Abstract
Uterine cancer was first subclassified based on anatomic site, separating those tumours arising from the endometrium from cervical cancers. There was then further subclassification of endometrial cancers based on cell type, and this correlated with the Type I and Type II categories identified through the epidemiological studies of Bokhman, with endometrioid carcinoma corresponding (approximately) to Type I and serous carcinoma to Type II. These histotypes are not clearly separable in practice, however, with considerable interobserver variability in histotype diagnosis, especially for high-grade tumours. There followed studies of immunomarkers and then mutational studies of single genes, in attempts to improve subclassification. While these have revealed significant differences in protein expression and mutation profiles between endometrioid and serous carcinomas, there is also considerable overlap, so that there remain challenges in subclassification of endometrial carcinoma. Gene panel testing, using next-generation sequencing, was applied to endometrial cancers and highlighted that there are tumours that show genetic alterations intermediate between classic Type I/endometrioid and Type II/serous carcinomas. The Cancer Genome Atlas studies of endometrioid and serous carcinoma offered revolutionary insight into the subclassification of endometrial carcinoma, i.e. that there are four distinct categories of endometrial carcinoma, rather than two, based on genomic architecture. In this review, we provide an overview of immunohistochemical and molecular markers in endometrial carcinoma and comment on the important future directions in endometrial carcinoma subclassification arising from The Cancer Genome Atlas results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The classification of endometrial carcinoma has evolved over time, with the goal of more precisely predicting patient prognosis and guiding management. The evolution of endometrial cancer classification has raised several questions such as (1) Can histotype of endometrial carcinoma be reproducibly defined? (2) How can stratification of patient risk of recurrence or death from disease be improved? (3) Which patients can be cured by surgery alone and who are candidates for fertility-preserving therapy? and (4) What is the appropriate surveillance for the patient after initial treatment? The Cancer Genome Atlas (TCGA)-based classification of endometrial carcinoma has shown promise in refining endometrial carcinoma classification and more accurately reflecting patient outcome. We review the evolution of endometrial carcinoma classification, from purely morphological to the recently proposed TCGA genomic-based classification, and provide an overview of immunohistochemical and molecular markers used in endometrial carcinoma classification. We also comment on future directions in endometrial carcinoma subclassification arising from these results.
The history of endometrial cancer classification
Uterine cancer was first subclassified based only on anatomical location, so that tumours from the cervix and uterine corpus were treated as separate entities; prior to this, cancers of the uterus were viewed by physicians as a single disease [1]. With regard to carcinoma of the uterine corpus, Bokhman first described two clinicopathological types of endometrial carcinoma based on epidemiological studies [2], namely, Type I endometrial carcinoma, associated with unopposed estrogen stimulation and corresponding to low-grade endometrioid carcinoma, and Type II which is unrelated to estrogen stimulation and corresponds to serous carcinoma [3]. This landmark study identified a high-grade endometrial carcinoma variant that was associated with increased frequency of myometrial invasion, metastasis and likelihood of death due to disease [2]; however, the Type I and Type II classification did not enter diagnostic practice as there was no clear boundary between the types and too many cases defied classification as either Type I or Type II. The corresponding histotypes, i.e. endometrioid or serous, are also not clearly separable in practice with some tumours having ambiguous morphology [4]; especially for high-grade tumours, interobserver variability in histotype diagnosis is considerable [5,6,7,8]. In one study, 56 cases of high-grade endometrial cancer were reviewed by three pathologists, and a consensus major histotype diagnosis was reached in only 62.5% of cases [5]. Another study showed that interobserver agreement in classification of high-grade endometrial carcinoma histotype based on morphology alone was only moderate [7]. Finally, Thomas et al. showed that upon review of 131 cases of grade 3 endometrioid carcinoma by two gynecologic pathologists, reclassification occurred 38% of the time [8]. Despite this, current endometrial carcinoma classification relies heavily on morphological features and the WHO 2014 classifies endometrial carcinoma based on histological subtype (Table 1), with endometrioid and serous carcinoma accounting for a large majority of cases.
Endometrial carcinoma histotypes (Fig. 1)
Endometrioid carcinoma is identified by its complex, branching glandular or villoglandular architecture composed of back to back glands with no intervening stroma. The cells lining the glands are crowded, stratified, columnar cells with mild to moderate nuclear atypia, inconspicuous nucleoli and eosinophilic cytoplasm. One characteristic feature of this tumour is the smooth contour of the lumen of the glands. The precursor lesion (atypical hyperplasia) may be seen. Variants of endometrioid carcinoma include those with squamous or secretory differentiation. The squamous component can be in the form of squamous morules or at the stromal interface. It is often a helpful feature in identifying the tumour as endometrioid carcinoma, but is not considered when grading the tumour. Secretory differentiation resembles the endometrium in the secretory phase of the menstrual cycle and occurs in less than 2% of endometrioid carcinomas [9].
Histotypes of endometrial carcinoma. a Low-grade endometrioid adenocarcinoma. b High-grade endometrioid adenocarcinoma. c Serous carcinoma. d Clear cell carcinoma. e Dedifferentiated carcinoma, showing low-grade (endometrioid) and high-grade (undifferentiated) components. f High-grade (undifferentiated) component of dedifferentiated carcinoma. a–e H&E, 100× magnification; f H&E, 200× magnification
Serous carcinoma is distinguished from endometrioid carcinoma by its marked nuclear pleomorphism, prominent nucleoli and scant cytoplasm. It characteristically has a papillary architecture, but can be solid and/or microcystic. In contrast to the round, smooth glandular lumens in endometrioid carcinoma, the luminal surfaces in serous carcinoma are irregular and slit-like. Mitoses are prominent. Serous carcinomas typically arise in a polyp or atrophic endometrium.
Paradigmatic low-grade endometrioid carcinomas and prototypic serous carcinomas are easily recognized based on their microscopic appearance, that correlates with different mutational and DNA expression profile [10, 11]. However, based on morphology alone, there is a subset of high-grade glandular tumours with ambiguous features that are difficult to classify (i.e. as endometrioid or serous) without ancillary tests [12].
