Abstract
Human enteroviruses, e.g. coxsackieviruses, induce a variety of severe acute and chronic forms of disease, including myocarditis, meningitis and diabetes mellitus type 1. To visualize enterovirus infection with a diagnostic intent, many studies have applied a commercially available antibody (anti-CVB5 VP1, clone 5-D8/1, Dako, Hamburg, Germany) that identifies VP1 of different enteroviral serotypes. Many antibodies, however, have been found to bind non-specifically to proteins of cardiomyocytes and in the interstitial space, resulting in non-specific staining in immunohistochemistry. In this paper we show that the anti-CVB5 VP1 antibody, recognizing VP1 of coxsackieviruses and widely used in diagnostics and research, shows strong cross-reactivity with cellular proteins in the heart (and pancreas) of humans and mice, which calls for a more specific antibody to be used for diagnostic purposes. We observed by Western blot analyses of lysates from human heart tissue samples and HeLa cells two cross-reactive bands when using clone 5-D8/1. Peptide mass fingerprinting (MALDI-TOF) identified these proteins as creatine kinase (B-type) and tubulin, confirming that this mAb detects cellular proteins in addition to viral VP1. In order to overcome the problems of false positive VP1 staining we generated a new highly specific and sensitive monoclonal antibody (Cox mAB 31A2) that recognizes VP1 from CVB3. The new antibody was characterized and was found to function well in immunohistochemistry, immunofluorescence staining, Western blotting, ELISA and FACS analyses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enteroviruses are small, non-enveloped viruses with an icosahedral capsid containing the positive-sense, single-stranded RNA genome. The capsid of all enteroviruses consists of 60 copies of the viral proteins 1–4 (VP1, VP2, VP3, VP4) [17]. Human enteroviruses, comprising more than 100 different serotypes, usually cause mild and self-limiting infections. However, enteroviruses may also induce a variety of severe acute and chronic diseases, including myocarditis, meningitis, and diabetes mellitus type 1 [16, 17, 23, 24]. During acute viral myocarditis, which is often induced by coxsackievirus B3, massive immune cell infiltration into the cardiac muscle can lead to reduced systolic function in individuals and can progress to dilated cardiomyopathy (DCM) [4]. However, the clinical diagnosis of myocarditis is still difficult and cannot be made without expert knowledge, due to its varied and often non-specific clinical presentation. Myocarditis is defined as inflammation of the heart muscle that is diagnosed using clinical, histopathological and immunohistological criteria [3, 27]. According to consensus statement papers the histopathological diagnosis of myocarditis is based on necrosis or degeneration (or both) of myocytes of non-ischemic origin accompanied by an adjacent inflammatory infiltrate [3]. An infectious cause of myocarditis can be confirmed or excluded using molecular virological methods, such as polymerase chain reaction (PCR) with subsequent sequencing of the amplified nucleic acids. In addition, in situ hybridization has become an important tool for diagnosis of viral infections [19].
As various enterovirus serotypes are involved in the pathogenesis of this life-threatening disease, many studies have sought to detect enterovirus group-specific viral capsid proteins using specific antibodies. An enterovirus group reactive monoclonal antibody (clone 5-D8/1) was reported to detect VP1 [26] from CVB5 but also from other enteroviruses [25]. This murine antibody is applied by many research groups for immunohistochemical and immunofluorescence staining of enteroviral VP1 in human and mouse heart tissue [1, 2]. In one study this antibody was used to suggest an etiological role for coxsackievirus B in Keshan disease [15]. This antibody targets one of the most immunodominant regions of enteroviruses (VP1) [26] and has also been used in ELISA or Western blotting, as it recognizes various coxsackie A and B viruses, polio- and echoviruses [31, 32]. The broad range of enterovirus infections detected by this antibody is useful for a preliminary search for a virus infection [32] but this goes along with a lack of specificity. We and others have observed strong cross-reactivity of this antibody with cellular proteins in tissue samples of heart, pancreas and gut, notably when used in immunohistochemistry [12]. Cross-reactivity of this antibody was detected in the myocardium of patients with familial cardiomyopathy, who were negative for viral RNA which confirmed absence of enterovirus infection. The anti-VP1 5-D8/1 mAb reportedly stains pancreatic centro-acinar cells, smooth muscle cells [22] and enterocyte nuclei [18], which should be regarded as non-specific as in these cell types enterovirus replication has never been observed by in situ hybridization or immunohistochemistry using polyclonal anti-CVB3 antibodies [30]. We also found enterovirus-unrelated reactivity by Western blotting in lysates from uninfected human heart samples and uninfected and infected HeLa cells.
