CTC were detected in 8% of pre-surgery specimens using the AdnaTest, in 17% using the ERCC1 test, and in 15% by both tests, with comparable values in post-chemotherapy samples. The presence of CTC detected by both assays in pre-treatment specimens was significantly associated with platinum resistance and shorter PFS and OS. Persistence of these cells post-chemotherapy was similarly associated with poor outcome [29].
Obermayr et al. recently studied blood samples from 137 patients with OC, described as serous or non-serous and graded based on the pre-WHO 2014 classification. Forty-three patients had blood samples at diagnosis and 6 months after completion of first-line chemotherapy, 59 only at diagnosis, and 35 only post-chemotherapy. CTCs were identified by immunofluorescent staining for EpCAM, EGFR, HER2, MUC1 and cytokeratins, a protocol found by the authors to be superior to a protocol based on EpCAM and CD45 alone. The presence of CTCs in specimens obtained at diagnosis was unrelated to outcome, whereas their detection in post-treatment samples was significantly related to worse PFS and OS [30].
Of note, an earlier study by the same group recommended a protocol in which 11 genes were added to EPCAM, with the requirement that at least 1 of them will be positive in order to characterize cells as CTCs. The genes in the suggested panel were PPIC, GPX8, CDH3, TUSC3, COL3A1, LAMB1, MAM, ESRP2, AGR2, BAIAP2L1, and TFF1. PPIC, encoding cyclophilin C, outperformed EPCAM in this study, and the presence of PPIC-positive CTCs was associated with chemoresistance [31].
A protocol combining the AdnaTest with single-cell characterization of CTCs using a gene panel including cancer stem cell (CSC) and epithelial-to-mesenchymal transition (EMT) markers was recently published [32].
Tumor heterogeneity appears to be a relevant issue complicating the assessment of CTCs, similarly to its central role in determining the behavior of cancer cells in solid lesions. Pecot and co-workers reported on the presence of aneuploid CK-negative tumor cells in the blood of patients diagnosed with ovarian, breast, and colorectal carcinoma. Loss of epithelial markers was the result of EMT undergone by cancer cells [33]. A study in which CTC detection using conventional markers was combined with fluorescent analysis of protein and mRNA expression of various cancer-associated molecules using immunostaining and in situ hybridization, respectively, was recently published [34].
Other gynecological cancers
The number of publications focusing on DTCs or CTCs in patients with non-ovarian carcinomas is considerably smaller than those which have investigated OC.
The study by Banys [16] analyzed, in addition to bone marrow aspirates from OC patients, specimens from endometrial, cervical, and vulvar cancer patients. As for OC, histology was not detailed. DTCs were found in 22/141 (16%), 19/102 (19%), and 1/22 (5%) cases of endometrial, cervical, and vulvar cancer, respectively. In endometrial cancer, no association with clinicopathologic factors or survival was found, whereas in cervical cancer, the presence of DTCs was associated with more advanced FIGO stage, larger tumor size, and lymph node metastasis.
In a more recent analysis of the clinical role of DTCs, a series of 603 bone marrow aspirates from patients with endometrial, cervical, and vulvar were analyzed applying the A45-B/B3 antibody. These patients, as those in the Banys series, were treated at Tübingen University Hospital, the difference between the series being recruiting in the years 2001–2007 vs. 2001–2012, making it likely that the larger study represents expansion of the Banys series. As in the previous study, histology was not specified. DTCs were found in 64/311 (21%), 37/228 (16%), and 10/64 (16%) cases of endometrial, cervical, and vulvar cancers, respectively. Their presence was associated with more advanced FIGO stage, lymphangiosis, and lymph node metastasis in cervical cancer, but not in the other two cancers. No association with survival was observed in any of the three malignancies [35].
The same results, i.e., association with disease stage and lymph node metastasis and absence of association with survival, was reported in a third study of DTCs in cervical cancer based on the Tübingen series, this time with inclusion of patients from Munich University Hospital (total = 325) [36].
In a fourth study based on the Tübingen cohort, 395 endometrial carcinoma patients were analyzed for the presence and clinical relevance of DTCs, detected using the A45-B/B3 antibody. In this study, tumors underwent central pathology review, in which 339 (86%), were classified as endometrioid, the remaining cases consisting of 35 serous, 5 clear cell, and 16 mixed histology carcinomas. The presence of DTCs was negatively related to a microcystic elongated and fragmented (MELF) pattern of invasion, but was unrelated to other clinicopathologic parameters, to L1CAM expression or to survival [37].