An uncommon histotype is clear cell carcinoma. This is a high-grade neoplasm and is considered to be a Type II endometrial carcinoma using Bokhman’s classification, as it is not associated with increased estrogen. The tumours have tubulocystic, papillary or solid growth, and polygonal or hobnail cells with marked nuclear pleomorphism, conspicuous nucleoli and clear cytoplasm (although eosinophilic cytoplasm may be present). Eosinophilic extracellular globules or hyaline bodies are also a characteristic feature, present in approximately two thirds of these tumours. Similar to serous carcinoma, these tumours arise in polyps or atrophic endometrium. Clear cell carcinoma can be mistaken for serous carcinoma or endometrioid carcinoma with clear cell squamous or secretory differentiation [9]. Rigorous criteria are recommended before establishing the diagnosis of clear cell carcinoma, with special emphasis on the typical architectural patterns.
A mixed carcinoma category recognizes the occurrence of tumours with heterogeneity. In these tumours, there must be two histological components with the second component comprising at least 5% of the tumour. One component must be either serous or clear cell carcinoma [9]. Rigorous histomorphological criteria are also highly recommended in the diagnosis of mixed carcinoma. There is considerable variability in the frequency with which mixed carcinoma is diagnosed; as with ovarian mixed carcinomas [13], it is evident that mixed carcinomas of the endometrium are clonal and the immunophenotype and molecular features are uniform throughout, in most cases [14, 15]. As such, “true” mixed carcinomas, when strictly defined as having two components that are distinct based on both light microscopy and molecular biomarker expression, are relatively uncommon.
Undifferentiated endometrial carcinomas are those in which no differentiation is present. The tumour contains highly mitotic, small- to intermediate-sized cells with condensed chromatin, arranged in dyshesive sheets. Dedifferentiated carcinoma is a variant of undifferentiated carcinoma with both an undifferentiated component and a component of well- or moderately differentiated endometrioid carcinoma [9].
The WHO histological classification system correlates well with the natural history of the disease and patient prognosis [16,17,18,19]. However, interobserver variability in classifying endometrial carcinomas remains problematic, particularly in the subset of high-grade carcinomas, including the grey zone between high-grade endometrioid and serous carcinomas [5,6,7,8]. This has far-reaching implications as inaccurate classification can impact our understanding of the natural history of these entities and is reflected by the fact that some studies examining outcomes of patients with different histotypes of high-grade endometrial carcinomas have shown differences in patient outcome, while others have not [20,21,22,23,24,25]. In a study of 187 high-grade endometrial carcinomas including FIGO grade 3 endometrioid, serous and clear cell carcinoma, Soslow et al. showed that when several variables were controlled for, high-grade endometrial cancers of different histologic subtypes had similar clinical outcomes [24]. Voss et al. showed similar clinical presentation and poor survival when comparing high-grade endometrial carcinomas of different histotypes. Based on this, they suggested that FIGO grade 3 endometrioid cancer should be regarded as a Type II cancer and treated with similar adjuvant chemotherapy as serous and clear cell carcinoma [25]. In contrast, other studies showed a significant survival difference between grade 3 endometrioid carcinoma and serous carcinoma (75 vs. 41% at 5 years, respectively, in the study by Boruta et al.) [23, 26].
Substantial interobserver variability in histotype diagnosis makes it impossible to enrol patients in clinical trials based on histotype, or deliver histotype-specific treatment consistently. Histotype (or any variable that cannot be reproducibly diagnosed) is of limited use in guiding more individualized treatment, given that treatment recommendations will vary depending on the reviewing pathologist, rather than the underlying tumour biology. It is therefore important to improve reproducibility of subclassification of endometrial carcinoma and studies on molecular markers that could guide improved endometrial carcinoma subclassification have been undertaken with this aim in mind. The initial focus was on single markers, using immunohistochemistry or mutational analysis, to improve histotype-based assignment. It should be acknowledged, however, that a purely molecular-based classification may supplant histotype, as has happened with breast and lung carcinomas, where the molecular markers, whether assessed by immunohistochemistry, fluorescence in situ hybridization (FISH) or mutational analysis, have far greater importance in guiding treatment than histopathological variables [27, 28].
Immunohistochemical markers
p53
The characteristic molecular events in serous carcinogenesis include mutation of TP53, while endometrioid carcinomas are associated with mutations in PTEN. In particular, p53 immunostaining can serve as an aid in differential diagnosis of endometrioid and serous carcinoma, yet it undoubtedly remains true that approximately 30% of grade 3 endometrioid carcinomas (morphologically typical, with predominantly solid growth and squamous metaplasia) show abnormal, mutant-pattern, p53 staining [29]. It is not recommended that p53 alone be used when the differential diagnosis includes serous versus high-grade endometrioid carcinoma as it is not helpful in this context [30]. In low-grade endometrioid carcinomas (FIGO grade 1 or 2), p53 expression is rarely abnormal [30]; however, it is relatively uncommon for there to be a morphological problem in distinguishing low-grade (i.e. grade 1 or 2) endometrioid carcinoma from serous carcinoma [12].
p53 immunohistochemical staining should be reported as abnormal (mutated) or wildtype. Abnormal p53 staining occurs when there is strong nuclear staining in all (or almost all) of the tumour or complete loss of staining. Wildtype p53 staining is weak and patchy (Fig. 2). A third pattern of abnormal p53 immunostaining, associated with mutations that impact on the nuclear translocation domain of the protein, is moderate to intense cytoplasmic staining without strong nuclear staining, but this pattern is uncommon [31]. Occasionally, there can be heterogeneous staining for p53, with different clones showing different staining patterns (Fig. 2). Clear cell and undifferentiated or dedifferentiated carcinomas are usually p53 wildtype. In serous carcinoma, p53 is mutated and shows intense diffuse staining in 80–90% of tumours and complete absence of staining in 10% of tumours [29].