This study firstly aimed to identify the nature of this unexplained cross-reactivity of the CVB5 5-D8/1 antibody by peptide mass fingerprinting (MALDI-TOF) of reactive proteins separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). A second aim was to develop a monospecific monoclonal anti-CVB3 antibody without cross-reactivity, as CVB3 is the major infectious agent to cause enteroviral myocarditis [11, 19]. Specificity and application range of the new antibody, Cox mAB 31A2, was investigated in different experimental approaches.
Material and methods
Immunohistochemistry
CVB3 VP1 protein was visualized in infected murine hearts and human heart tissue samples from explanted hearts or from patients with proven enterovirus infection by immunohistochemistry. Tissue sections were deparaffinized, subjected to heat induced epitope retrieval (in 10 mM citrate buffer) and incubated with monoclonal mouse anti-enterovirus antibody (1:400, clone 5-D8/1, Dako, Hamburg, Germany) or monoclonal mouse Cox mAB 31A2 antibody (1:400, Mediagnost, Reutlingen, Germany) for 1 h at room temperature. On mouse tissue sections immunoreactivity was visualized using the MaxHomo Mouse on Mouse Polymer HRP Detection Kit (MaxVision, Washington, USA) according to the manufacturer’s protocol followed by HistoGreen as chromogen (Linaris, Dossenheim, Germany). On human heart tissue sections this was done using the Zytochem Plus HRP Polymer System (Zytomed, Berlin, Germany) with di-aminobenzidine (brown signals, Fig. 1) (Dako, Hamburg, Germany) or HistoGreen as chromogen (green signals, Fig. 4). All tissue sections were counterstained with hematoxylin. As negative control, isotype-specific IgG was used instead of the primary antibody. Slides were viewed with a Zeiss Axioskop 40 microscope.
Lethal coxsackievirus myocarditis in a male neonate. Immunohistochemistry (IH) detects enteroviral VP1 in myocytes (clone 5-D8/1, brown signals). No staining is detected in the isotype-specific IgG negative control. Radioactive RNA/RNA in situ hybridization visualizes enteroviral RNA genome in myocytes (black silver grains) (a). Strong background staining in the heart of a patient with systemic sclerosis and chronic inflammation using clone 5-D8/1 (brown signals). No viral RNA is detected by radioactive in situ hybridization in consecutive tissue sections (b). Unspecific staining (green) of necrotic myocytes from mice 3 months post CVB3 infection by clone 5-D8/1. CVB3 RNA is not found by radioactive in situ hybridization (c).(a,b,c ×200)
For the detailed protocols of all other used methods please refer to Suppl. File 1 [5, 9, 14, 29].
Results
The 5-D8/1 antibody stains CVB3 infected but also non-infected myocytes in human and mouse tissue samples
The 5-D8/1 antibody stained myocytes in immunohistochemical studies, of which infection was proven by detection of enteroviral RNA by RT-PCR (data not shown) and radioactive in situ hybridization as shown in Fig. 1a. Control immunohistochemistry using isotype-specific IgG instead of the first antibody was negative. As shown in Fig.1b, however, the antibody resulted in non-specific staining as it stained myocytes in tissue samples from autoimmune-mediated chronic myocarditis, such as in a patient with systemic sclerosis. Absence of viral infection was supported by negative radioactive in situ hybridization or RT-PCR for the detection of enteroviral RNA. Non-specific staining was also observed in necrotic or apoptotic myocytes of mice months after CVB3 inoculation, in which no viral RNA was detected by RT-PCR or in situ hybridization (Fig. 1c). Importantly, unaffected myocytes in human or mouse hearts did not show non-specific staining.
Peptide mass fingerprinting identifies clone 5D8/1 cross-reactive cellular proteins
We analyzed HeLa cells and virus-negative heart tissue samples by SDS-PAGE and subsequent Western blotting. Figure 2a shows a cross-reactive band (1) in lysates of HeLa cells which were uninfected or infected with different enterovirus subtypes. Subsequent 2D-gel electrophoresis showed a ~ 40 kDa band corresponding to the Western blot, which was cut out for enzymatic digestion with trypsin and analysis by mass spectrometry (MALDI-TOF). This procedure identified creatine kinase B-type (KCRB_HUMAN) with sequence coverage of 50 % (Fig. 2c). In addition, in lysates of uninfected human heart analyzed by SDS-PAGE a second cross-reactive band (2) with a higher molecular weight (~50 kDa) band was noted (Fig. 2b) which by MALDI-TOF was found to represent tubulin beta chain (TBB5_HUMAN) with a sequence coverage >70 % (Fig. 2c).
