Abstract
We report a case of tumour in the head of the pancreas observed in a 57-year-old man with a history of worsening jaundice and elevated alpha-fetoprotein (AFP) serum level, who underwent Whipple pancreatoduodenectomy. Histologically, the tumour was predominantly composed of solid sheets of large eosinophilic cells with a prominent lymphoid infiltration without association neither with DNA microsatellite instability nor Epstein-Barr virus infection. The tumour was diffusely and strongly positive for hepatocyte paraffin-1 (Hep Par-1) and glypican-3 leading to the diagnosis of hepatoid carcinoma. Strong cytoplasmic staining for AFP was focally observed. Moreover, tumour cells showed countless cytoplasmic eosinophilic globules immunoreactive for the stress protein p62. A primary hepatocellular carcinoma of the liver was ruled out by careful clinical analysis. Hepatoid carcinoma is an extremely rare pancreatic neoplasm, and here, we describe the first case of such variant associated with lymphoid stroma. The characteristic histologic features and the immunophenotypic profile help in distinguishing this carcinoma from other pancreatic tumours, notably from medullary carcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatoid carcinoma (HC) is a primary extrahepatic carcinoma of non-germ cell origin showing, at least focally, cytological and/or architectural features of hepatocellular carcinoma. More frequently reported in the stomach [1], it has also been described in many other sites (e.g. ovary and gallbladder). Those occurring in the pancreas are extremely rare and represent a heterogeneous group of tumours. The first case of pancreatic hepatoid carcinoma (PHC) was reported in 1987 by Hruban et al [2]. Since then, 22 additional cases have been reported in the medical literature [3–23]. The definition of “hepatoid” differentiation is usually based on both the morphological and immunohistochemical evidence of hepatocellular lineage: large polygonal cells with abundant eosinophilic cytoplasm, often with periodic acid-Schiff (PAS) positive, resistant to diastase digestion (dPAS), intracytoplasmic globules and bile production, coupled with immunoreactivity for hepatocellular markers, such as alpha-fetoprotein (AFP), hepatocyte paraffin-1 (Hep Par-1), polyclonal carcinoembryonic antigen (pCEA), and CD10, the latter two with a typical canalicular pattern. Few data on the prognosis of these carcinomas are available and even less is known about their molecular features.
In this paper, we present a case of HC of the pancreas with an unusual, never previously described, morphology: a prominent lymphoid infiltration of the tumour stroma, without association with neither DNA microsatellite instability (MSI) nor Epstein-Barr virus (EBV) infection.
Clinical history
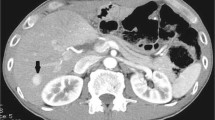
A 57-year-old man presented to the Surgery Department of the San Matteo University Hospital of Pavia (Italy) with a recent history of worsening jaundice without other symptoms. His past medical history was only significant for essential hypertension. He denied any underlying liver or pancreatic disease. Physical examination was unremarkable, except for jaundice. An abdominal computed tomography (CT) scan showed a round low-density mass in the head of the pancreas with an axial diameter of approximately 20-25 mm, impinging the intra-pancreatic common bile duct with a dilation of intra and extrahepatic biliary tract upstream; no narrowing of the main pancreatic duct was found. No invasion of the main vascular walls, in particular no connections with the superior mesenteric vessels or the celiac axis, no significant lymphadenopathies or hepatic focal lesions were detected.
Laboratory tests showed substantially normal serum levels of CEA (5.3 ng/mL; normal <5.0 ng/mL) and carbohydrate antigen 19-9 (CA19-9) (15.4 U/mL; normal <37.0 U/mL), but an elevated serum level of AFP (147.8 IU/mL; normal <12 IU/mL). Hepatitis B and C serology was negative. Blood cell counts, blood coagulation tests, serum amylase and lipase, and liver function tests were normal, except for blood bilirubin that was 9 mg/dl (direct bilirubin 6.4 mg/dl). As clinical evaluation and imaging did not show abnormal findings in the liver, the possibility of a primary hepatocellular carcinoma of the liver was excluded in this patient.
