Abstract
Key quality parameters in diagnostic pathology include timeliness, accuracy, completeness, conformance with current agreed standards, consistency and clarity in communication. In this review, we argue that with worldwide developments in eHealth and big data, generally, there are two further, often overlooked, parameters if our reports are to be fit for purpose. Firstly, population-level studies have clearly demonstrated the value of providing timely structured reporting data in standardised electronic format as part of system-wide quality improvement programmes. Moreover, when combined with multiple health data sources through eHealth and data linkage, structured pathology reports become central to population-level quality monitoring, benchmarking, interventions and benefit analyses in public health management. Secondly, population-level studies, particularly for benchmarking, require a single agreed international and evidence-based standard to ensure interoperability and comparability. This has been taken for granted in tumour classification and staging for many years, yet international standardisation of cancer datasets is only now underway through the International Collaboration on Cancer Reporting (ICCR). In this review, we present evidence supporting the role of structured pathology reporting in quality improvement for both clinical care and population-level health management. Although this review of available evidence largely relates to structured reporting of cancer, it is clear that the same principles can be applied throughout anatomical pathology generally, as they are elsewhere in the health system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As diagnostic pathologists, our primary activity is the analysis of human fluids, cells and tissues. Our primary function, however, is to gather, process, synthesise and communicate pathology information which is required for, or useful in, the management of patient health. In diagnostic pathology, information is everything. It is our raison d'être, and our reports, in communicating this information, underpin our contribution to the quality of clinical care and public health.
Key quality parameters for reporting include timeliness, accuracy, completeness, conformance with current agreed standards, consistency and clarity in communication.
Over the last two decades, national pathology organisations in many countries have initiated programmes to develop standardised cancer datasets in order to ensure the quality of pathology reporting [1–4]. This is because, as the following sections will demonstrate, there is ample evidence that standardised structured reporting (SR) using agreed published datasets significantly improves the quality of individual pathology reports as defined above. In addition, although these datasets were developed initially as checklists to assist completeness of reporting, it is clear that, since they define and distil all the essential elements of what we produce as pathologists, they are central to quality activities within the laboratory. Many of the monitoring and other system-wide, quality-related activities detailed elsewhere in this special issue of Virchows Archiv depend upon the extraction, collation and analysis of individual elements of information contained in pathology reports. Adoption and electronic implementation of standardised SR are thus a powerful enabler for many of these quality activities.
Furthermore, with accompanying explanation and commentary, the same datasets are an important tool for the education and training of individual pathologists and, when made freely available globally, the same or modified datasets can be used to inform resource allocation within laboratories and the priorities of health planners in developing countries.
To date, many standards for SR and much of the published literature have centred on cancer and, for this reason, these form the basis for this article, but informatics standards have recently been developed to support SR across all of anatomical pathology, including support for digital imaging [5–7]. It is acknowledged that the same principles clearly apply to SR across all disciplines of diagnostic anatomical pathology and indeed in healthcare in general. For example, surgeons [8–10] and gastroenterologists [11–13] in selected practices have successfully adopted structured electronic reporting templates for operation notes and, although initially slow to adopt SR, diagnostic radiology is now embracing the same paradigm [14, 15]. In tumour radiology in particular, there is a need for accurate objective measurement and comparison over time to assess response to therapy, and clinical users require accurate structured data [16]. Reflecting this imperative, current DICOM standards in radiology include fields for structured diagnostic data [17].
Structured reporting is not a new concept in pathology. Clinical laboratories have always reported results as structured atomic data elements. In morphological pathology, Bethesda systems are an excellent example of standardised structured reporting in both gynaecological [18] and thyroid [19] cytology, the former having been the basis for successful cervical screening programmes worldwide for many years.
In the following sections, we discuss the differences between structured formatting of reports and structured data in the context of published literature, as well as the nature of datasets and the processes required to standardise content. Implementation issues are examined, and the benefits of SR in quality improvement are discussed both in relation to individual clinical care and population-level quality initiatives. A global perspective of standardisation in SR follows, with a brief overview of future possibilities and directions.