Cross-reactivity of clone 5-D8/1 with cellular proteins of HeLa cells and human myocardium by Western blotting of HeLa cells infected with different enteroviruses and mock-infected cells. A VP1-specific band is detected in infected cells, in addition to a cross-reactive band with a molecular weight of ~40 kDa (a). By Western blotting of human myocardium negative for enteroviral RNA by RT-PCR and in situ hybridization two cross-reactive bands are observed in absence of a VP1-specific band (b). The cross-reactive bands were cut out and analyzed by peptide mass fingerprinting (MALDI-TOF). The cross-reactive band of ~40 kDa was identified as creatine kinase B-type (15 peptides matched/82 masses submitted, expect value: 2*10−6). Human tubulin beta was found as a major component of the cross-reactive band with a molecular weight of ~50 kDa (23 peptides matched/88 masses submitted, expect value: 1.6*10−15 (c)
Generation of a monoclonal antibody recognizing CVB3 protein
In order to generate a new monoclonal antibody recognizing CVB3 we immunized mice twice with CVB3 Nancy strain. Immunoglobulin production of anti-CVB3 antibodies was shown by immunofluorescence staining of infected and uninfected HeLa cells, only infected cells showing specific staining (Fig. 3a). Spleen cells of the mouse showing the highest antibody titer were fused to mouse myeloma cells in order to generate hybridomas. Supernatants were tested for antibody production by immunofluorescence staining of CVB3-infected or uninfected fixed cells, using 5-D8/1 as positive control. Clones producing supernatant with specific staining were subcloned by limited dilution. After three subcloning cycles, clone Cox 31 A2, producing an IgG of subclass 2, was selected for further study.
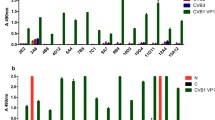
Immunofluorescence staining of infected Vero cells to test the ability of the serum from immunized mice to detect viral proteins. CVB3-infected and fixed monolayers were incubated with different dilutions of sera (positive cells are red), nuclei were counterstained with DAPI. Positive control of infection was done by staining the infected monolayers with clone 5-D8/1 (a). Comparison of cross-reactivity of the clones 5-D8/1 and Cox mAB 31A2 in cultured enterovirus–infected HeLa cells. Detection of VP1 (green) of different enteroviruses in HeLa cells by indirect immunofluorescence staining with Cox mAB 31A2 (upper row) or 5-D8/1 (lower row), nuclei are counterstained with DAPI (b). Western Blot analysis detecting VP1 of different enterovirus serotypes in HeLa cells, indicating that Cox mAB 31A2 detects only CVB3 (Nancy and PD strain) (c). ELISA experiments reveal a strong reaction with CVB3 and a minor reaction for CVB5 with Cox mAB 31A2 whereas the 5-D8/1 reacts also with CVB2, 3, 4 and 5 (d). The results are shown from three independent experiments
The Cox mAB 31A2 antibody only recognizes CVB3 viral proteins
Clone Cox mAB 31A2 antibodies were purified by protein A-based affinity chromatography and tested for cross-reactivity on HeLa cells infected with enteroviruses CVB2, CVB4, EV7 and CVA9. As shown in Fig. 3b, by immunofluorescence no staining was observed with other enteroviruses, indicating high specificity for CVB3 proteins. Only minimal CVB5 cross-reactivity was observed. Specificity was confirmed in Western blotting experiments on lysates from enterovirus infected HeLa (Fig. 3c). By ELISA Cox mAB 31A2 efficiently detected CVB3 with slight cross-reactivity with CVB5 (Fig. 3d).
MALDI-TOF identifies VP1 CVB3 protein as recognized by Cox mAB 31A2
We again applied peptide mass fingerprinting to identify the CVB3 protein detected by the Cox mAB 31A2 antibody. Virus containing supernatants of CVB3-infected cells were concentrated by ultrafiltration, subjected to SDS-PAGE and Western blotting and the detected band was cut out and after digestion with trypsin or chymotrypsin subjected to MALDI-TOF. VP1 was identified as the CVB3-protein that is recognized by Cox mAB 31A2 (Suppl. File 2, Fig.1).