Twenty days later, the patient underwent conventional Whipple pancreatoduodenectomy. At a post-operative check, serum AFP was significantly decreased (24 IU/mL). As an early complication, he developed a low flow pancreatic fistula (with a maximum value of amylase in the peri-anastomotic drainage of 11,713 mU/mL on the 7th post-operative day), which spontaneously healed in a few days. In the 10th post-operative day, the patient was discharged.
Materials and methods
The surgical specimen was fixed in buffered formalin (formaldehyde 4 % w/v and acetate buffer 0.05 M) and, after macroscopic examination, cut specimens were routinely processed to paraffin. Histological sections were stained with haematoxylin-eosin (H and E), alcian-blue/periodic acid-Schiff (AB-PAS), periodic acid-Schiff (PAS), and PAS resistant to diastase digestion (dPAS) stains for the morphological evaluations.
Immunohistochemistry
A comprehensive panel of immunohistochemical stains was performed employing the antibodies listed in Table 1 and using a polymer-based method (Envision, DAKO) and diaminobenzidine as the chromogen. Negative and positive controls were performed for each immunohistochemical stain.
DNA isolation and KRAS mutation analysis
DNA was extracted using QuickGene DNA tissue kit S (DT-S; KURABO) on the FUJIFILM QuickGene-810 extraction platform (Fujifilm) according to the manufacturer’s instructions.
Genetic analysis of the KRAS gene was performed by PCR amplification of exons 2, 3 and 4 followed by direct sequencing of the PCR products. The PCR products were purified by QIAquick Gel Extraction kit (QIAGEN) and subjected to bidirectional dye-terminator sequencing (BigDye Terminator Version 1.1 Cycle Sequencing Kit) with the same primers used for the amplification. Sequencing fragments were detected by capillary electrophoresis using the automatic sequencer AB 3130 Genetic Analyzer (Life Technologies).
MSI analysis
MSI analysis was carried out using a pentaplex panel of monomorphic mononucleotide repeats (BAT25, BAT26, NR-21, NR-22 and NR-24) as previously reported [24].
EBV detection
In order to detect latent EBV infection, non-isotopic in situ hybridization was performed on formalin-fixed paraffin-embedded tissue sections according to standardized protocols with fluorescein-labelled EBER (Epstein-Barr encoded small RNAs, EBER-1 and EBER-2) peptidic nuclear acid probe (DAKO). Negative and positive controls were performed as well.
Results
Gross pathology
The gross specimen consisted of an 8 × 6.5 × 3-cm pancreatic head, with attached segment of gastric antrum-duodenum, common bile duct, cystic duct and gallbladder. On sectioning of the pancreas, a solid, lobulated, firm, grey-tan mass measuring 3.5 × 3 × 3 cm was identified (Fig. 1a). The tumour had well-delineated borders and appeared to focally infiltrate the duodenal wall. The tumour was entirely blocked in. Thirty lymph nodes were found in the peri-pancreatic and peri-common bile duct adipose tissue.
Macroscopic and histologic features of PHC with lymphoid stroma. a Gross photograph of a cut section of the pancreas showing a solid tan mass. b The tumour cells contain abundant eosinophilic cytoplasm and exhibit a trabecular and pseudoglandular growth pattern. A bile plug may be identified within a pseudoglandular lumen. c, d Heavy lymphoid infiltration of the tumour stroma (low power image in c), including a lymphoid follicle with germinal centre (shown in d)
Microscopic findings
H and E stained sections showed a tumour predominantly composed of solid sheets and islands of large eosinophilic cells (Fig. 1b). A pseudoglandular growth pattern was focally observed. We could identify varying degrees of cellular differentiation, ranging from hepatocyte-like large cells with abundant, eosinophilic or clear cytoplasm (sometimes with a microvesicular appearance), distinct cytoplasmic borders, central round vesicular nuclei and variably prominent nucleoli, to less differentiated and haphazardly arranged pleomorphic cells.