Levels of implementation of structured reporting—the Ontario scale
In 2009, Srigley et al. [20] described the spectrum of pathology cancer reporting, from traditional narrative reports with neither standardised content nor formatting (Level 1), through to Level 6 with an agreed, standardised dataset and electronic implementation with terminology binding (Fig. 1). Each incremental level leads to an improvement in reporting, but as the following sections will demonstrate, it is only at Level 6 that the full benefits for quality improvement can be achieved.
Implementation of structured reporting in the Ontario project [20] identified six levels. Level 1 follows the traditional text-based model in which there is no defined content or formatting. Synoptic-like reporting in most countries currently conforms to Level 3. The benefits of data aggregation, analysis and population-level quality measurement and interventions require Level 6 reporting
Standardising the content of reports (Ontario Level 2)
Pathology datasets contain information that is required for, or useful in, the management of patient health. In cancer datasets, this includes key macroscopic elements as well the microscopic findings including specific cancer type, TNM staging and all prognostic or predictive information relevant to patient management. Most published datasets recognise mandatory versus non-mandatory data elements. The International Collaboration on Cancer Reporting (ICCR) uses the terms “Required” and “Recommended” (Fig. 2), and the former are those which are essential for clinical management—such as TNM stage or classification, as well as prognostic or predictive information which satisfies evidentiary standards to Level III-2 [21]. Thus, the sum of all Required elements represents a minimum dataset. An important component of any dataset is a “Commentary” specific for each element. Commentary defines the element and the context in which it is used, describes how it is assessed or measured and provides the evidence to support its inclusion in the dataset according to published evidentiary standards.
This excerpt from the ICCR melanoma dataset illustrates the combination of data elements to form a structured dataset viz. element names, prescribed value lists and measurement constraints as well as conditional dependencies between different elements and Required (bold) versus Recommended (grey) elements. The book icons are hyperlinks to Commentary (bubble at right) describing what the element is and how it is measured and providing evidence for its inclusion in the dataset
Pathology cancer dataset content is necessarily reviewed at least with every new AJCC/TNM staging, and WHO “Blue Book” edition, but the rapid development of targeted therapies, personalised medicine and prognostic assays using in vitro diagnostics will drive ever-shortening development and review cycles. Structured reporting will be an essential component in the capture of biomarker data for both clinical management and assessment of efficacy and cost analysis across the healthcare system [22].
The process used to standardise content
The quality of a structured report depends upon the quality of the underlying standardised dataset template, which, in turn, depends upon the process that governs the development of that template.
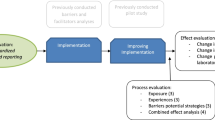
In the ICCR [23–27] and other established programmes [1–3], a defined series of steps is required (Fig. 3), beginning with the selection of a chair of the Dataset Authoring Committee (DAC) and its multidisciplinary experts according to defined criteria. Key steps include the assessment of existing datasets worldwide, development of a draft dataset in conjunction with the chair and iterative review by the DAC with evidentiary assessment using published standards [21]. The adoption of evidentiary standards to ensure the validity of required elements is a fundamental component of dataset development. To ensure consistent quality and efficiency, an ICCR Project manager assists and guides the DAC process and a member of the ICCR Steering Committee is seconded to each DAC. The resulting draft dataset is then published worldwide for a period of 6 weeks open consultation and all responses collated, reviewed and DAC responses and changes published openly prior to final publication on the ICCR website.
Structured formatting or synoptic reporting (Ontario Level 3)
Valenstein in 2008 [28] reviewed the structured formatting of pathology reports in the light of cognitive psychology and principles adopted by the media, aviation and other industries. The four guidelines presented in this review include the use of diagnostic headlines and “white space”, the standardisation of layout and continuity over time, the optimisation of information density and the reduction of “clutter”.
Unstructured formatting by comparison is not organised in any pre-defined way and may contain large blocks of text—exemplified by traditional written reports. In this “paragraphic” or narrative style, the lack of headings, white space or other formatting makes the content impenetrable to normal eye scanning; hence, the entire text must be read to find an individual element, and it may be overlooked—or absent. Indeed in 1991, Markel and Hirsch [29] noted that “… surgeons and other clinicians frequently asked why certain information, which was actually in the body of our reports, was not.” In response to this, they described the now familiar “synoptic” reporting format similar to that originally described by Hutter and Rickert several years previously [30].