Several smaller studies investigated the presence and clinical relevance of CTCs in endometrial and cervical cancer. Lemech et al. found CTCs in 18 of 30 patients with advanced-stage (FIGO III–IV) disease, and their presence was more frequently associated with non-endometrioid histology, larger tumors, stage IV disease, and shorter survival, though differences were not statistically significant [38].
In the study of Alonso-Alconada, 34 pre-treatment blood samples from patients with high-risk endometrial carcinoma were analyzed for the presence of CTCs and the molecular characteristics of the latter. Tumors consisted of 19 endometrioid, 10 serous, and 5 clear cell carcinomas, diagnosed at stage IB-IV. Among genes overexpressed in specimens from patients with stage III–IV carcinomas and recurrences compared with healthy controls were molecules related to EC pathogenesis (BRAF, CTNNB1, GDF15), the NF-κB family member RELA, RUNX1, genes related to hormone pathways (STS), and CSC markers (ALDH, CD44). CTCs additionally expressed EMT markers, and expression of ZEB2, as well as RUNX1, was associated with disease recurrence [39].
A protocol applying digital direct RT-PCR to detection of CTCs containing HPV RNA was published by Pfitzner et al. [40].
Another protocol, combining pan-CK and adenovirus detecting cells expressing telomerase was recently published in a study of 23 cervical squamous cell carcinomas [41]. CTCs were found in six patients and harbored the same HPV type as the primary tumor in five of these cases. Notably, the isolated cells were CK-negative, consisting of a population which may remain undetected using standard protocols, as in the report by Pecot [33].
Detection of sub-cellular components
Numerous studies which have focused on detection of different sub-cellular tumor components rather than whole cells have been published in the last 20 years.
Early studies applied PCR-based assays to detection of specific gene products expressed by carcinoma cells, such as HPV [42], squamous cell carcinoma antigen [43], CK19 [44,45,46], CK20 [45, 47], and EGFR [45]. While such analyses may provide evidence that a patient has cancer in the setting of primary diagnosis or disease recurrence, they do not constitute evidence of the presence of tumor cells in the blood, as these molecules may have their origin in the primary tumor or in metastases. In addition to this limitation, molecules such as CKs are also expressed in normal cells, a fact which may reduce the specificity of such assays, as reported in the case of CK19 [45].
The application of more advanced technology, particularly next generation sequencing, has dramatically changed this field, suggesting the feasibility of early detection of cancer using a non-invasive test in the primary diagnostic setting or in monitoring of patients for disease recurrence by analyzing cfDNA, or specifically its tumor-originated fraction, ctDNA [48, 49].
As with CTCs, studies of OC are far more numerous than those focusing on other gynecological malignancies. Selected publications from recent years are discussed below.
OC
Kamat et al. measured the levels of plasma cell-free DNA (cfDNA) using qPCR in a series of 288 specimens from patients diagnosed with OC (n = 164), benign ovarian tumors (n = 49), and controls (n = 75) divided into training and validation sets. OC were classified as serous or non-serous, low-grade or high-grade. Specimens from OC patients had significantly higher cfDNA levels compared to the two other groups, and levels > 22,000 genome equivalents/mL were significantly associated with poor outcome, a finding retained in multivariate analysis [50].
High levels of cfDNA were similarly associated with poor PFS and OS, a finding which remained significant in multivariate analysis, in a study of 144 patients with multiresistant OC treated with Bevacizumab, the majority diagnosed with serous carcinoma [51].
The presence of small extrachromosomal circular DNA (eccDNA), called micro-DNA, in the serum of lung cancer (n = 12) and OC (n = 11) patients was reported by Kumar et al. OC consisted of serous and endometrioid carcinomas, as well as two cases designated as “ovarian cancer.” MicroDNA levels decreased following surgical removal of the tumor [52].
Wimberger and co-workers analyzed the presence of cfDNA and nucleosomes in matched pre- and post-chemotherapy serum samples from 62 OC patients. Nucleosome levels increased, whereas DNA levels decreased following chemotherapy. High serum DNA levels pre-chemotherapy were associated with higher residual disease volume and higher risk of relapse, whereas high post-chemotherapy levels were significantly related to poor OS [53].
Vanderstichele et al. analyzed the diagnostic role of copy-number alteration (CNA) profiling in cfDNA in patients with adnexal mass, including 54 diagnosed with carcinoma, 3 with borderline tumor, and 11 with benign tumors, as well as 44 controls. Chromosomal instability, quantitated as genome-wide z-scores, was significantly higher in patients with carcinoma compared to those with benign tumors or controls, and this test outperformed CA 125 measurement or the risk of malignancy (RMI) index [54].