Although stromal desmoplasia was focally detected, the tumour stroma was mainly composed of a dense population of non-neoplastic lymphocytes (Fig. 1c). In particular, the lymphoid infiltrate was diffusely represented both inside the tumour and at its periphery, though with no evidence of lymphocyte infiltration inside individual epithelial neoplastic nests. A few lymphoid follicles, often with germinal centres, were also seen within the dense lymphoid infiltrate (Fig. 1d).
Well-circumscribed eosinophilic globules of variable size, only focally positive for dPAS staining, were frequently found in the cytoplasm of tumour cells (Fig. 3a); a yellowish brown pigment consistent with bile was sometimes identified within the pseudoglandular lumina, although no ectopic normal liver tissue was present. The neoplastic cells exhibited more than 10 mitoses per 10 high power fields and patchy areas of necrosis. Peri-neural and angio-lymphatic vessel invasion were identified. Tumour cells displayed a tongue-like invasion into the surrounding pancreatic lobules, infiltrated the duodenal muscular wall and extended into the peri-pancreatic soft tissues. Two lymph node metastases were also detected. According to the 7th edition of AJCC TNM staging system, tumour stage was IIB (pT3pN1M0). Surgical margins were negative.
Immunohistochemical findings
Hep Par-1 and glypican-3 antibodies showed diffuse cytoplasmic positivity in most tumour cells (Fig. 2a, b). Strong cytoplasmic staining for AFP was focally observed (Fig. 2c). Low molecular weight cytokeratins (cytokeratins 8 and 18), cytokeratin 19 (Fig. 2d), epithelial membrane antigen (EMA), were diffusely expressed, whereas cytokeratin 7 immunoreactivity was only focally present. Pseudoglandular structures were immunoreactive for pCEA. No immunoreactivity for acinar cell carcinoma markers (trypsin and B cell lymphoma/leukemia (Bcl)10), neuroendocrine markers (chromogranin A, synaptophysin, CD56), cytokeratin 20, CD10 and CD117 was observed; nuclear expression of beta-catenin was not observed as well. These immunohistochemical results support the hepatocellular differentiation of the tumour. As there was neither evidence of other differentiated (non-hepatoid) components nor clinicoradiologic evidence of a primary liver lesion, the histologic and immunohistochemical profile was diagnostic for a pure form of primary PHC. Non-neoplastic liver ectopic tissue within the pancreas from whom the carcinoma may have developed was carefully searched but not identified.
Cytoplasmic eosinophilic globules were immunoreactive for the stress protein p62 (Fig. 3b) and unreactive for cytokeratin 8 and 18, thus recalling the intracellular hyaline bodies present in the tumour cells of some primary hepatocellular carcinomas [25].
Immunophenotyping of tumour infiltrating lymphocytes showed a predominance of CD3-positive T cells, most of them CD8 positive, admixed among fewer CD20-positive B cells, which focally formed lymphoid follicles. No reactivity for the EBV antigen LMP-1 (latent membrane protein-1) was present. Mismatch repair proteins (MLH1, PMS2, MSH2, MSH6) were normally expressed in most tumour cells.
Molecular findings
No mutations in the analyzed exons of KRAS oncogene or MSI were identified. EBV in situ hybridization was negative in both tumour cells and lymphoid stroma.
Clinical follow-up and management
After surgery, the patient received chemotherapy with gemcitabine 1000 mg/mq (6 cycles). The patient underwent imaging follow-up with CT scan and abdominal RMN 1 month after the last chemotherapy cycle with no evidence of recurrence. The patient is currently alive and recurrence-free 18 months after resection. The last serum AFP level (1 month after the 6th chemotherapy cycle) was normal (8.76 IU/mL).