The term “synoptic reporting” is often used synonymously with “structured reporting”, yet they are not identical [31]. Synoptic reporting as described by Hutter and Rickert refers to the commonly used two-column layout structure in which each line contains a single, separate data element. In most laboratories, this is still achieved using a text-based word processor, with no discrete data fields [32]. When standardised datasets or checklists are combined with synoptic formatting in a text-based system, the reports conform to Level 3 reporting on the Ontario scale [20] (Fig. 1). This achieves many key elements of quality in reporting including completeness, consistency, conformance and clarity, yet it is not structured data and cannot provide the many benefits of Level 6 electronic implementation using structured data.
Fully structured reporting (Ontario Levels 5 and 6)
“Structured data” is a computer informatics term that describes indivisible or “atomic” data elements combined in pre-defined way, multiple elements being combined to form a “dataset” (Fig. 2). Any relationships or interdependencies between the different data elements of any given data set are defined by a “data model”, sometimes referred to as an “archetype”.
Electronic implementation as a computer database is an excellent example of structured data, being made up of discrete data fields and, usually, defined value lists. Unstructured data on the other hand is best exemplified by free text, manipulated and stored in a word processor.
Implementation of structured data at Level 6, by enabling machine readability, unlocks many possibilities for pathologists including consistent and automatic structured formatting of reports, decision support and improved efficiency. When implemented at the population level, however, it opens up a new vista for analysis at all levels of system-level health management and in particular, as discussed below, possibilities for measuring and improving the quality of pathology reporting and practice.
Impact of SR upon quality of patient care
A number of publications provide good evidence that SR significantly improves the completeness of cancer reports [4, 20, 33–36]. In one study in particular [33], free text reports of pancreatic resections provided information on TNM stage, all resection margins, lymphovascular invasion and perineural invasion in 44, 11, 66 and 84 % of cases compared with 100, 94, 100 and 100 % for structured reports. One could argue that there are reasons why this might be the case other than the intrinsic value of SR: selection bias being the most obvious. However, longitudinal studies identifying changes in performance after staged introduction of SR also demonstrate highly significant improvements in completeness of reports [20, 34].
There are a number of possible reasons why this might be so. Datasets provide a “reminder” function akin to the checklists used by airline pilots prior to take off, but they also provide an educational component. The task of keeping abreast of advances across the full range of cancers has become onerous for pathologists, and cancer datasets ensure that all pathologists are kept abreast of the latest minimum reporting standards for all tumours, including those outside their areas of special interest. If the repetition and predictability of the question set and the possible responses inherent in each specimen type make structured reporting possible, it is the increasing complexity of pathology reporting datasets which makes structured reporting an imperative. Furthermore, the advent of targeted therapies and a rapidly expanding repertoire of accompanying biomarkers are currently adding significantly to the complexity of pathology reports and the need for both clarity and completeness in communication.
In addition to completeness, an important component of reporting quality is conformance to standardised nomenclature in order to avoid ambiguity. In the survey described by Gill et al., 28 different names were used to designate resection margins in 109 free text reports [33]. Standardised SR defines not only the data elements but also the nomenclature, agreed value lists and method of measurement.
Since standardised cancer pathology reports generally contain the date of surgery and the date of pathology reporting, the turnaround times can be derived and analysed at jurisdictional, institutional or individual pathologist levels, thus providing information on timeliness.
Accuracy is one of the most important determinants of quality in reporting. Clearly, SR cannot substitute for training, pathologist knowledge or skill in interpretation. Indeed, at one symposium [37], a clinician made the comment that “…at least with text reports, it is possible to tell whether the pathologist knows what they are talking about.” This is recognised in the RCPA datasets in which emphasis is placed upon providing the facility for a free text overarching comment or synthesis attached to the diagnosis line so that issues of uncertainty and nuance can be finessed as required, independently of the discrete data elements. SR does, however, contribute to accuracy by standardising methods of measurement (e.g. capsular invasion in prostate cancer, or visceral pleural involvement in lung cancer) and units (e.g. mitotic count per square millimetre vs high-power field). Moreover, although not yet widely explored or utilised, SR can enable decision support. This could be as simple as auto-calculation of the diagnosis line or TNM stage derived from the discrete data elements entered. More complex computer modelling of the patterns of data entered in real time could prompt the pathologist if unusual, inconsistent or forbidden combinations of tumour data are entered. Accumulation of discrete data elements across a number of pathologists over a period of time enables monitoring of reporting patterns that can form an important part of targeted quality peer review which may relate directly to accuracy.