Cohen et al. demonstrated the feasibility of using a non-invasive pre-natal platform for detecting both early- and advanced-stage high-grade serous carcinoma (HGSC). In this study, sub-chromosomal changes, defined as genomic gains or losses of ≥ 15 MB, were identified in 13/32 samples from HGSC patients compared to 2/32 controls. Changes in whole chromosomes were less informative [55].
The diagnostic potential of plasma or serum cell-free miRNA in OC was previously reviewed [7]. miRNAs are often packed in exosomes, 30–100 nm endosome-derived vesicles carrying mRNA, miRNA, long non-coding RNA (lncRNA), proteins, and lipid. While exosomes may be isolated from blood, they are also present in effusion specimens in OC and contain both miRNAs and lncRNAs, which are informative of chemoresponse and outcome in this cancer [56, 57].
Other gynecological cancers
Nucleosomes were shown to be present in the serum of patients with cervical carcinoma (n = 11; squamous, adenosquamous, or adenocarcinoma), and their levels were higher than those of controls. Their levels increased in five and decreased in six patients following chemotherapy, with no significant difference among these groups with respect to treatment response [58].
The presence of viral-cellular integration sequences, which are the result in HPV integration in the human genome, was observed in 5/21 cell-free sera from cervical cancer patients, and their presence was associated with significantly shorter recurrence-free survival [59].
In analysis of 109 endometrial carcinomas, cfDNA was significantly more frequently found in serous (n = 19) or clear cell (n = 3) compared to endometrioid (n = 87) carcinomas (36.4% vs. 13.8%, respectively), and its levels were significantly higher in grade 2 or 3 endometrioid carcinomas compared to grade 1 tumors. No association was observed with disease stage [60].
Concluding remarks and future directions
While several groups, particularly in Europe, have gained considerable experience and technical competence in detecting CTCs and DTCs, this approach has failed to achieve more universal acceptance in the primary diagnosis or disease monitoring of gynecological cancers. The reasons for this likely include the costs, in terms of personnel and equipment, and the technical complexity of this procedure, as well as the mixed results of the above-discussed studies. Data for vulvar, cervical, and endometrial cancer are by and large negative, and while data for OC are on the whole more positive, drawing any certain conclusions from published papers is difficult at best. Many of the studies of gynecological cancers, including those not cited in this review, lack central pathology review of the cases included and/or adequate description of histology. Combining tumors currently accepted to represent profoundly different disease at the molecular and clinical levels presents another difficulty. Variation in technical aspects further hampers the ability to compare data. Finally, studies in which CK-negative tumor cells were found in the circulation further compound this issue, questioning the sensitivity of some of these assays. More than 15 years after modern analyses of CTCs and DTCs were first published, their universal inclusion in standard stratification of gynecological patients appears unlikely.
Cutting-edge technology now appears to be instead directed to measurement of cfDNA levels, or even more so, to identification of more specific gene signatures which may aid in early detection, identify disease recurrence early, and provide information with respect to actionable mutations or genetic changes marking chemoresistance.
In the primary diagnosis setting, analyses of methylation signatures were reported to aid in the early diagnosis of OC, e.g., the combination of COL23A1, C2CD4D, and WNT6 methylation identified by Widschwendter and co-workers [61].
Färkkilä et al. analyzed 120 serial plasma samples collected prospectively from 35 patients with adult granulosa cell tumor for the presence of FOXL2 402C>G (C134W) mutation, diagnostic of this tumor, using digital droplet PCR [62]. FOXL2 mutation in ctDNA was detected in 12/33 (36%) patients with measurable disease at the time of sample collection, both at the primary diagnosis (6/17; 35%) and recurrence (6/31; 19%) setting, suggesting that this assay can be used as non-invasive test in this tumor.
Detection of TP53 mutations in primary cancer diagnosis or in disease monitoring has been the subject of several studies [63,64,65]. Forshew and co-workers designed a set of 48 primer pairs covering the coding regions of TP53 and PTEN, and selected regions in EGFR, BRAF, KRAS, and PIK3CA. Following assay testing in formalin-fixed paraffin-embedded (FFPE) ovarian tumor tissue, the test was applied to seven plasma samples, all of which were positive for TP53 mutations in ctDNA. A de novo EGFR mutation absent in the primary tumor was additionally found in a recurrent case. Subsequent analysis of 62 plasma samples from 37 HGSC patients identified 39 mutations. Monitoring of ctDNA over time was additionally feasible [63].