Discussion
After the first case reported by Hruban et al. [2], additional 22 PHCs have been reported in the medical literature [3–23]. Herein, we describe the 24th case diagnosed based on morphology and immunoreactivity for hepatocellular markers, such as Hep Par-1, AFP, and glypican-3. Furthermore, to the best of our knowledge, immunoreactivity for stress protein p62, a major constituent of intracellular hyaline bodies observed in a few hepatocellular carcinomas [25], had never been previously reported in any PHC. Notably, a primary hepatocellular carcinoma of the liver (metastatic to the pancreas) was ruled out, as clinical work-up failed to reveal any tumour in the liver. A clinicopathologic summary of the reported PHCs, including our case, is provided in Table 2.
A striking and new feature of our tumour was the massive lymphocytic infiltration, mainly composed of CD3+ CD8+ T lymphocytes. The lymphocytic infiltrate was diffusely present both in the intratumoural stroma and at the edge of the neoplastic growth. Because of this peculiar feature, medullary carcinoma of the pancreas was considered among the possible differential diagnoses. Medullary carcinoma of the pancreas is characterized by poor structural differentiation, well-defined borders, a syncitial growth pattern and at least focal necrosis. It generally shows several intratumoural T lymphocytes and harbours wild-type KRAS gene and microsatellite instability [26]. Although our case showed abundant lymphoplasmacytic infiltrates, akin to medullary carcinoma, it exhibited both histological and cytochemical features of hepatocellular differentiation. Thus, we bona fide classified it as a HC with lymphoid stroma. It is worth noting that lymphoplasmacytic infiltration has been observed in hepatoid carcinomas of other organs, such as the lung [27] and kidney [28], and in primary hepatocellular carcinoma as well [29].
Worth of note, the patient did not receive any pre-operative treatment, ruling out the possibility that the intratumoural lymphoid infiltrate could be due to secondary changes after chemotherapy. The possibility that the lymphoid tissue was a part of the peri-pancreatic lymph nodes was reasonably ruled out as (1) the mass was largely located in the centre of the pancreatic parenchyma (Fig. 1a), (2) the carcinoma with accompanying lymphoid stroma was also found in infiltrated duodenal muscular wall and (3) no overt lymph node structures could be identified.
Our case had wild-type KRAS gene, as it is often observed in medullary carcinoma of the pancreas [26] conversely to ductal adenocarcinoma of the pancreas, which frequently harbours KRAS mutations [30]. Notably, this is the first reported case of PHC in which a molecular analysis has been performed. Moreover, we could find no evidence of EBV infection using both LMP-1 immunohistochemistry and EBER in situ hybridization, which is known to be a highly sensitive and specific method to detect EBV [31]. To date, only a few cases of lymphoepithelioma-like carcinoma arising from the pancreas and hepatobiliary system have been reported [29, 32–35], most of them being EBV-positive biliary cholangiocarcinomas. In the literature, EBV RNA was identified in only two carcinomas of the pancreas, both without hepatocellular differentiation [26, 32].
The general, prognosis of PHC is difficult to establish due to its rarity. In general, HCs of the gastrointestinal tract carry a poor prognosis, likely due to an advanced stage at diagnosis. Nine out of 23 cases of PHC previously reported in the literature showed distant metastases at diagnosis and 11 died of disease. Interestingly, a few patients, as our, had a more indolent behaviour. Kelly et al [19] suggested that pure PHC might have a more favourable prognosis than mixed tumours (with ductal, acinar or neuroendocrine components). It has also been suggested that pancreatic ductal adenocarcinomas characterized by a CD8-positive T cell infiltration display a better clinical behaviour [36]. In hepatocellular carcinoma, a marked lymphocytic infiltration appears to be a favourable prognostic factor [37] seemingly owing to an antitumour effect mediated by T lymphocytes [38]. We may argue that the prominent lymphocytic infiltration in our case could at least partially account for the absence of tumour recurrence. In our case, there was no evidence of infiltration by lymphocytes inside the epithelial neoplastic nests, as usually seen in EBV-positive or medullary carcinomas. This pattern of lymphocytic infiltration is elsewhere defined as “intratumoural” rather than “intraepithelial” [39]; its meaning and its impact on prognosis remain unclear.