Finally, clinician demand for SR will be well known to all pathologists and in many cases has been the driving force for SR programmes. Clinician approval is generally self-evident; however, the few published studies available indicate clinician satisfaction with completeness and readability of both structured and synoptic reports [29, 38].
Impact of population-wide structured reporting using quality indicators
There are many secondary users of pathology report information including registries, health planners, government agencies, research organisations and epidemiologists, and most of these require consolidated data from large populations of patients.
For secondary users, the same quality parameters apply as in clinical use: timeliness, accuracy, completeness, conformance with current agreed standards, consistency and clarity in communication. However, for large, population-wide use, there are two additional key considerations if the report quality is to be “fit for purpose”.
Firstly, the burden of manual coding of pathology reports in registries is prohibitively expensive and difficult, causing long delays in registration and analysis. To be fit for purpose to secondary users, the reports are best electronically encoded as standardised structured information at Level 6 (Fig. 1). By making pathology data available at atomic level on a real-time basis with timely analysis, the role of cancer registries and data repositories is changing from historical review to real-time dynamic analysis and feedback, thus enabling quality improvement in health systems.
In 2004, Cancer Care Ontario (CCO) [20] initiated a project aimed at improving the quality of cancer pathology reporting by standardising the content, format and transmission of reports to a central registry and enabling the information to be available for planning, quality measurement and quality improvement [39]. Clinically relevant quality indicators were developed, and structured pathology data were electronically captured at the point of reporting and used to automate the timely generation of clinical performance indicator analyses to support quality improvement in surgical oncology. Quality indicators were agreed (colorectal lymph node retrieval rates >12, and pT2 prostatectomy margin positivity rates <25 %), and institutional and aggregated data were made available to participating institutions. Underperforming entities were assisted through a knowledge transfer programme and in a cultural environment described as “shedding light not heat”. Over the 2-year period reported, the province-wide figures for lymph node retrieval rate (>12) increased from 76 to 87 %, and pT2 prostatectomy margin positivity rates decreased from 37 to 21 % [39]. The early implementation of Level 3, then Level 6 pathology structured reporting was an essential foundational component of this quality improvement process [20]. Significantly, although initially analysed and published at an institutional level, the same data can be used to monitor performance at individual practitioner level [40].
The second key consideration in report quality for secondary users is the level at which standardisation is applied. Whilst it is possible to agree upon standardised cancer datasets for clinical use at any level: institutional, state, provincial or national, it is clear that population-wide studies, particularly for benchmarking, require a single agreed international standard to ensure interoperability and comparability. This has been taken for granted in tumour classification [41] and staging [42–44] for many years, yet international standardisation of cancer datasets is only now underway through the International Collaboration on Cancer Reporting [27].
Global standardisation of structured reporting
The International Collaboration on Cancer Reporting was formed in 2011 to develop common, internationally standardised and evidence-based cancer protocols for reporting in surgical pathology [27]. ICCR experience has demonstrated that collaboration on a single international reporting standard can
-
1.
Attract the world’s best pathology domain experts and pathology societies to the development process
-
2.
Remove elements which are outmoded or lack evidentiary support
-
3.
Be readily achieved—often more simply than in smaller jurisdictions
-
4.
Significantly reduce cost and duplication of effort in dataset development worldwide
-
5.
Provide reporting standards for those countries which lack the necessary resources
-
6.
Enable global interoperability and flow of core cancer data
-
7.
Provide a single authoritative and evidence-based overview or template for education in cancer reporting worldwide
-
8.