In the study by Parkinson [64], the same research group assessed the possibility to monitor treatment response in HGSC based on TP53 status in ctDNA. Serial plasma samples collected from 40 patients with HGSC were tested for 31 TP53 mutations identified in their tumors in analysis of FFPE specimens. The TP53 mutant allele fraction (TP53MAF) correlated with radiologic tumor volume measurements and the TP53MAF to disease volume ratio was higher in relapsed compared to untreated patients. In relapsed disease, pre-treatment TP53MAF concentration, but not CA 125, was associated with time to progression. Response to chemotherapy was seen earlier with ctDNA than with CA 125.
Park and co-workers recently reported on good agreement between ctDNA, fresh frozen tissue and FFPE tissue in TP53 mutation analysis [65].
Monitoring of BRCA status is another potential setting in which ctDNA may have a role. Christie et al. analyzed plasma and tumor specimens from 30 patients diagnosed with HGSC who had BRCA1 or BRCA2 germline mutation, including 14 patients with samples prior to primary debulking surgery and 16 patients with disease recurrence. BRCA mutations were found in all tumors. However, reversion mutations predicted to restore the BRCA1/2 open reading frame were found only in five cases, all from patients with recurrent disease. cfDNA showed this reversion in three of these five cases, and test sensitivity depended on the abundance of tumor-derived DNA. Reversion of BRCA status was associated with resistance to platinum or poly-ADP ribose polymerase (PARP) inhibitor therapy [66].
Weigelt et al. analyzed BRCA status in cfDNA from 19 patients with stage III/IV platinum-resistant or platinum-refractory OC (18 HGSC, 1 endometrioid) and 5 patients with breast cancer using massively parallel sequencing. Putative BRCA1 or BRCA2 reversion mutations were identified in 4 OC and 2 breast cancer patients. All 19 OC had TP53 mutation. Other mutations detected were in the NF1, ERCC4, RB1, and CHEK2 genes [67].
Martignetti and co-workers reported on FGFR2 fusion detected in ctDNA from a patient with serous OC. This assay had superior sensitivity compared to CA 125 in disease monitoring [68].
Morikawa et al. analyzed the presence of PIK3CA and KRAS mutations in tumor tissue and cfDNA in a series of 33 clear cell OC. PIK3CA mutation was found in tumor tissue in five cases and was demonstrated in the cfDNA in two of these patients. KRAS mutation was found in three tumors, and in one of these cases was also detected in cfDNA [69].
PIK3CA mutations were also detected in ctDNA from cervical cancer patients in 26/117 (22%) cases, the majority constituting squamous cell carcinomas. The presence of PIK3CA mutations was significantly associated with larger tumor size and shorter DFS and OS [70]. In another study, HPV DNA levels, measured in cfDNA, were reported to be affected by immunotherapy, suggesting a possibility to measure treatment response using this assay [71].
Cohen et al. recently identified a cancer-specific assay consisting of detection of mutations in ctDNA and measurement of circulating proteins, which they termed CancerSEEK. Applied to 1005 samples from patients with eight types of non-metastatic cancer, including OC, this test had sensitivity at 70%. The assay was positive in only 7/812 controls from healthy subjects, i.e., specificity of 99% [72].
This rapidly evolving field is likely to assume a central role in cancer management in the future.