p53 immunohistochemical staining of endometrial carcinomas. a Mutant p53 staining, strong and diffuse (overexpression pattern). b Mutant p53 staining, null (loss of expression pattern—note positive staining of benign cells, which serves as an internal control). c Wildtype p53 staining, weak and patchy. d Heterogeneous p53 staining, with both overexpression and loss of expression. This pattern is encountered infrequently
Estrogen receptor
Estrogen receptor (ER) is expressed in more than 95% of low-grade endometrioid carcinomas [32]; however, grade 3 endometrioid carcinomas can lack expression (15–50%) [33]. It was formerly thought that ER is usually negative in serous carcinomas; however, with more sensitive immunostaining protocols, more than half of serous carcinomas can show positivity [33]. Clear cell and undifferentiated or dedifferentiated carcinomas are typically negative for ER [3, 30, 34].
p16
Although HPV is not involved in the pathogenesis of endometrial carcinoma, p16 immunohistochemical staining of endometrial tumours may be useful. It can be diffusely and strongly positive in serous carcinoma (Fig. 3), with up to 82% specificity in some studies [30, 33, 35], but further validation, looking specifically at the differential diagnosis of grade 3 endometrioid and serous carcinoma, is needed. Endometrioid carcinoma usually exhibits focal positivity in less than 50% of tumour cells (Fig. 3) [36]. Occasional cases of mucinous adenocarcinoma of the endometrium or endometrioid adenocarcinoma with mucinous differentiation can show strong diffuse p16 immunoreactivity [37]. Clear cell carcinomas show a similar staining pattern to endometrioid carcinoma.
Napsin A/HNF-1β
Napsin A and HNF-1β have been used to identify clear cell carcinoma (> 90% specificity) and usually give a diffuse moderate to strong staining pattern [38,39,40]. These markers appear to be less specific for clear cell carcinoma of the endometrium, than for clear cell carcinoma of the ovary, where they are well validated, as they can be positive in a subset of other endometrial carcinoma histotypes including endometrioid and serous [38, 39]. Alpha-methylacyl-CoA racemase (AMACR) has also been suggested to be useful for diagnosing clear cell carcinomas, in some studies.
Abnormal SWI/SNF subunit expression: INI1 (SMARCB1), BRG1 (SMARCA4) and BAF250a (ARID1A)/ARID1B immunostaining—a feature of dedifferentiated endometrial carcinoma
Recent studies have examined the immunohistochemical and molecular profiles of undifferentiated and dedifferentiated carcinomas of the endometrium [41,42,43]. While these tumours often lose PAX8 and ER expression (seen in over 60% of these tumours), it has also been found that a large portion lose INI1 and BRG1 expression [41,42,43,44,45,46]. Aberrant p53 staining can also occur in a small subset of these BRG1/INI1 deficient tumours; however, wildtype p53 expression predominates [41,42,43,44].
PTEN
Loss of expression of the tumour suppressor, PTEN (phosphatase and tensin homolog), occurs most frequently in endometrioid carcinoma, but can also occur in undifferentiated and mixed carcinomas [30, 47]. PTEN immunohistochemistry detects tumours with genetic loss of PTEN, but also with functional PTEN loss as a consequence of epigenetic mechanisms, and may be superior to gene sequencing for identifying tumours with PTEN abnormalities [47]. PTEN immunohistochemistry has been reported to show variable results in different laboratories. However, recent studies have proposed new protocols designed to improve inter-laboratory performance [48].
Other markers and panels of antibodies
Immunohistochemistry may be used as an aid in histotype diagnosis and subclassification of endometrial carcinoma; however, no single marker is completely sensitive or specific for a given histotype [33]. Lack of expression of mismatch repair genes has been suggested as evidence against the diagnosis of serous carcinoma. Additional proteins that have been proposed in the differential diagnosis between endometrioid and serous carcinomas are HER2, claudin 3 and 4, FOLR-1, HMGA-2, cyclin E, IMP2 and IMP3, but none of them has shown good sensitivity and specificity. p53 is the most extensively validated immunomarker for serous versus endometrioid, but, as noted previously, is limited in that abnormal p53 expression is seen in a significant minority of high-grade endometrioid carcinomas.
Panels of immunostains have been recommended and have been shown to improve interobserver agreement regarding histotype diagnosis, but there is no agreement on the best composition of such a panel, and how to handle cases where the results of individual stains from the panel are not consistent [5, 49, 50]. The most frequent proteins included in these panels are p53, p16 and PTEN [51].
Genetic markers
CTNNB1 (β-catenin)
Several studies have identified the presence of β-catenin mutations in endometrioid carcinoma. Mutation can occur in up to 66% of low-grade endometrioid tumours [30, 52].
ARID1A
ARID1A mutations are acquired in both low- and high-grade endometrioid carcinomas, occurring in 40–46.7% of low-grade tumours and up to 60% of high-grade tumours [49, 52, 53]. It has also been found to be mutated in up to one quarter of clear cell carcinomas [39, 54]. These mutations are rarely seen in serous carcinoma.
PTEN
PTEN mutations are the most common recurrent genetic event in endometrioid carcinomas. Clear cell carcinoma also have PTEN mutations at a high frequency, whereas these are rarely found in serous carcinoma [30, 39, 52].
PIK3CA
PIK3CA mutations can be found in both endometrioid and serous carcinomas, occurring in 30–60% of endometrioid carcinomas and 24–40% of serous carcinomas [29, 55,56,57,58]. High-grade endometrioid carcinoma shows a significantly higher PIK3CA mutation frequency when compared with serous carcinoma. Furthermore, there is an association between ARID1A and PTEN/PIK3CA mutation, further strengthening the relationship between these mutations and the endometrioid histotype [52].
TP53
While high- and low-grade endometrioid carcinomas have a somewhat similar mutation profile, several studies have shown a significantly increased TP53 mutation frequency with increased FIGO grade of endometrioid carcinoma [29, 49, 52, 55]. Furthermore, TP53 mutations occur much more frequently in serous carcinomas when compared to endometrioid carcinoma and is the hallmark of this histotype, being present in 80–90% or more of these tumours [29, 55, 58]. Interestingly, there are some differences in the spectrum of TP53 mutations between endometrioid and serous carcinomas, with hot-spot mutations more frequent in serous tumours [59].
PPP2R1A
Up to 41% of serous carcinomas have a missense mutation in PPP2R1A, while this occurs in a much smaller fraction (5%) of endometrial endometrioid carcinomas [55, 60, 61].