In conclusion, we described here the first case of PHC with lymphoid stroma, increasing our knowledge about pancreatic hepatoid carcinomas. The prominent hepatocellular differentiation helps in distinguishing this peculiar type of carcinoma from other pancreatic tumours, notably the medullary carcinoma of the pancreas.
References
Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y et al (1986) Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer 58:119–126
Hruban RH, Molina JM, Reddy MN et al (1987) A neoplasm with pancreatic and hepatocellular differentiation presenting with subcutaneous fat necrosis. Am J Clin Pathol 88:639–645
Tanno S, Obara T, Fujii T et al (1999) Alpha-fetoprotein-producing adenocarcinoma of the pancreas presenting focal hepatoid differentiation. Int J Pancreatol 26:43–47
Yano T, Ishikura H, Wada T et al (1999) Hepatoid adenocarcinoma of the pancreas. Histopathology 35:90–92
Paner GP, Thompson KS, Reyes CV (2000) Hepatoid carcinoma of the pancreas. Cancer 88:1582–1589
Lam K, Lo C, Wat M et al (2001) Malignant insulinoma with hepatoid differentiation: a unique case with alpha-fetoprotein production. Endocr Pathol 12:351–354
Hughes K, Kelty S, Martin R (2004) Hepatoid carcinoma of the pancreas. Am Surg 70:1030–1033
Matsueda K, Yamamoto H, Yoshida Y et al (2006) Hepatoid carcinoma of the pancreas producing protein induced by vitamin K absence or antagonist II (PIVKA-II) and α-fetoprotein (AFP). J Gastroenterol 41:1011–1019
Oh HJ, Cheung DY, Kim TH (2006) A case of hepatoid carcinoma of the pancreas. Korean J Gastroenterol 47:389–393
Shih NN, Tsung JS, Yang AH et al (2006) A unique pancreatic tumour with exclusive hepatocytic differentiation. Ann Clin Lab Sci 36:216–221
Cardona D, Grobmyer S, Crawford JM (2007) Hepatocellular carcinoma arising from ectopic liver tissue in the pancreas. Virchows Arch 450:225–229
Hameed O, Xu H, Saddeghi S et al (2007) Hepatoid carcinoma of the pancreas: a case report and literature review of a heterogeneous group of tumours. Am J Surg Pathol 31:146–152
Kubota K, Kita J, Rokkaku K (2007) Ectopic hepatocellular carcinoma arising from pancreas: a case report and review of the literature. World J Gastroenterol 13:4270–4273
Liu CZ, Hu SY, Wang L et al (2007) Hepatoid carcinoma of the pancreas: a case report. Chin Med J 120:1850–1852
Zhang Y, Zhou P, Sun Y (2007) Hepatoid carcinoma of the pancreas: a case report. Chin J Clin Oncol 4:445–447
Jung JY, Kim YJ, Kim HM et al (2010) Hepatoid carcinoma of the pancreas combined with neuroendocrine carcinoma. Gut Liver 4:98–102
Huang SC, Chang HC, Yeh TS et al (2012) Hepatoid microcarcinoma of the pancreas: a case report and review of the literature. Chang Gung Med J 35:285–291
Kai K, Nakamura J, Ide T et al (2012) Hepatoid carcinoma of the pancreas penetrating into the gastric cavity: a case report and literature review. Pathol Int 62:485–490
Kelly PJ, Spence R, Dasari BV et al (2012) Primary hepatocellular carcinoma of the pancreas: a case report and review of the heterogeneous group of pancreatic hepatoid carcinomas. Histopathology 60:1012–1015
Petrelli F, Ghilardi M, Colombo S et al (2012) A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer 43:97–102
Majumder S, Dasanu C (2013) Hepatoid variant of pancreatic cancer: insights from a case and literature review. JOP 14:442–445