Simplify and reduce the cost of necessary software development, terminology binding and electronic implementation
The quality of standardised pathology reporting templates is further supported by strategic alliances which ensure close working relations between other international standard setting organisations including World Health Organisation (WHO)/International Association for Research in Cancer (IARC), American Joint Commission on Cancer (AJCC)/International Union for the Control of Cancer (UICC), International Association of Cancer Registries (IACR) and the many downstream clinical and secondary users including registries, research organisations and health planners throughout the world. ICCR publishes datasets in synchrony with the WHO Blue Books, necessitating WHO/IARC representation on the ICCR Dataset Steering Committee and cross representation between ICCR and a number of strategic partners such as the UICC TNM Core Group Committee.
Data linkage and standardised structured pathology reporting
Whilst initial studies focussed upon the importance of standardised SR in managing quality through performance indicators which are themselves derived directly from pathology reports [39], it is only through data linkage that pathology SR data can be linked meaningfully to health outcomes including survival curves. This is achieved through combining standardised pathology SR data with information from multiple sources such as cancer registries, screening programmes, clinical and multidisciplinary team (MDT) registries, radiotherapy and radiology services, public and private health insurers, hospital and pharmaceutical databases and death registrations [45]. It is clear that pathology is fundamental to any such data linkage analysis by providing key tumour stage and profile information, central to all downstream analyses.
A recent data linkage study of bladder cancer outcomes illustrates the importance of reporting the accurate pathological stage as a prerequisite for clinical management [46]. Significantly poorer patient outcomes were found in cases where muscularis propria was not mentioned in pathology reports leading to uncertainty between categories pT1 and pT2, with probable understaging and undertreatment.
Stage capture [43, 47] is a required element in SR cancer datasets and has been shown to improve significantly with the adoption of SR [20, 33, 34]. TNM stage, when combined with other health data through data linkage, can be used to assess the value of screening programmes and compliance with treatment guidelines [48], and to direct and assess the value of interventions in the health system [49, 50]. In a recent analysis of ovarian and breast cancer outcomes across six countries, the International Cancer Benchmarking Partnership excluded 54 and 52 % respectively of all patients due to lack of stage data from the relevant registries [51–53]. With recognition of the importance of TNM stage in population health management, there is a worldwide drive to capture this data, and structured pathology reporting is a key element [54]. Cancer Care Ontario (CCO) launched the Stage Capture programme in 2007 with the aim of recording full TNM stage on all patients by 2012 [55]. As a result, in 2010, CCO was able to report a high stage at presentation for First Nations women in Ontario, highlighting a need for health intervention in this group [50, 56].
Similar programmes are underway in many countries including the USA, UK and Australia, and through the Global Initiative for Cancer Registration (GICR) initiated by IARC [57].
Thus, pathology SR data lies at the core of data linkage studies that are essential to system-wide, population-based healthcare management. At one level, these can be used to assess the quality and appropriateness of healthcare across different geographical regions and socioeconomic or ethnic groups, or adherence to clinical practice guidelines. At another level, financial data coupled with SR information is integral to the benchmarking of health system performance and cost-effectiveness of different clinical pathways and healthcare interventions.
Implementation issues and the future
We have presented evidence that standardised Level 3 or “synoptic” reporting provides real benefit for the clinical management of patients and secondary users. There are no technological barriers to Level 3 synoptic reporting as this can be achieved using any word processor or text-based system—even a typewriter, and many centres, institutions and individuals around the world already practise this. Factors influencing adoption and retention of SR have been reported [4, 38, 58], and it is clear that some of the most important considerations include the need for a local pathologist leader or champion, the use of incentives such as co-payments, jurisdictional mandation and pathologist perception of the impact of SR upon their efficiency and workflow.
The question of whether SR represents a burden on pathologist time is a complex one, and published evidence has been inconclusive. Key to this is the need for an improved interface between the pathologist and laboratory information system or middleware solution. Providing electronic dropdown menus (Level 4), with or without speech recognition, can lead to improved laboratory efficiency and timeliness; however, this requires some investment in IT infrastructure. Given the many benefits of aggregated analysis, it is logical that IT investment would be best directed to a coordinated programme of Level 6 reporting linked to system-wide registry and eHealth implementation, directed by government or other large health organisations who are the major beneficiaries of population-level data linkage and analysis.