Cox mAB 31A2 specifically stains VP1 CVB3 protein in human and mouse hearts by immunohistochemistry
We performed immunohistochemical staining on formalin-fixed paraffin embedded myocardial tissue of patients with proven enterovirus infection using Cox mAB 31A2. As shown in Fig. 4b and d, the antibody stains CVB3-infected myocytes without background staining. As positive control, CVB3 viral RNA was detected by radioactive in situ hybridization (Fig. 4a). Immunohistochemistry was consistently negative when the first antibody was replaced by an isotype-specific IgG antibody (Fig. 4c). Similar experiments on mouse hearts with acute viral myocarditis (8 days post-CVB3 infection) revealed immunohistochemical staining patterns of CVB3 RNA congruent with those obtained by radioactive in situ hybridization (e) and VP1 (Fig. 4e-l). Negative controls showed no staining (f). Figure 4h shows single infected myocytes already on day 4 after infection. Likewise, similar patterns of infection were observed in acutely CVB3-infected pancreas by in situ hybridization for the detection of viral RNA (i) and immunohistochemistry with the Cox mAB 31A2 antibody (j). Specific staining of different mouse organs was confirmed by immunofluorescence staining of CVB3-infected murine tissue (Fig. 4k and l).
Visualization of CVB3 infection in acute myocarditis. Human myocardium with a with CVB3 infection is identified by positive radioactive in situ hybridization showing CVB3 RNA (black silver grains) (a). Immunohistochemistry of the same heart shows infected myocytes (green) without staining of surrounding tissue (b) as confirmed at higher magnification in (d). Negative control of the same tissue (c). (a, b, c ×200, d ×600). Comparison of radioactive in situ hybridization (ISH) and immunohistochemistry (IH) using Cox mAB 31A2 in acutely (8 days pi) CVB3-infected mice. ISH was used to the presence of CVB3 in a virus positive control heart (e), pancreas (i). The same patterns of infection are detected in mouse organs by specific IH in the myocardium (green myocytes in g and h) and also in the exocrine pancreas (j) without background staining. Negative control of the heart (f). Detection of infected myocytes by indirect immunofluorescence (positive cells in green, nuclei counterstained with DAPI) in acutely CVB3-infected mouse hearts (l). Mock-infected mouse hearts were negative (k). (e, f, g, k, l) ×200, (h) ×800, (i, j) ×600
Cox mAB 31A2 does not show non-specific staining as observed with mAb 5-D8/1
We compared immunohistochemical staining patterns of both antibodies on myocardial tissue from patients with different enterovirus-negative heart diseases. As shown in Fig. 5a and c, the 5-D8/1 antibody stains numerous myocytes in myocardium of a patient with virus-negative chronic myocarditis while neither the Cox mAB 31A2 (d) antibody nor the negative control (b) on consecutive sections show any staining. Similar findings are shown in Fig. 5e-j, on myocardial tissue samples of patients with enterovirus-negative chronic myocarditis, dilated cardiomyopathy and ischemic cardiomyopathy: 5-D8/1 antibody shows strong non-specific staining of affected and disrupted myocytes while staining with Cox mAB 31A2 antibody is negative.
Comparison of staining patterns with Cox mAB 31A2 and 5-D8/1 in tissue samples of patients suffering from non-viral chronic myocarditis (a-f), dilated cardiomyopathy (g,h) or ischemic cardiomyopathy (i, j). Unspecific staining of myocytes by 5-D8/1 (a, c; c is inset of a). Negative control in the same tissue from (a) is shown in (b). IH using Cox mAB 31A2 does not stain myocytes in this heart in comparison to (a) (d). Similar non-specific staining patterns in affected myocytes are observed with 5-D8/1 in patients with chronic myocarditis, dilated cardiomyopathy and ischemic cardiomyopathy (e, g, i) whereas the Cox mAB 31A2 does not cross-react with uninfected but damaged myocytes (f, h, i).(Bar size: (a-d) = 30 μm; (c) = insert of (a). (a, b, c, d) = 30 μm; (i, j) =100 μm)
FACS analyses
To determine suitability of Cox mAB 31A2 antibody for FACS use, HeLa cells were infected with different MOI (1, 5 and 10) of CVB3 Nancy strain and analyzed by FACS. The antibody showed highly sensitive MOI-dependent detection of VP1 positive but not of mock infected cells by FACS. No staining was detected in mock-infected cells (Suppl. File 2, Fig. 2).