Molecular markers have aided in the classification of endometrial carcinoma and there are strong correlations between histotype and mutations in single genes; however, just as with immunohistochemistry, no single marker is completely sensitive or specific for classification (Table 2).
Gene panel testing
The introduction of next-generation sequencing into clinical practice has enabled routine sequencing of multiple genes for the same cost as a single gene. This makes possible the use of a panel of genetic markers as an aid in classification, with the goal of decreased interobserver variation and greater diagnostic accuracy [52, 62]. For example, McConechy et al. studied 393 endometrial carcinomas with targeted exon sequencing of nine genes [52]. PTEN and ARID1A are associated with endometrioid type, while TP53 and PPP2RIA are associated with serous type, so that the presence of either or both of the former mutations and absence of the latter (i.e. TP53 and PPP2R1A) support a diagnosis of endometrioid carcinoma. Such an approach, of sequencing a small number of genes, is not able to reliably classify endometrial carcinoma, however, as there are large numbers of indeterminate cases where the mutation profile does not permit unequivocal assignment of histotype and occasional cases with outright discordance between histologic diagnosis and mutation profile. Various molecular profiles of endometrial carcinoma histotypes are shown in Fig. 4. Intra-tumoural heterogeneity has also been suggested as a potential problem, since some important mutational driver events may be restricted to specific regions of the tumour [63].
Mutation profiles of endometrial subtypes. a Low-grade endometrioid carcinoma, including grade 1 and 2 tumours. b High-grade endometrioid carcinoma, grade 3. c Serous carcinoma. d Carcinosarcoma. e Undifferentiated and mixed histology subtypes: undifferentiated carcinomas (a), mixed low-grade endometrioid carcinoma with serous carcinoma (b), mixed endometrioid and clear cell carcinoma (c) and mixed serous and clear cell carcinoma (d). Rows indicate genes and columns represent tumour cases. Coloured bars indicate mutations including missense, truncating, indels and splice site mutations. Grey bars indicate no mutations were detected. +carcinosarcomas with heterologous differentiation elements. *serous carcinoma outliers with ARID1A mutations. #low-grade endometrioid carcinoma and high-grade endometrioid carcinoma mutation outliers with serous-type mutations (TP53 or PPP2R1A). (Reproduced with permission. McConechy MK, Ding J, Cheang MCU, et al. (2012) Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 228:20–30. doi: https://doi.org/10.1002/path.4056)
The Cancer Genome Atlas (TCGA) genomic-based classification of endometrial carcinoma
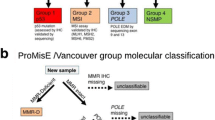
A multiplatform analysis of 373 endometrial carcinomas through the TCGA resulted in a proposed molecular classification of endometrial carcinoma. Four groups were described based on integrated genomic architecture rather than single genetic mutations, and this subclassification has direct clinical and prognostic implications [55, 64]. The four groups are (1) ultramutated/polymerase ε mutated (POLE), (2) hypermutated/microsatellite instability, (3) low-copy number abnormalities and (4) high-copy number abnormalities (Table 3). The ultramutated/POLE group comprised approximately 10% of the studied tumours and was frequently of high-grade endometrioid histologic subtype, with few somatic copy number alterations but a very high mutation burden (ten times the number of mutations, on average, as hypermutated tumours and 100-fold more mutations than the tumours in the low-copy number group). Furthermore, these tumours had a significantly better prognosis than the other three TCGA groups [65,66,67]. The hypermutated/microsatellite instability (MSI) group is characterized by the histologic features of Lynch syndrome-associated carcinomas, including tumour heterogeneity, ambiguous histology and tumour-infiltrating lymphocytes [68, 69]. High-somatic-copy number abnormalities were seen in serous-like tumours and correspond broadly to the Type II tumours described by Bokhman, while the low-somatic-copy number tumours correspond to Type I endometrial carcinomas. Notably, however, up to 35% of high-grade endometrioid carcinomas also had high-copy number alterations and were therefore more accurately classified, genomically, as being within the high-copy number group (despite having unequivocal endometrioid features, including squamous differentiation in some instances) [55].
This genomic approach to classification is potentially more robust than single-gene or single-protein analysis, has shown significant correlation with patient outcome and is clinically actionable. Unfortunately, however, due to logistic and resource constraints, such as the need for fresh frozen tissue, cost, long turn-around time and lack of applicability to biopsies or curettings, so that classification is not available before definitive surgical treatment, this approach is not currently applicable in routine practice. Identification of surrogate markers that accurately reflect molecular subtype is the only feasible way to overcome this barrier.
Development of a clinically applicable surrogate for TCGA classification
More cost-effective and convenient methods, allowing testing on formalin-fixed paraffin-embedded tissue, are required for genomic-based classification of endometrial carcinomas into molecular subtype to enter routine practice. Two groups, working independently, have identified the same approach to molecular classification [64, 70, 71]. Abnormal p53 staining by immunohistochemistry is a surrogate for identifying tumours with high-copy number alterations, immunohistochemistry for mismatch repair proteins (MLH1, MSH2, MSH6, PMS2) can be used to detect hypermutated/MSI tumours [72], sequencing for mutations in the POLE exonuclease domains is a surrogate for identifying the ultramutated/POLE group [61], and the copy number low group consists of those tumours lacking any of the above molecular features.
These methods are more widely accessible and inexpensive and can be performed expeditiously and on small samples, with results based on biopsy/curettings being highly concordant with results based on the hysterectomy specimen [73]. Although further validation is required, the evidence to date indicates the potential for this classification to be highly reproducible. For example, p53 immunohistochemistry can show a very high correlation with TP53 mutation status [31]; however, this is not being achieved in all clinical laboratories, and further improvements in quality of staining and interpretation should be sought [74]. Another caveat is that this classification does not apply to all endometrial carcinomas, specifically dedifferentiated/undifferentiated endometrial carcinoma. A unique molecular profile with frequent aberrant mismatch repair protein expression and loss of SWItch-sucrose non-fermentable (SWI/SNF) protein expression characterizes these tumours, and they do not fit into the TCGA-based classifier [42,43,44].