References
Talmadge JE, Fidler IJ (2010) AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669
Wang H, Stoecklein NH, Lin PP, Gires O (2017) Circulating and disseminated tumor cells: diagnostic tools and therapeutic targets in motion. Oncotarget 8:1884–1912
Lianidou ES, Strati A, Markou A (2014) Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci 51:160–171
Pantel K, Alix-Panabières C (2014) Bone marrow as a reservoir for disseminated tumor cells: a special source for liquid biopsy in cancer patients. Bonekey Rep 3:584
Speicher MR, Pantel K (2014) Tumor signatures in the blood. Nat Biotechnol 32:441–443
Traver S, Assou S, Scalici E, Haouzi D, al-Edani T, Belloc S, Hamamah S (2014) Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum Reprod Update 20:905–923
Nakamura K, Sawada K, Yoshimura A, Kinose Y, Nakatsuka E, Kimura T (2016) Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol Cancer 15:48
Turchetti G, Palagi R, Gacci G (1968) Cancer cells in the blood stream: review and personal findings with the fluorochrome method. Gynaecologia 165:253–260
Yagi M (1963) Studies on tumor cells in the circulating blood of patients with malignant tumor. I. Cytological observations of tumor cells in the circulating blood. Jpn Obstet Gynecol Soc 10:69–76
Doran TA, Thompson DW (1966) Malignant cells in the peripheral blood of patients with endometrial carcinoma. Am J Obstet Gynecol 94:985–990
Song J, Nettles JB (1969) Circulating tumor cells in patients with carcinoma in situ of the cervix uteri. Am J Obstet Gynecol 104:713–726
Cui L, Kwong J, Wang CC (2015) Prognostic value of circulating tumor cells and disseminated tumor cells in patients with ovarian cancer: a systematic review and meta-analysis. J Ovarian Res 8:38
Romero-Laorden N, Olmos D, Fehm T, Garcia-Donas J, Diaz-Padilla I (2014) Circulating and disseminated tumor cells in ovarian cancer: a systematic review. Gynecol Oncol 133:632–639
Braun S, Schindlbeck C, Hepp F, Janni W, Kentenich C, Riethmüller G, Pantel K (2001) Occult tumor cells in bone marrow of patients with locoregionally restricted ovarian cancer predict early distant metastatic relapse. J Clin Oncol 19:368–375
Marth C, Kisic J, Kaern J, Tropé C, Fodstad Ø (2002) Circulating tumor cells in the peripheral blood and bone marrow of patients with ovarian carcinoma do not predict prognosis. Cancer 94:707–712
Banys M, Solomayer EF, Becker S, Krawczyk N, Gardanis K, Staebler A, Neubauer H, Wallwiener D, Fehm T (2009) Disseminated tumor cells in bone marrow may affect prognosis of patients with gynecologic malignancies. Int J Gynecol Cancer 19:948–952
Judson PL, Geller MA, Bliss RL et al (2003) Preoperative detection of peripherally circulating cancer cells and its prognostic significance in ovarian cancer. Gynecol Oncol 91:389–394
Fan T, Zhao Q, Chen JJ, Chen WT, Pearl ML (2009) Clinical significance of circulating tumor cells detected by an invasion assay in peripheral blood of patients with ovarian cancer. Gynecol Oncol 112:185–191
Pearl ML, Zhao Q, Yang J, Dong H, Tulley S, Zhang Q, Golightly M, Zucker S, Chen WT (2014) Prognostic analysis of invasive circulating tumor cells (iCTCs) in epithelial ovarian cancer. Gynecol Oncol 134:581–590
Pearl ML, Dong H, Tulley S, Zhao Q, Golightly M, Zucker S, Chen WT (2015) Treatment monitoring of patients with epithelial ovarian cancer using invasive circulating tumor cells (iCTCs). Gynecol Oncol 137:229–238
Pearl ML, Dong H, Zhao Q, Tulley S, Dombroff MK, Chen WT (2017) iCTC drug resistance (CDR) testing ex vivo for evaluation of available therapies to treat patients with epithelial ovarian cancer. Gynecol Oncol 147:426–432
He W, Wang H, Hartmann LC, Cheng JX, Low PS (2007) In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A 104:11760–11765
He W, Kularatne SA, Kalli KR, Prendergast FG, Amato RJ, Klee GG, Hartmann LC, Low PS (2008) Quantitation of circulating tumor cells in blood samples from ovarian and prostate cancer patients using tumor-specific fluorescent ligands. Int J Cancer 123:1968–1973