Standardised structured information is fundamental to information exchange in eHealth, but in time, technological improvements are likely to change the way that structured information is generated. Intelligent assistants such as Siri manipulate the interface between humans and computers, converting unstructured speech into structured data—and back again. Implementation of Level 6 reporting with an intelligent assistant could change the current paradigm, ensuring that all the quality parameters are met, including timeliness.
For the clinical users of our reports on paper or screen, there are further challenges in assimilating large amounts of complex information. Already, some datasets are too large for the traditional synoptic format, and more effective report rendering will be needed. For specific users, reporting of only a subset of the data may suffice, or more flexible electronic or web-based displays may ultimately need to be considered.
Appropriate technology platforms will be key to many aspects of reporting and communication in the developing eHealth environment. Nonetheless, globally standardised, evidence-based datasets and good clinical governance in their development are fundamental to both the quality of reporting in diagnostic pathology, and downstream data linkage studies essential for population health management.
References
RCPA (Royal College of Pathologists of Australasia) (2009) Structured pathology reporting of cancer. http://www.rcpa.edu.au/Publications/StructuredReporting.htm. Accessed 4 June 2015
RCPath (Royal College of Pathologists) datasets and tissue pathways. http://www.rcpath.org/index.asp?PageID=254. Accessed 4 June 2015
Cancer Committee C (2009) Cancer protocols and checklists. Cancer checklists. http://www.cap.org/apps/cap.portal?_nfpb=true&cntvwrPtlt_actionOverride=%2Fportlets%2FcontentViewer%2Fshow&_windowLabel=cntvwrPtlt&cntvwrPtlt%7BactionForm.contentReference%7D=committees%2Fcancer%2Fcancer_protocols%2Fprotocols_index.html&_state=maximized&_pageLabel=cntvwr Accessed 4 June 2015
Casati B, Bjugn R (2012) Structured electronic template for histopathology reporting on colorectal carcinoma resections: five-year follow-up shows sustainable long-term quality improvement. Arch Pathol Lab Med 136:652–656
Daniel C, Shrader T (2010) White paper: anatomic pathology structured reports. http://wiki.ihe.net/index.php?title=Anatomic_Pathology_Structured_Reports. Accessed 4 June 2014 2015
Daniel C, Macary F, Rojo MG, Klossa J et al (2011) Recent advances in standards for collaborative digital anatomic pathology. Diagn Pathol 6(Suppl 1):S17
DICOM Standards Committee WG, Pathology (2010) Digital imaging and communications in medicine, supplement 145: whole slide image IOD and SOP classes. ftp://medical.nema.org/medical/dicom/final/sup145_ft.pdf. Accessed 4 June 2015
Temple WJ, Chin-Lenn L, Mack LA, Cancer Surgery A (2014) Evaluating population-based breast cancer surgical practice in real time with a web-based synoptic operative reporting system. Am J Surg 207:693–696, discussion 696–697
Edhemovic I, Temple WJ, de Gara CJ, Stuart GC (2004) The computer synoptic operative report—a leap forward in the science of surgery. Ann Surg Oncol 11:941–947
Hoffer DN, Finelli A, Chow R, Liu J et al (2012) Structured electronic operative reporting: comparison with dictation in kidney cancer surgery. Int J Med Inform 81:182–191
Aabakken L, Barkun AN, Cotton PB, Fedorov E et al (2014) Standardized endoscopic reporting. J Gastroenterol Hepatol 29:234–240
Kumarasinghe MP, Brown I, Raftopoulos S, Bourke MJ et al (2014) Standardised reporting protocol for endoscopic resection for Barrett oesophagus associated neoplasia: expert consensus recommendations. Pathology 46:473–480
Soekhoe JK, Groenen MJ, van Ginneken AM, Khaliq G et al (2007) Computerized endoscopic reporting is no more time-consuming than reporting with conventional methods. Eur J Intern Med 18:321–325