This TCGA surrogate seems to be particularly informative in the group of high-grade endometrial carcinomas, in the spectrum of tumours that includes high-grade endometrioid carcinoma and serous carcinoma. It is also a good tool to identify patients with tumours (ultramutated and hypermutated) that may benefit from immunotherapy [75, 76].
Summary and conclusions
We have seen the evolution of endometrial cancer classification from being purely based on anatomical location to histologic cell type-based classification, to classification incorporating ancillary molecular testing, such as gene panel testing and genomic analysis. Molecular classification holds promise for more accurately subtyping endometrial carcinoma to better reflect patient prognosis and outcome and may be the mainstay of endometrial carcinoma classification in the future.
References
Karamanou M, Tsoucalas G, Laios K, Deligeoroglou E, Agapitos E, Androutsos G (2015) Uterine cancer in the writings of Byzantine physicians. J BUON 20(6):1645–1648
Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17. https://doi.org/10.1016/0090-8258(83)90111-7
Lax SF, Pizer ES, Ronnett BM, Kurman RJ (1998) Comparison of estrogen and progesterone receptor, Ki-67, and p53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum Pathol 29(9):924–931. https://doi.org/10.1016/S0046-8177(98)90197-6
Soslow RA (2010) Endometrial carcinomas with ambiguous features. Semin Diagn Pathol 27(4):261–273. https://doi.org/10.1053/j.semdp.2010.09.003
Gilks CB, Oliva E, Soslow RA (2013) Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 37(6):874–881. https://doi.org/10.1097/PAS.0b013e31827f576a
Lomo L, Nucci MR, Lee KR, Lin MC, Hirsch MS, Crum CP, Mutter GL (2008) Histologic and immunohistochemical decision-making in endometrial adenocarcinoma. Mod Pathol 21(8):937–942. https://doi.org/10.1038/modpathol.2008.97
Han G, Sidhu D, Duggan MA, Arseneau J, Cesari M, Clement PB, Ewanowich CA, Kalloger SE, Köbel M (2013) Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 26(12):1594–1604. https://doi.org/10.1038/modpathol.2013.102
Thomas S, Hussein Y, Bandyopadhyay S, Cote M, Hassan O, Abdulfatah E, Alosh B, Guan H, Soslow RA, Ali-Fehmi R (2016) Interobserver variability in the diagnosis of uterine high-grade endometrioid carcinoma. Arch Pathol Lab Med 140(8):836–843. https://doi.org/10.5858/arpa.2015-0220-OA
Kurman RJ, Carcangiu ML, Herrington CS, Young RH (2014) WHO classification of tumours of female reproducitve organs. International Agency for Research on Cancer, Lyon
Moreno-Bueno G, Sánchez-Estévez C, Cassia R, Rodríguez-Perales S, Díaz-Uriarte R, Domínguez O, Hardisson D, Andujar M, Prat J, Matias-Guiu X, Cigudosa JC, Palacios J (2003) Differential gene expression profile in endometrioid and nonendometrioid endometrial carcinoma: STK15 is frequently overexpressed and amplified in nonendometrioid carcinomas. Cancer Res 63(18):5697–5702
Yeramian A, Moreno-Bueno G, Dolcet X, Catasus L, Abal M, Colas E, Reventos J, Palacios J, Prat J, Matias-Guiu X (2013) Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene 32(4):403–413. https://doi.org/10.1038/onc.2012.76
Garg K, Leitao MM, Wynveen CA, Sica GL, Shia J, Shi W, Soslow RA (2010) P53 overexpression in morphologically ambiguous endometrial carcinomas correlates with adverse clinical outcomes. Mod Pathol 23(1):80–92. https://doi.org/10.1038/modpathol.2009.153
Mackenzie R, Talhouk A, Eshragh S, Lau S, Cheung D, Chow C, Le N, Cook LS, Wilkinson N, McDermott J, Singh N, Kommoss F, Pfisterer J, Huntsman DG, Köbel M, Kommoss S, Gilks CB, Anglesio MS (2015) Morphological and molecular characteristics of mixed epithelial ovarian cancers. Am J Surg Pathol 39(11):1548–1557. https://doi.org/10.1097/PAS.0000000000000476
Köbel M, Meng B, Hoang LN, Almadani N, Li X, Soslow RA, Gilks CB, Lee CH (2016) Molecular analysis of mixed endometrial carcinomas shows clonality in most cases. Am J Surg Pathol 40:166–180. https://doi.org/10.1097/PAS.0000000000000536
Coenegrachts L, Garcia-Dios DA, Depreeuw J, Santacana M, Gatius S, Zikan M, Moerman P, Verbist L, Lambrechts D, Matias-Guiu X, Amant F (2015) Mutation profile and clinical outcome of mixed endometrioid-serous endometrial carcinomas are different from that of pure endometrioid or serous carcinomas. Virchows Arch 466(4):415–422. https://doi.org/10.1007/s00428-015-1728-5
Kurman RJ, Scully RE (1976) Clear cell carcinoma of the endometrium: an analysis of 21 cases. Cancer 37(2):872–882. https://doi.org/10.1002/1097-0142(197602)37:2<872::AID-CNCR2820370236>3.0.CO;2-L
Christopherson WM, Alberhasky RC, Connelly PJ (1982) Carcinoma of the endometrium: I. A clinicopathologic study of clear-cell carcinoma and secretory carcinoma. Cancer 49(8):1511–1523. https://doi.org/10.1002/1097-0142(19820415)49:8<1511::AID-CNCR2820490802>3.0.CO;2-6
Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R (1982) Uterine papillary serous carcinoma: a highly malignant form of endometrial adenocarcinoma. Am J Surg Pathol 6(2):93–108. https://doi.org/10.1097/00000478-198203000-00002
Sato Y, Ozaki M, Ueda G, Tanizawa O (1985) A clinicopathologic study of endometrial carcinoma with special reference to new histological variants. Nihon Sanka Fujinka Gakkai Zasshi 37(6):1015–1019