Poveda A, Kaye SB, McCormack R, Wang S, Parekh T, Ricci D, Lebedinsky CA, Tercero JC, Zintl P, Monk BJ (2011) Circulating tumor cells predict progression free survival and overall survival in patients with relapsed/recurrent advanced ovarian cancer. Gynecol Oncol 122:567–572
Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA (2013) Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol 131:352–356
Massard C, Borget I, Farace F, Aspeslagh S, le Deley MC, le Tourneau C, Bidard FC, Pierga JY, Dieras V, Hofman P, Spano JP, Ferte C, Lacroix L, Soria JC (2017) RECIST response and variation of circulating tumour cells in phase 1 trials: a prospective multicentric study. Eur J Cancer 83:185–193
Lee M, Kim EJ, Cho Y et al (2017) Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol 145:361–365
Aktas B, Kasimir-Bauer S, Heubner M, Kimmig R, Wimberger P (2011) Molecular profiling and prognostic relevance of circulating tumor cells in the blood of ovarian cancer patients at primary diagnosis and after platinum-based chemotherapy. Int J Gynecol Cancer 21:822–830
Chebouti I, Kuhlmann JD, Buderath P, Weber S, Wimberger P, Bokeloh Y, Hauch S, Kimmig R, Kasimir-Bauer S (2017) ERCC1-expressing circulating tumor cells as a potential diagnostic tool for monitoring response to platinum-based chemotherapy and for predicting post-therapeutic outcome of ovarian cancer. Oncotarget 8:24303–24313
Obermayr E, Bednarz-Knoll N, Orsetti B et al (2017) Circulating tumor cells: potential markers of minimal residual disease in ovarian cancer? A study of the OVCAD consortium. Oncotarget 8:106415–106428
Obermayr E, Castillo-Tong DC, Pils D, Speiser P, Braicu I, van Gorp T, Mahner S, Sehouli J, Vergote I, Zeillinger R (2013) Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—a study of the OVCAD consortium. Gynecol Oncol 128:15–21
Blassl C, Kuhlmann JD, Webers A, Wimberger P, Fehm T, Neubauer H (2016) Gene expression profiling of single circulating tumor cells in ovarian cancer—establishment of a multi-marker gene panel. Mol Oncol 10:1030–1042
Pecot CV, Bischoff FZ, Mayer JA, Wong KL, Pham T, Bottsford-Miller J, Stone RL, Lin YG, Jaladurgam P, Roh JW, Goodman BW, Merritt WM, Pircher TJ, Mikolajczyk SD, Nick AM, Celestino J, Eng C, Ellis LM, Deavers MT, Sood AK (2011) A novel platform for detection of CK+ and CK- CTCs. Cancer Discov 1:580–586
Wu Y, Park KJ, Deighan C et al (2016) Multiparameter evaluation of the heterogeneity of circulating tumor cells using integrated RNA in situ hybridization and immunocytochemical analysis. Front Oncol 6:234
Walter CB, Taran FA, Wallwiener M, Rothmund R, Kraemer B, Krawczyk N, Blassl C, Melcher C, Wallwiener D, Fehm T, Hartkopf AD (2014) Prevalence and prognostic value of disseminated tumor cells in primary endometrial, cervical and vulvar cancer patients. Future Oncol 10:41–48
Fehm T, Banys M, Rack B, Jäger B, Hartkopf A, Taran FA, Janni W (2014) Presence of disseminated tumor cells in bone marrow correlates with tumor stage and nodal involvement in cervical cancer patients. Int J Cancer 134:925–931
Kommoss S, Hartkopf AD, Krämer B, Bunz AK, Grevenkamp F, Kommoss F, Pasternak J, Arbabi SM, Wallwiener M, Staebler A, Lax SF, Brucker SY, Taran FA (2017) Disseminated tumor cells are not associated with established risk factors, L1CAM immunoreactivity and outcome in endometrial carcinoma. J Cancer Res Clin Oncol 143:2183–2188
Lemech CR, Ensell L, Paterson JC, Eminowicz G, Lowe H, Arora R, Arkenau HT, Widschwendter M, MacDonald N, Olaitan A, Mould T, Meyer T, Hartley J, Mitra A, Ledermann JA, McCormack M, Kristeleit RS (2016) Enumeration and molecular characterisation of circulating tumour cells in endometrial cancer. Oncology 91:48–54
Alonso-Alconada L, Muinelo-Romay L, Madissoo K et al; ENITEC Consortium (2014) Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol Cancer 13:223
Pfitzner C, Schröder I, Scheungraber C et al (2014) Digital-direct-RT-PCR: a sensitive and specific method for quantification of CTC in patients with cervical carcinoma. Sci Rep 4:3970
Takakura M, Matsumoto T, Nakamura M, Mizumoto Y, Myojyo S, Yamazaki R, Iwadare J, Bono Y, Orisaka S, Obata T, Iizuka T, Kagami K, Nakayama K, Hayakawa H, Sakurai F, Mizuguchi H, Urata Y, Fujiwara T, Kyo S, Sasagawa T, Fujiwara H (2018) Detection of circulating tumor cells in cervical cancer using a conditionally replicative adenovirus targeting telomerase-positive cells. Cancer Sci 109:231–240