Discussion
The anti-enterovirus 5-D8/1 antibody is widely used for the detection of enterovirus infection in diagnostic and experimental settings. This antibody does detect enteroviral VP1 by immunohistochemistry, immunofluorescence and Western blotting notably in infected cultured cells [21, 28]. However, strong cross-reactivity of this antibody has been reported in tissue samples of the pancreas, heart and other organs, especially by immunohistochemistry [12]. These findings suggest that the antibody cross-reacts with epitope(s) of cellular proteins homologous with residues 40–48 of VP1 [26]. Some controversy exists with regard to the exact nature of the cross-reacting antigens. In the pancreas cross-reactivity of this antibody with two mitochondrial proteins, creatine kinase B-type and ATP synthase beta subunit, was reported by Western blotting, two-dimensional gel electrophoresis, and mass spectrometry [10] but this was not confirmed in another study, which found cross reactivity in lysates of HeLa and HepG2 cell by Western blotting [21]. Here, the authors conclude that the 5-D8/1 antibody recognizes only denatured forms of CKB. We have not been able either to immunoprecipitate or detect in ELISA defined cellular proteins with the 5-D8/1 antibody from lysates of uninfected HeLa cells (unpublished data).
In order to identify target molecules of the 5D8/1 antibody, we performed peptide mass fingerprinting and identified beta tubulin (TBB5) and creatine kinase B-type as reacting with the antibody, confirming earlier published data obtained from human pancreas islets by using LC/MS/MS [10]. This finding provides an explanation for the strong background staining in heart tissue with the 5-D8/1 antibody. Another issue with the 5-D8/1 antibody is that in different diseases of the heart, including ischemic heart disease, not all myocytes are uniformly stained as shown in Fig. 5. The patchy staining pattern suggests that only affected (apoptotic/necrotic) myocytes are stained, which we have confirmed by parallel immunohistochemical staining of damaged myocytes (unpublished results). This might be due to differential exposure in affected myocytes of epitopes of specific cardiac proteins, which can then be detected by cross-reacting antibodies. This is supported by the finding that in patients with myocarditis or cardiomyopathy anti-heart autoantibodies such as anti-myosin antibodies develop [7] as a consequence of myocyte destruction. In addition, CVB3 VP1 appears to share about 40 % identity with cardiac myosin in a 14- to 15-amino acid overlap, which explains cross-reactivity between VP1 and cardiac myosin [8].
Cross-reactivity between antiviral antibodies and cellular epitopes was not only found in enterovirus infections but also in herpesvirus infections. An anti-Varicella Zoster Virus (VZV) antibody cross-reacted with various myocytes (smooth including arterial, skeletal and cardiac), purportedly due to shared epitopes between VZV proteins and myocytes [20]. The problem of shared epitopes leading to cross-reactivity in immunohistochemistry is well-known and is a main concern when validating antibodies for diagnostic and research purposes [6].
To overcome the problems with 5-D8/1 antibody we generated a new monoclonal antibody, Cox mAB 31A2, which recognizes coat protein VP1 from CVB3 as shown by peptide mass fingerprinting. We performed comprehensive validation of the Cox mAB 31A2 antibody according to the “Rimm Lab Algorithm”, including Western blot analyses of different cells lines infected with various enteroviruses over a range of dilutions in different assays and antibody lots [6]. Finally, the staining pattern obtained using the Cox mAB 31A2 antibody was compared that of the 5-D8/1 antibody in immunohistochemistry and immunofluorescence and with localization of viral RNA by in situ hybridization in consecutive tissue sections of CVB3 positive tissue. The new antibody shows no cross-reactivity with other coxsackievirus serotypes by Western blotting using lysates of infected HeLa cells. In immunofluorescence staining experiments and ELISA, minimal cross-reactivity with CVB5 was observed, which is not surprising as VP1 of CVB3 and CVB5 have a high sequence similarity.