McGunigal M, Liu J, Kalir T, Chadha M, Gupta V (2017) Survival differences among uterine papillary serous, clear cell and grade 3 endometrioid adenocarcinoma endometrial cancers. Int J Gynecol Cancer 27(1):85–92. https://doi.org/10.1097/IGC.0000000000000844
Goto T, Takano M, Aoyama T, Miyamoto M, Watanabe A, Kato M, Sasaki N, Hirata J, Sasa H, Furuya K (2012) Prognosis of high-grade endometrial cancer: a comparison of serous-type and clear cell type to grade 3 endometrioid-type. Eur J Gynaecol Oncol 33(6):579–583
Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK (2006) Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer 94(5):642–646. https://doi.org/10.1038/sj.bjc.6603012
Boruta DM, Gehrig PA, Groben PA, Bae-Jump V, Boggess JF, Fowler WC, van le L (2004) Uterine serous and grade 3 endometrioid carcinomas: is there a survival difference? Cancer 101(10):2214–2221. https://doi.org/10.1002/cncr.20645
Soslow RA, Bissonnette JP, Wilton A, Ferguson SE, Alektiar KM, Duska LR, Oliva E (2007) Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol 31(7):979–987. https://doi.org/10.1097/PAS.0b013e31802ee494
Voss MA, Ganesan R, Ludeman L, McCarthy K, Gornall R, Schaller G, Wei W, Sundar S (2012) Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer—a clinical and pathological evaluation. Gynecol Oncol 124(1):15–20. https://doi.org/10.1016/j.ygyno.2011.07.030
Alkushi A, Köbel M, Kalloger SE, Gilks CB (2010) High-grade endometrial carcinoma: serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int J Gynecol Pathol 29(4):343–350. https://doi.org/10.1097/PGP.0b013e3181cd6552
Nicolini A, Ferrari P, Duffy MJ (2017) Prognostic and predictive biomarkers in breast cancer: past, present and future. Semin Cancer Biol. https://doi.org/10.1016/j.semcancer.2017.08.010
Kumar M, Ernani V, Owonikoko TK (2015) Biomarkers and targeted systemic therapies in advanced non-small cell lung cancer. Mol Asp Med 45:55–66. https://doi.org/10.1016/j.mam.2015.06.009
Lax SF, Kendall B, Tashiro H, Slebos RJC, Ellenson LH (2000) The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88(4):814–824. https://doi.org/10.1002/(SICI)1097-0142(20000215)88:4<814::AID-CNCR12>3.0.CO;2-U
Mittal K, Soslow R, McCluggage WG (2008) Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med 132(3):402–423. https://doi.org/10.1043/1543-2165(2008)132[402:AOITGP]2.0.CO;2
Köbel M, Piskorz AM, Lee S, Lui S, LePage C, Marass F, Rosenfeld N, Mes Masson AM, Brenton JD (2016) Optimized p53 immunohistochemistry is an accurate predictor of TP53 mutation in ovarian carcinoma. J Pathol Clin Res 2(4):247–258. https://doi.org/10.1002/cjp2.53
Alkushi A, Clarke BA, Akbari M, Makretsov N, Lim P, Miller D, Magliocco A, Coldman A, van de Rijn M, Huntsman D, Parker R, Gilks CB (2007) Identification of prognostically relevant and reproducible subsets of endometrial adenocarcinoma based on clustering analysis of immunostaining data. Mod Pathol 20(11):1156–1165. https://doi.org/10.1038/modpathol.3800950
Wei J-J, Paintal A, Keh P (2013) Histologic and immunohistochemical analyses of endometrial carcinomas: experiences from endometrial biopsies in 358 consultation cases. Arch Pathol Lab Med 137(11):1574–1583. https://doi.org/10.5858/arpa.2012-0445-OA
Kapucuoglu N, Bulbul D, Tulunay G, Temel MA (2008) Reproducibility of grading systems for endometrial endometrioid carcinoma and their relation with pathologic prognostic parameters. Int J Gynecol Cancer 18(4):790–796. https://doi.org/10.1111/j.1525-1438.2007.01067.x
Yoon G, Won Koh C, Yoon N, Kim JY, Kim HS (2017) Stromal p16 expression is significantly increased in endometrial carcinoma. Oncotarget. https://doi.org/10.18632/oncotarget.13594
McCluggage WG, Jenkins D (2003) p16 immunoreactivity may assist in the distinction between endometrial and endocervical adenocarcinoma. Int J Gynecol Pathol 22(3):231–235. https://doi.org/10.1097/01.PGP.0000055172.04957.2F
Chekmareva M, Ellenson LH, Pirog EC (2008) Immunohistochemical differences between mucinous and microglandular adenocarcinomas of the endometrium and benign endocervical epithelium. Int J Gynecol Pathol 27(4):547–554. https://doi.org/10.1097/PGP.0b013e318177
Fadare O, Desouki MM, Gwin K, Hanley KZ, Jarboe EA, Liang SX, Quick CM, Zheng W, Parkash V, Hecht JL (2014) Frequent expression of napsin A in clear cell carcinoma of the endometrium: potential diagnostic utility. Am J Surg Pathol 38(2):189–196. https://doi.org/10.1097/PAS.0000000000000085
Hoang LN, McConechy MK, Meng B, McIntyre JB, Ewanowich C, Gilks CB, Huntsman DG, Köbel M, Lee CH (2015) Targeted mutation analysis of endometrial clear cell carcinoma. Histopathology 66(5):664–674. https://doi.org/10.1111/his.12581
Al-Maghrabi JA, Butt NS, Anfinan N, Sait K, Sait H, Marzouki A, Khabaz MN (2016) Infrequent immunohistochemical expression of napsin A in endometrial carcinomas. Appl Immunohistochem Mol Morphol 1(9):632–638. https://doi.org/10.1097/PAI.0000000000000350
Stewart CJR, Crook ML (2015) SWI/SNF complex deficiency and mismatch repair protein expression in undifferentiated and dedifferentiated endometrial carcinoma. Pathology 47(5):439–445. https://doi.org/10.1097/PAT.0000000000000270