Steen S, Wolin E, Geller SA et al (2013) Primary hepatocellular carcinoma (“hepatoid” carcinoma) of the pancreas: a case report and review of the literature. Clin Case Rep 1:66–71
Soofi Y, Kanehira K, Abbas A et al (2015) Pancreatic hepatoid carcinoma: a rare form of pancreatic neoplasm. Diagn Cytopathol 43:251–256. doi:10.1002/dc.23195
Suraweera N, Duval A, Reperant M et al (2002) Evaluation of tumour microsatellite instability using five quasimonomorphic mononucleotide repeats and pentaplex PCR. Gastroenterology 123:1804–1811
Denk H, Stumptner C, Fuchsbichler A et al (2006) Are the Mallory bodies and intracellular hyaline bodies in neoplastic and non-neoplastic hepatocytes related? J Pathol 208:653–661
Wilentz RE, Goggins M, Redston M et al (2000) Genetic, immunohistochemical, and clinical features of medullary carcinoma of the pancreas: a newly described and characterized entity. Am J Pathol 156:1641–1651
Haninger DM, Kloecker GH, Bousamra Ii M et al (2014) Hepatoid adenocarcinoma of the lung: report of five cases and review of the literature. Mod Pathol 27:535–542
Shim J, Go H, Lim YS et al (2014) Hepatoid differentiation in renal cell carcinoma: a rare histologic pattern with clinical significance. Ann Diagn Pathol 18:363–368
Chan AW, Tong JH, Pan Y et al (2015) Lymphoepithelioma-like hepatocellular carcinoma: an uncommon variant of hepatocellular carcinoma with favorable outcome. Am J Surg Pathol 39:304–312
Pellegata NS, Sessa F, Renault B et al (1994) K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumours progress through different genetic lesions. Cancer Res 54:1556–1560
Loughrey M, Trivett M, Lade S et al (2004) Diagnostic application of Epstein-Barr virus-encoded RNA in situ hybridisation. Pathology 36:301–308
Kekis PB, Murtin C, Künzli BM et al (2004) Epstein-Barr virus-associated lymphoepithelial carcinoma in the pancreas. Pancreas 28:98–102
Vortmeyer AO, Kingma DW, Fenton RG et al (1998) Hepatobiliary lymphoepithelioma-like carcinoma associated with Epstein-Barr virus. Am J Clin Pathol 109:90–95
Jeng YM, Chen CL, Hsu HC (2001) Lymphoepithelioma-like cholangiocarcinoma: an Epstein-Barr virus-associated tumour. Am J Surg Pathol 25:516–520
Si MW, Thorson JA, Lauwers GY et al (2004) Hepatocellular lymphoepithelioma-like carcinoma associated with Epstein Barr virus: a hitherto unrecognized entity. Diagn Mol Pathol 13:183–189
Ino Y, Yamazaki-Itoh R, Shimada K et al (2013) Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer 108:914–923
Emile JF, Adam R, Sebagh M et al (2000) Hepatocellular carcinoma with lymphoid stroma: a tumour with good prognosis after liver transplantation. Histopathology 37:523–529
Wada Y, Nakashima O, Kutami R et al (1998) Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology 27:407–414
Chiaravalli AM, Klersy C, Vanoli A et al (2012) Histotype-based prognostic classification of gastric cancer. World J Gastroenterol 18:896–904
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vanoli, A., Argenti, F., Vinci, A. et al. Hepatoid carcinoma of the pancreas with lymphoid stroma: first description of the clinical, morphological, immunohistochemical, and molecular characteristics of an unusual pancreatic carcinoma. Virchows Arch 467, 237–245 (2015). https://doi.org/10.1007/s00428-015-1788-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1788-6