Detailed immunohistochemical experiments revealed that Cox mAB 31A2 recognizes only VP1 in human as well as in murine tissue samples but no other cellular proteins (see Figs. 4 and 5). Specificity for CVB3 infection was confirmed by the presence of viral RNA as detected by RT-PCR and radioactive in situ hybridization [11, 13]. Immunohistochemical staining of heart tissue of patients with non-infectious myocarditis revealed no background staining, which makes the new antibody a reliable screening tool for CVB3 infection in patients with suspected myocarditis and dilated cardiomyopathy. The antibody can also be used in Western blotting, shows high specificity and sensitivity in FACS analyses and can be used in ELISA.
In conclusion, the monoclonal 5-D8/1 antibody should not be used for diagnosing enterovirus infections in diagnostic samples. The new Cox mAB 31A2 antibody provides a sensitive and specific alternative. Moreover, the detection of enterovirus infection especially in the heart by immunohistochemistry should be complemented by other molecular approaches, such as (quantitative) RT-PCR and in situ hybridization.
References
Andreoletti L, Bourlet T, Moukassa D, Rey L, Hot D, Li Y, Lambert V, Gosselin B, Mosnier JF, Stankowiak C, Wattre P (2000) Enteroviruses can persist with or without active viral replication in cardiac tissue of patients with end-stage ischemic or dilated cardiomyopathy. J Infect Dis 182:1222–1227. doi:10.1086/315818
Andreoletti L, Hober D, Becquart P, Belaich S, Copin MC, Lambert V, Wattre P (1997) Experimental CVB3-induced chronic myocarditis in two murine strains: evidence of interrelationships between virus replication and myocardial damage in persistent cardiac infection. J Med Virol 52:206–214
Aretz HT (1987) Myocarditis: the Dallas criteria. Hum Pathol 18:619–624
Blauwet LA, Cooper LT Myocarditis Prog Cardiovasc Dis 52:274–288. doi:10.1016/j.pcad.2009.11.006
Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins. RNA and DNA in polyacrylamide gels Electrophoresis 8:93–99. doi:10.1002/elps.1150080203
Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D (2010) Antibody validation BioTechniques 48:197–209. doi:10.2144/000113382
Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, Thiene G, Iliceto S (2008) Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity 41:35–45. doi:10.1080/08916930701619235
Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ (1992) Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A 89:1320–1324
de StGroth SF, Scheidegger D (1980) Production of monoclonal antibodies: strategy and tactics. J Immunol Methods 35:1–21
Hansson SF, Korsgren S, Ponten F, Korsgren O (2013) Enteroviruses and the pathogenesis of type 1 diabetes revisited: cross-reactivity of enterovirus capsid protein (VP1) antibodies with human mitochondrial proteins. J Pathol 229:719–728. doi:10.1002/path.4166
Klingel K, Hohenadl C, Canu A, Albrecht M, Seemann M, Mall G, Kandolf R (1992) Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A 89:314–318
Klingel K, Sauter M, Bock CT, Szalay G, Schnorr JJ, Kandolf R (2004) Molecular pathology of inflammatory cardiomyopathy. Med Microbiol Immunol 193:101–107. doi:10.1007/s00430-003-0190-1
Klingel K, Stephan S, Sauter M, Zell R, McManus BM, Bultmann B, Kandolf R (1996) Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets. J Virol 70:8888–8895
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li Y, Bourlet T, Andreoletti L, Mosnier JF, Peng T, Yang Y, Archard LC, Pozzetto B, Zhang H (2000) Enteroviral capsid protein VP1 is present in myocardial tissues from some patients with myocarditis or dilated cardiomyopathy. Circulation 101:231–234
McManus BM, Chow LH, Radio SJ, Tracy SM, Beck MA, Chapman NM, Klingel K, Kandolf R (1991) Progress and challenges in the pathological diagnosis of myocarditis. Eur Heart J 12(Suppl D):18–21
Melnick J (1996) Enteroviruses: poliovirus, coxsackieviruses, echoviruses and newer enteroviruses. In: Fields BN, Knipe D, Howley P (eds) Fields virology. Lippincott-Raven, New York, pp. 655–712
Mercalli A, Lampasona V, Klingel K, Albarello L, Lombardoni C, Ekstrom J, Sordi V, Bolla A, Mariani A, Bzhalava D, Dillner J, Roivainen M, Bosi E, Piemonti L No evidence of enteroviruses in the intestine of patients with type 1 diabetes. Diabetologia 55:2479–2488. doi:10.1007/s00125-012-2591-4