Hoang LN, Lee Y-S, Karnezis AN, Tessier-Cloutier B, Almandani N, Coatham M, Gilks CB, Soslow RA, Stewart CJR, Köbel M, Lee CH (2016) Immunophenotypic features of dedifferentiated endometrial carcinoma—insights from BRG1/INI1-deficient tumours. Histopathology 69(4):560–569. https://doi.org/10.1111/his.12989
Ramalingam P, Croce S, McCluggage WG (2017) Loss of expression of SMARCA4 (BRG1), SMARCA2 (BRM) and SMARCB1 (INI1) in undifferentiated carcinoma of the endometrium is not uncommon and is not always associated with rhabdoid morphology. Histopathology 70(3):359–366. https://doi.org/10.1111/his.13091
Karnezis AN, Hoang LN, Coatham M, Ravn S, Almadani N, Tessier-Cloutier B, Irving J, Meng B, Li X, Chow C, McAlpine J, Kuo KT, Mao TL, Djordjevic B, Soslow RA, Huntsman DG, Blake Gilks C, Köbel M, Lee CH (2016) Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Mod Pathol 29(3):302–314. https://doi.org/10.1038/modpathol.2015.155
Ramalingam P, Masand RP, Euscher ED, Malpica A (2016) Undifferentiated carcinoma of the endometrium: an expanded immunohistochemical analysis including PAX-8 and basal-like carcinoma surrogate markers. Int J Gynecol Pathol 35(5):410–418. https://doi.org/10.1097/PGP.0000000000000248
Li Z, Zhao C (2016) Clinicopathologic and immunohistochemical characterization of dedifferentiated endometrioid adenocarcinoma. Appl Immunohistochem Mol Morphol AIMM 24(8):562–568. https://doi.org/10.1097/PAI.0000000000000232
Djordjevic B, Hennessy BT, Li J, Barkoh BA, Luthra R, Mills GB, Broaddus RR (2012) Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol 25(5):699–708. https://doi.org/10.1038/modpathol.2011.208
Maiques O, Santacana M, Valls J, Pallares J, Mirantes C, Gatius S, García Dios d, Amant F, Pedersen HC, Dolcet X, Matias-Guiu X (2014) Optimal protocol for PTEN immunostaining; role of analytical and preanalytical variables in PTEN staining in normal and neoplastic endometrial, breast, and prostatic tissues. Hum Pathol 45(3):522–532. https://doi.org/10.1016/j.humpath.2013.10.018
Hoang LN, McConechy MK, Köbel M, Han G, Rouzbahman M, Davidson B, Irving J, Ali RH, Leung S, McAlpine JN, Oliva E, Nucci MR, Soslow RA, Huntsman DG, Gilks CB, Lee CH (2013) Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol 37(9):1421–1432. https://doi.org/10.1097/PAS.0b013e31828c63ed
Nastic D, Shanwell E, Wallin K-L, Valla M, Måsbäck A, Mateoiu C, Lidang M, Liakka A, Lappi-Blanco E, Grove A, Davidson B, Carpen O, Bertelsen BI, Bak J, Abusland AB, Selling J, Carlson JW (2017) A selective biomarker panel increases the reproducibility and the accuracy in endometrial biopsy diagnosis. Int J Gynecol Pathol 36(4):339–347. https://doi.org/10.1097/PGP.0000000000000334
Santacana M, Maiques O, Valls J, Gatius S, Abó AI, López-García MÁ, Mota A, Reventós J, Moreno-Bueno G, Palacios J, Bartosch C, Dolcet X, Matias-Guiu X (2014) A 9-protein biomarker molecular signature for predicting histologic type in endometrial carcinoma by immunohistochemistry. Hum Pathol 45(12):2394–2403. https://doi.org/10.1016/j.humpath.2014.06.031
McConechy MK, Ding J, Cheang MCU, Wiegand KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T, McDonald H, Schmidt AP, Mutch DG, McAlpine JN, Hirst M, Shah SP, Lee CH, Goodfellow PJ, Gilks CB, Huntsman DG (2012) Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol 228:20–30. https://doi.org/10.1002/path.4056
Guan B, Mao T-L, Panuganti PK, Kuhn E, Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL, Shih IM (2011) Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am J Surg Pathol 35(5):625–632. https://doi.org/10.1097/PAS.0b013e318212782a
Hoang LN, Han G, McConechy M, Lau S, Chow C, Gilks CB, Huntsman DG, Köbel M, Lee CH (2014) Immunohistochemical characterization of prototypical endometrial clear cell carcinoma—diagnostic utility of HNF-1β and oestrogen receptor. Histopathology 64(4):585–596. https://doi.org/10.1111/his.12286
Cancer Genome Atlas Research Network T (2013) Integrated genomic characterization of endometrial carcinoma. doi: https://doi.org/10.1038/nature12113
Oda K, Stokoe D, Taketani Y, McCormick F (2005) High frequency of coexistent mutations of PIK3CA and PTEN genes in endometrial carcinoma. Cancer Res 65(23):10669–10673. https://doi.org/10.1158/0008-5472.CAN-05-2620
Rudd ML, Price JC, Fogoros S, Godwin AK, Sgroi DC, Merino MJ, Bell DW (2011) A unique spectrum of somatic PIK3CA (p110 ) mutations within primary endometrial carcinomas. Clin Cancer Res 17(6):1331–1340. https://doi.org/10.1158/1078-0432.CCR-10-0540
Gatius S, Matias-Guiu X (2016) Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod Pathol 29:S45–S58. https://doi.org/10.1038/modpathol.2015.141
Schultheis AM, Martelotto LG, De Filippo MR, Piscuglio S, Ng CKY, Hussein YR, Reis-Filho JS, Soslow RA, Weigelt B (2016) TP53 mutational spectrum in endometrioid and serous endometrial cancers. Int J Gynecol Pathol 35(4):289–300. https://doi.org/10.1097/PGP.0000000000000243
McConechy MK, Anglesio MS, Kalloger SE, Yang W, Senz J, Chow C, Heravi-Moussavi A, Morin GB, Mes-Masson AM, Australian Ovarian Cancer Study Group, Carey MS, McAlpine JN, Kwon JS, Prentice LM, Boyd N, Shah SP, Gilks CB, Huntsman DG (2011) Subtype-specific mutation of PPP2R1A in endometrial and ovarian carcinomas. J Pathol 223(5):567–573. https://doi.org/10.1002/path.2848