Pao CC, Hor JJ, Yang FP, Lin CY, Tseng CJ (1997) Detection of human papillomavirus mRNA and cervical cancer cells in peripheral blood of cervical cancer patients with metastasis. J Clin Oncol 15:1008–1012
Stenman J, Lintula S, Hotakainen K, Vartiainen J, Lehväslaiho H, Stenman UH (1997) Detection of squamous-cell carcinoma antigen-expressing tumour cells in blood by reverse transcriptase-polymerase chain reaction in cancer of the uterine cervix. Int J Cancer 74:75–80
Ji XQ, Sato H, Tanaka H, Konishi Y, Fujimoto T, Takahashi O, Tanaka T (2006) Real-time quantitative RT-PCR detection of disseminated endometrial tumor cells in peripheral blood and lymph nodes using the LightCycler system. Gynecol Oncol 100:355–360
Mitsuhashi A, Tanaka N, Suzuka K, Matsui H, Seki K, Sekiya S (2003) Detection of epidermal growth factor receptor mRNA in peripheral blood of cervical cancer patients. Gynecol Oncol 89:480–485
Yuan CC, Wang PH, Ng HT, Li YF, Huang TS, Chen CY, Tsai LC, Shyong WY (2002) Detecting cytokeratin 19 mRNA in the peripheral blood cells of cervical cancer patients and its clinical-pathological correlation. Gynecol Oncol 85:148–153
Klein A, Fishman A, Zemer R, Zimlichman S, Altaras MM (2000) Detection of tumor circulating cells by cytokeratin 20 in the blood of patients with endometrial carcinoma. Gynecol Oncol 78:352–355
Bettegowda C, Sausen M, Leary RJ (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. In: Sci Transl med 6:224ra24
Phallen J, Sausen M, Adleff V et al (2017) Direct detection of early-stage cancers using circulating tumor DNA. Sci Transl Med 9. pii: eaan2415
Kamat AA, Baldwin M, Urbauer D, Dang D, Han LY, Godwin A, Karlan BY, Simpson JL, Gershenson DM, Coleman RL, Bischoff FZ, Sood AK (2010) Plasma cell-free DNA in ovarian cancer: an independent prognostic biomarker. Cancer 116:1918–1925
Steffensen KD, Madsen CV, Andersen RF, Waldstrøm M, Adimi P, Jakobsen A (2014) Prognostic importance of cell-free DNA in chemotherapy resistant ovarian cancer treated with bevacizumab. Eur J Cancer 50:2611–2618
Kumar P, Dillon LW, Shibata Y, Jazaeri AA, Jones DR, Dutta A (2017) Normal and cancerous tissues release extrachromosomal circular DNA (eccDNA) into the circulation. Mol Cancer Res 15:1197–1205
Wimberger P, Roth C, Pantel K, Kasimir-Bauer S, Kimmig R, Schwarzenbach H (2011) Impact of platinum-based chemotherapy on circulating nucleic acid levels, protease activities in blood and disseminated tumor cells in bone marrow of ovarian cancer patients. Int J Cancer 128:2572–2580
Vanderstichele A, Busschaert P, Smeets D, Landolfo C, van Nieuwenhuysen E, Leunen K, Neven P, Amant F, Mahner S, Braicu EI, Zeilinger R, Coosemans A, Timmerman D, Lambrechts D, Vergote I (2017) Chromosomal instability in cell-free DNA as a highly specific biomarker for detection of ovarian cancer in women with adnexal masses. Clin Cancer Res 23:2223–2231
Cohen PA, Flowers N, Tong S, Hannan N, Pertile MD, Hui L (2016) Abnormal plasma DNA profiles in early ovarian cancer using a non-invasive prenatal testing platform: implications for cancer screening. BMC Med 14:126
Vaksman O, Tropé C, Davidson B, Reich R (2014) Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis 35:2113–2120
Filippov-Levy N, Cohen-Schussheim H, Tropé CG, Hetland Falkenthal TE, Smith Y, Davidson B, Reich R (2018) Expression and clinical role of long non-coding RNA in high-grade serous carcinoma. Gynecol Oncol 148:559–566
Trejo-Becerril C, Pérez-Cárdenas E, Treviño-Cuevas H et al (2003) Circulating nucleosomes and response to chemotherapy: an in vitro, in vivo and clinical study on cervical cancer patients. Int J Cancer 104:663–668
Carow K, Gölitz M, Wolf M et al (2017) Viral-cellular DNA junctions as molecular markers for assessing intra-tumor heterogeneity in cervical cancer and for the detection of circulating tumor DNA. Int J Mol Sci 18. pii: E2032