Pankuweit S, Klingel K (2013) Viral myocarditis: from experimental models to molecular diagnosis in patients. Heart Fail Rev 18:683–702. doi:10.1007/s10741-012-9357-4 Review
Pisapia DJ, Lavi E (2016) VZV, temporal arteritis, and clinical practice: false positive immunohistochemical detection due to antibody cross-reactivity. Exp Mol Pathol 100:114–115. doi:10.1016/j.yexmp.2015.12.007
Richardson SJ, Leete P, Dhayal S, Russell MA, Oikarinen M, Laiho JE, Svedin E, Lind K, Rosenling T, Chapman N, Bone AJ, Foulis AK, Frisk G, Flodstrom-Tullberg M, Hober D, Hyoty H, Morgan NG (2014) Evaluation of the fidelity of immunolabelling obtained with clone 5D8/1, a monoclonal antibody directed against the enteroviral capsid protein, VP1, in human pancreas. Diabetologia 57:392–401. doi:10.1007/s00125-013-3094-7
Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG (2009) The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia 52:1143–1151. doi:10.1007/s00125-009-1276-0
Roivainen M, Klingel K (2009) Role of enteroviruses in the pathogenesis of type 1 diabetes. Diabetologia 52:995–996. doi:10.1007/s00125-009-1332-9
Rotbart HA (1995) Enteroviral infections of the central nervous system. Clin Infect Dis 20:971–981
Samuelson A, Forsgren M, Johansson B, Wahren B, Sallberg M (1994) Molecular basis for serological cross-reactivity between enteroviruses. Clin Diagn Lab Immunol 1:336–341
Samuelson A, Forsgren M, Sallberg M (1995) Characterization of the recognition site and diagnostic potential of an enterovirus group-reactive monoclonal antibody. Clin Diagn Lab Immunol 2:385–386
Schultz JC, Hilliard AA, Cooper LT Jr, Rihal CS (2009) Diagnosis and treatment of viral myocarditis. Mayo Clin Proc 84:1001–1009. doi:10.1016/S0025-6196(11)60670-8
Steinke K, Sachse F, Ettischer N, Strutz-Seebohm N, Henrion U, Rohrbeck M, Klosowski R, Wolters D, Brunner S, Franz WM, Pott L, Munoz C, Kandolf R, Schulze-Bahr E, Lang F, Klingel K, Seebohm G (2013) Coxsackievirus B3 modulates cardiac ion channels. FASEB J 27:4108–4121. doi:10.1096/fj.13-230193
Ursu ON, Sauter M, Ettischer N, Kandolf R, Klingel K (2014) Heme oxygenase-1 mediates oxidative stress and apoptosis in coxsackievirus B3-induced myocarditis cellular. Physiology and Biochemistry 33:52–66
Werner S, Klump WM, Schonke H, Hofschneider PH, Kandolf R (1988) Expression of coxsackievirus B3 capsid proteins in Escherichia coli and generation of virus-specific antisera. DNA 7:307–316
Yousef GE, Brown IN, Mowbray JF (1987) Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology 28:163–170
Yousef GE, Mann GF, Brown IN, Mowbray JF (1987) Clinical and research application of an enterovirus group-reactive monoclonal antibody. Intervirology 28:199–205
Acknowledgments
This work was supported in part by grants of the DFG (KL 595/2-3) and the German Federal Ministry of Education and Research (BMBF ID 13EZ0817). The authors thank Sandra Bundschuh for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
For the technical investigations we used leftover material which was not required any more for routine diagnosis of cardiac diseases (Kandolf R et al., Eur Heart J. 1991 Aug;12 Suppl D:49–55) and from mouse studies (Gruhle S et al., Basic Res Cardiol. 2012 Sep.;107(5):287).
Disclosure/Conflict of interest
A. Normann and B. Flehmig are employees of Mediagnost, Reutlingen, Germany. Mediagnost, Germany did not participate in the study design, data collection, data analysis, data interpretation, decision to publish or funding. K Klingel received a honorarium for a staff training lecture from Mediagnost. All other authors have no conflicts of interest to declare.
Electronic supplementary material
Suppl. File 1
(DOCX 24 kb)
Suppl. File 2
(PPTX 1212 kb)
Rights and permissions
About this article
Cite this article
Ettischer-Schmid, N., Normann, A., Sauter, M. et al. A new monoclonal antibody (Cox mAB 31A2) detects VP1 protein of coxsackievirus B3 with high sensitivity and specificity. Virchows Arch 469, 553–562 (2016). https://doi.org/10.1007/s00428-016-2008-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-016-2008-8