Singh N, Gilks CB, Gilks SN (2017) The changing landscape of gynaecological cancer diagnosis: implications for histopathological practice in the 21st century. Histopathology 70(1):56–69. https://doi.org/10.1111/his.13080
Hoang LN, Kinloch MA, Leo JM, Grondin K, Lee CH, Ewanowich C, Köbel M, Cheng A, Talhouk A, McConechy M, Huntsman DG, McAlpine JN, Soslow RA, Gilks CB (2017) Interobserver agreement in endometrial carcinoma histotype diagnosis varies depending on The Cancer Genome Atlas (TCGA)-based molecular subgroup. Am J Surg Pathol 41(2):245–252. https://doi.org/10.1097/PAS.0000000000000764
Mota A, Colás E, García-Sanz P, Campoy I, Rojo-Sebastián A, Gatius S, García Á, Chiva L, Alonso S, Gil-Moreno A, González-Tallada X, Díaz-Feijoo B, Vidal A, Ziober-Malinowska P, Bobiński M, López-López R, Abal M, Reventós J, Matias-Guiu X, Moreno-Bueno G (2017) Genetic analysis of uterine aspirates improves the diagnostic value and captures the intra-tumor heterogeneity of endometrial cancers. Mod Pathol 30(1):134–145. https://doi.org/10.1038/modpathol.2016.143
Stelloo E, Nout RA, Osse EM, Jürgenliemk-Schulz IJ, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, Nijman HW, Putter H, Bosse T, Creutzberg CL, Smit VTHBM (2016) Improved risk assessment by integrating molecular and clinicopathological factors in early-stage endometrial cancer-combined analysis of the PORTEC cohorts. Clin Cancer Res 22(16):4215–4224. https://doi.org/10.1158/1078-0432.CCR-15-2878
Bakhsh S, Kinloch M, Hoang LN, Soslow RA, Köbel M, Lee CH, McAlpine JN, McConechy MK, Gilks CB (2016) Histopathological features of endometrial carcinomas associated with POLE mutations: implications for decisions about adjuvant therapy. Histopathology 68(6):916–924. https://doi.org/10.1111/his.12878
McConechy MK, Talhouk A, Leung S, Chiu D, Yang W, Senz J, Reha-Krantz LJ, Lee CH, Huntsman DG, Gilks CB, McAlpine JN (2016) Endometrial carcinomas with POLE exonuclease domain mutations have a favorable prognosis. Clin Cancer Res 22(12):2865–2873. https://doi.org/10.1158/1078-0432.CCR-15-2233
Church DN, Stelloo E, Nout RA, Valtcheva N, Depreeuw J, ter Haar N, Noske A, Amant F, Tomlinson IPM, Wild PJ, Lambrechts D, Jürgenliemk-Schulz IM, Jobsen JJ, Smit VTHBM, Creutzberg CL, Bosse T (2015) Prognostic significance of POLE proofreading mutations in endometrial cancer. J Natl Cancer Inst 107(1):402. https://doi.org/10.1093/jnci/dju402
Shikama A, Minaguchi T, Matsumoto K, Akiyama-Abe A, Nakamura Y, Michikami H, Nakao S, Sakurai M, Ochi H, Onuki M, Satoh T, Oki A, Yoshikawa H (2016) Clinicopathologic implications of DNA mismatch repair status in endometrial carcinomas. Gynecol Oncol 140(2):226–233. https://doi.org/10.1016/j.ygyno.2015.11.032
Garg K, Soslow RA (2009) Lynch syndrome (hereditary non-polyposis colorectal cancer) and endometrial carcinoma. J Clin Pathol 62(8):679–684. https://doi.org/10.1136/jcp.2009.064949
Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, Huntsman DG, Gilks CB, McAlpine JN (2015) A clinically applicable molecular-based classification for endometrial cancers. Br J Cancer 113(2):299–310. https://doi.org/10.1038/bjc.2015.190
Talhouk A, McConechy MK, Leung S, Yang W, Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, Karnezis AN, Huntsman DG, Gilks CB, McAlpine JN (2017) Confirmation of ProMisE: a simple, genomics-based clinical classifier for endometrial cancer. Cancer 123(5):802–813. https://doi.org/10.1002/cncr.30496
McConechy MK, Talhouk A, Li-Chang HH, Leung S, Huntsman DG, Gilks CB, McAlpine JN (2015) Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol Oncol 137(2):306–310. https://doi.org/10.1016/j.ygyno.2015.01.541
Talhouk A, Hoang LN, McConechy MK, Nakonechny Q, Leo J, Cheng A, Leung S, Yang W, Lum A, Köbel M, Lee CH, Soslow RA, Huntsman DG, Gilks CB, McAlpine JN (2016) Molecular classification of endometrial carcinoma on diagnostic specimens is highly concordant with final hysterectomy: earlier prognostic information to guide treatment. Gynecol Oncol 143(1):46–53. https://doi.org/10.1016/j.ygyno.2016.07.090
Lee S, Piskorz AM, Le Page C, Mes Masson AM, Provencher D, Huntsman D, Chen W, Swanson PE, Gilks CB, Köbel M (2016) Calibration and optimization of p53, WT1, and napsin A immunohistochemistry ancillary tests for histotyping of ovarian carcinoma: Canadian Immunohistochemistry Quality Control (CIQC) experience. Int J Gynecol Pathol 35(3):209–221. https://doi.org/10.1097/PGP.0000000000000251
Piulats JM, Matias-Guiu X (2016) Immunotherapy in endometrial cancer: in the nick of time. Clin Cancer Res 22(23):5623–5625. https://doi.org/10.1158/1078-0432.CCR-16-1820
Piulats JM, Guerra E, Gil-Martín M, Roman-Canal B, Gatius S, Sanz-Pamplona R, Velasco A, Vidal A, Matias-Guiu X (2017) Molecular approaches for classifying endometrial carcinoma. Gynecol Oncol 145(1):200–207. https://doi.org/10.1016/j.ygyno.2016.12.015
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Goebel, E.A., Vidal, A., Matias-Guiu, X. et al. The evolution of endometrial carcinoma classification through application of immunohistochemistry and molecular diagnostics: past, present and future. Virchows Arch 472, 885–896 (2018). https://doi.org/10.1007/s00428-017-2279-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-017-2279-8