Powell DK, Silberzweig JE (2015) State of structured reporting in radiology, a survey. Acad Radiol 22:226–233
Larson DB, Towbin AJ, Pryor RM, Donnelly LF (2013) Improving consistency in radiology reporting through the use of department-wide standardized structured reporting. Radiology 267:240–250
Brook OR, Brook A, Vollmer CM, Kent TS et al (2015) Structured reporting of multiphasic CT for pancreatic cancer: potential effect on staging and surgical planning. Radiology 274:464–472
von Wangenheim A, Barcellos CL Jr, Andrade R, de Carlos Back Giuliano I et al (2013) Implementing DICOM structured reporting in a large-scale telemedicine network. Telemed J E Health 19:535–541
Nayar R, Wilbur DC (2015) The Pap Test and Bethesda 2014. “The reports of my demise have been greatly exaggerated.” (after a quotation from Mark Twain). Acta Cytol 59:121–132
Ali SZ (2011) Thyroid cytopathology: Bethesda and beyond. Acta Cytol 55:4–12
Srigley JR, McGowan T, Maclean A, Raby M et al (2009) Standardized synoptic cancer pathology reporting: a population-based approach. J Surg Oncol 99:517–524
Merlin T, Weston A, Tooher R (2009) Extending an evidence hierarchy to include topics other than treatment: revising the Australian ‘levels of evidence’. BMC Med Res Methodol 9:34
Simpson RW, Berman MA, Foulis PR, Divaris DX et al (2015) Cancer biomarkers: the role of structured data reporting. Arch Pathol Lab Med 139:587–593
Scolyer RA, Judge MJ, Evans A, Frishberg DP et al (2013) Data set for pathology reporting of cutaneous invasive melanoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Am J Surg Pathol 37:1797–1814
McCluggage WG, Colgan T, Duggan M, Hacker NF et al (2013) Data set for reporting of endometrial carcinomas: recommendations from the International Collaboration on Cancer Reporting (ICCR) between United Kingdom, United States, Canada, and Australasia. Int J Gynecol Pathol 32:45–65
Jones KD, Churg A, Henderson DW, Hwang DM et al (2013) Data set for reporting of lung carcinomas: recommendations from International Collaboration on Cancer Reporting. Arch Pathol Lab Med 137:1054–1062
Kench JG, Delahunt B, Griffiths DF, Humphrey PA et al (2013) Dataset for reporting of prostate carcinoma in radical prostatectomy specimens: recommendations from the International Collaboration on Cancer Reporting. Histopathology 62:203–218
ICCR (2011) International Collaboration on Cancer Reporting. http://www.iccr-cancer.org. Accessed 4 June 2015
Valenstein PN (2008) Formatting pathology reports: applying four design principles to improve communication and patient safety. Arch Pathol Lab Med 132:84–94
Markel SF, Hirsch SD (1991) Synoptic surgical pathology reporting. Hum Pathol 22:807–810
Hutter RV, Rickert RR (1983) Organization and management of the surgical pathology laboratory. In: Silverberg SG (ed) Principles and practice of surgical pathology. Wiley, New York, pp 17–18
Ellis DW (2011) Surgical pathology reporting at the crossroads: beyond synoptic reporting. Pathology 43:404–409
Cancer Committee C (2011) Definition of synoptic reporting. http://www.cap.org/apps/docs/committees/cancer/cancer_protocols/synoptic_report_definition_and_examples.pdf. Accessed 4 June 2015
Gill AJ, Johns AL, Eckstein R, Samra JS et al (2009) Synoptic reporting improves histopathological assessment of pancreatic resection specimens. Pathology 41:161–167
Hammond EH, Flinner RL (1997) Clinically relevant breast cancer reporting: using process measures to improve anatomic pathology reporting. Arch Pathol Lab Med 121:1171–1175
Karim RZ, van den Berg KS, Colman MH, McCarthy SW et al (2008) The advantage of using a synoptic pathology report format for cutaneous melanoma. Histopathology 52:130–138
Cross SS, Feeley KM, Angel CA (1998) The effect of four interventions on the informational content of histopathology reports of resected colorectal carcinomas. J Clin Pathol 51:481–482
NSW CI (2007) Structured pathology reporting—report on a round table discussion. http://www.cancerinstitute.org.au/supporting-best-practice/treatments-and-protocols/structured-pathology-reporting. Accessed 4 June 2015