Dobrzycka B, Terlikowski SJ, Mazurek A, Kowalczuk O, Niklinska W, Chyczewski L, Kulikowski M (2010) Circulating free DNA, p53 antibody and mutations of KRAS gene in endometrial cancer. Int J Cancer 127:612–621
Widschwendter M, Zikan M, Wahl B, Lempiäinen H, Paprotka T, Evans I, Jones A, Ghazali S, Reisel D, Eichner J, Rujan T, Yang Z, Teschendorff AE, Ryan A, Cibula D, Menon U, Wittenberger T (2017) The potential of circulating tumor DNA methylation analysis for the early detection and management of ovarian cancer. Genome Med 9:116
Färkkilä A, McConechy MK, Yang W et al (2017) FOXL2 402C>G mutation can be identified in the circulating tumor DNA of patients with adult-type granulosa cell tumor. J Mol Diagn 19:126–136
Forshew T, Murtaza M, Parkinson C et al (2012) Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med 4:136ra68
Parkinson CA, Gale D, Piskorz AM, Biggs H, Hodgkin C, Addley H, Freeman S, Moyle P, Sala E, Sayal K, Hosking K, Gounaris I, Jimenez-Linan M, Earl HM, Qian W, Rosenfeld N, Brenton JD (2016) Exploratory analysis of TP53 mutations in circulating tumour DNA as biomarkers of treatment response for patients with relapsed high-grade serous ovarian carcinoma: a retrospective study. PLoS Med 13:e1002198
Park YR, Kim YM, Lee SW, Lee HY, Lee GE, Lee JE, Kim YT (2018) Optimization to detect TP53 mutations in circulating cell-free tumor DNA from patients with serous epithelial ovarian cancer. Obstet Gynecol Sci 61:328–336
Christie EL, Fereday S, Doig K, Pattnaik S, Dawson SJ, Bowtell DDL (2017) Reversion of BRCA1/2 germline mutations detected in circulating tumor DNA from patients with high-grade serous ovarian cancer. J Clin Oncol 35:1274–1280
Weigelt B, Comino-Méndez I, de Bruijn I, Tian L, Meisel JL, García-Murillas I, Fribbens C, Cutts R, Martelotto LG, Ng CKY, Lim RS, Selenica P, Piscuoglio S, Aghajanian C, Norton L, Murali R, Hyman DM, Borsu L, Arcila ME, Konner J, Reis-Filho JS, Greenberg RA, Robson ME, Turner NC (2017) Diverse BRCA1 and BRCA2 reversion mutations in circulating cell-free DNA of therapy-resistant breast or ovarian Cancer. Clin Cancer Res 23:6708–6720
Martignetti JA, Camacho-Vanegas O, Priedigkeit N et al (2014) Personalized ovarian cancer disease surveillance and detection of candidate therapeutic drug target in circulating tumor DNA. Neoplasia 16:97–103
Morikawa A, Hayashi T, Shimizu N, Kobayashi M, Taniue K, Takahashi A, Tachibana K, Saito M, Kawabata A, Iida Y, Ueda K, Saito M, Yanaihara N, Tanabe H, Yamada K, Takano H, Nureki O, Okamoto A, Akiyama T (2018) PIK3CA and KRAS mutations in cell free circulating DNA are useful markers for monitoring ovarian clear cell carcinoma. Oncotarget 9:15266–15274
Chung TKH, Cheung TH, Yim SF, Yu MY, Chiu RWK, Lo KWK, Lee IPC, Wong RRY, Lau KKM, Wang VW, Worley MJ Jr, Elias KM, Fiascone SJ, Smith DI, Berkowitz RS, Wong YF (2017) Liquid biopsy of PIK3CA mutations in cervical cancer in Hong Kong Chinese women. Gynecol Oncol 146:334–339
Kang Z, Stevanović S, Hinrichs CS, Cao L (2017) Circulating cell-free DNA for metastatic cervical Cancer detection, genotyping, and monitoring. Clin Cancer Res 23:6856–6862
Cohen JD, Li L, Wang Y, Thoburn C, Afsari B, Danilova L, Douville C, Javed AA, Wong F, Mattox A, Hruban RH, Wolfgang CL, Goggins MG, Dal Molin M, Wang TL, Roden R, Klein AP, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Vogelstein JT, Browne JD, Schoen RE, Brand RE, Tie J, Gibbs P, Wong HL, Mansfield AS, Jen J, Hanash SM, Falconi M, Allen PJ, Zhou S, Bettegowda C, Diaz LA Jr, Tomasetti C, Kinzler KW, Vogelstein B, Lennon AM, Papadopoulos N (2018) Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359:926–930
Author information
Authors and Affiliations
Contributions
BD: Performed the literature search and wrote the manuscript.
Corresponding author
Ethics declarations
Not applicable.
Conflict of interest
The author declares that he has no conflict of interest.
Rights and permissions
About this article
Cite this article
Davidson, B. Circulating tumor cells and cell-free nucleic acids in patients with gynecological malignancies. Virchows Arch 473, 395–403 (2018). https://doi.org/10.1007/s00428-018-2447-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-018-2447-5