Lankshear S, Srigley J, McGowan T, Yurcan M et al (2013) Standardized synoptic cancer pathology reports—so what and who cares? A population-based satisfaction survey of 970 pathologists, surgeons, and oncologists. Arch Pathol Lab Med 137:1599–1602
Srigley J, Lankshear S, Brierley J, McGowan T et al (2013) Closing the quality loop: facilitating improvement in oncology practice through timely access to clinical performance indicators. J Oncol Pract 9:e255–e261
McFadyen C, Lankshear S, Divaris D, Berry M et al (2015) Physician level reporting of surgical and pathology performance indicators: a regional study to assess feasibility and impact on quality. Can J Surg 58:31–40
Bosman F, Jaffe ES, Lakhani SR, Ohgaki H (2015) WHO/IARC Classification of tumours. IARC Press, Lyon
Greene FL, Sobin LH (2009) A worldwide approach to the TNM staging system: collaborative efforts of the AJCC and UICC. J Surg Oncol 99:269–272
Edge SD, Byrd D, Compton C, Fritz AG et al (2009) AJCC cancer staging manual. Springer, New York
International Union against Cancer (UICC) (2009) TNM classification of malignant tumours. Wiley-Blackwell, Chichester
Roder DM, Fong KM, Brown MP, Zalcberg J et al (2014) Realising opportunities for evidence-based cancer service delivery and research: linking cancer registry and administrative data in Australia. Eur J Cancer Care (Engl) 23:721–727
Chamie K, Ballon-Landa E, Bassett JC, Daskivich TJ et al (2015) Quality of diagnostic staging in patients with bladder cancer: a process-outcomes link. Cancer 121:379–385
Sobin LH, Gospodarowicz MK, Wittekind C (2009) TNM classification of malignant tumours. Wiley-Blackwell, Hoboken
Beckmann KR, Bennett A, Young GP, Roder DM (2014) Treatment patterns among colorectal cancer patients in South Australia: a demonstration of the utility of population-based data linkage. J Eval Clin Pract 20:467–477
Mackillop WJ, O’Sullivan B, Gospodarowicz M (1998) The role of cancer staging in evidence-based medicine. Cancer Prev Control 2:269–277
Brierley JD, Srigley JR, Yurcan M, Li B et al (2013) The value of collecting population-based cancer stage data to support decision-making at organizational, regional and population levels. Healthc Q 16:27–33
Walters S, Maringe C, Butler J, Brierley JD et al (2013) Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer 132:676–685
Maringe C, Walters S, Butler J, Coleman MP et al (2012) Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol 127:75–82
Walters S, Maringe C, Butler J, Rachet B et al (2013) Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: a population-based study. Br J Cancer 108:1195–1208
Roder D, Buckley E (2015) Translation from clinical trials to routine practice: how to demonstrate community benefit. Asia Pac J Clin Oncol 11:1–3
Lankshear S, Brierley JD, Imrie K, Yurcan M (2010) Changing physician practice: an evaluation of knowledge transfer strategies to enhance physician documentation of cancer stage. Healthc Q 13:84–92
Sheppard AJ, Chiarelli AM, Marrett LD, Mirea L et al (2010) Detection of later stage breast cancer in First Nations women in Ontario, Canada. Can J Public Health 101:101–105
IARC (2014) Global Initiative for Cancer Registry Development (GICR). http://gicr.iarc.fr/. Accessed 4 June 2015
Casati B, Haugland HK, Barstad GM, Bjugn R (2014) Factors affecting the implementation and use of electronic templates for histopathology cancer reporting. Pathology 46:165–168
Acknowledgments
The authors would like to thank Meagan Judge for her considerable contributions to structured pathology reporting and for advice on this manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ellis, D.W., Srigley, J. Does standardised structured reporting contribute to quality in diagnostic pathology? The importance of evidence-based datasets. Virchows Arch 468, 51–59 (2016). https://doi.org/10.1007/s00428-015-1834-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1834-4