Abstract
Breast cancer is occasionally complicated by sclerosing adenosis (SA). Although both lesions usually originate in the terminal duct lobular unit, their pathogenetic relationship has not yet been elucidated. The present study analyzed 63 breast cancer patients with SA (involving a total of 75 breasts) to clarify if coexisting SA increased the frequency of multicentric breast cancer or not. Using the topographical classification proposed in our previous study, breast cancers with SA were classified into the following three types: type A (n = 22), cancer area was completely surrounded by the SA; type B (n = 26), cancer area partially overlapped the SA; and type C (n = 27), cancer area was located separate from the SA. Breast cancers with SA had a significant (P < 0.001) increase in frequency of harboring bilateral and multicentric cancers [17 of 63 (27 %) and 15 of 63 (24 %), respectively] when compared to breast cancer patients without SA, regardless of topographical type. Breast cancers with SA were less invasive (P < 0.001), of lower histological grade (P = 0.034), and had similar frequency of estrogen receptor-positive (P = 0.21) and HER2-positive (P = 0.74) tumors. In conclusion, contralateral and ipsilateral multicentric breast cancers occurred at a higher frequency in those with SA. Our data suggest that SA is, in addition to lobular neoplasia, a predictor of multicentric breast cancers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The widespread use of screening mammography (MG), as well as improvement of the equipment and advances in image interpretation techniques, has led to improved detection of diseases which were previously only incidentally found during pathological examination. One such disease is breast cancer associated with sclerosing adenosis (SA), characterized by architectural distortion or microcalcifications on MG [1–4].
SA is a lobulocentric proliferative lesion derived from the terminal ductal-lobular unit (TDLU) and is classified as a benign epithelial proliferation. It shows a biphasic lesion of variably atrophic epithelium and myoepithelium and is characterized by lobular fibrosis and sclerosis [5]. SA has been previously known to be a low general risk factor for the development of invasive breast cancer [6–8]. Our previous studies of SA have indicated that cancer with macroscopic SA had contralateral breast cancer at a high frequency [9, 10]. Additionally, a few case studies have suggested that bilateral breast cancer is more frequent in breast cancer patients with SA [11, 12]. However, at present, there is no information regarding the frequency of ipsilateral multicentric breast cancer in patients with SA that may be responsible for cancer recurrence after breast-conserving surgery. Furthermore, it is possible that bilateral or multicentric breast cancer may occur even if the SA is located separate from the breast cancer area, although the frequency of this has not been clarified.
In the present study, we analyzed the clinicopathological characteristics of breast cancer patients with SA to clarify any association between SA and bilateral or multicentric breast cancer. We utilized the topographical classification system from our previous study on breast cancer with SA [9].
Materials and methods
The present retrospective and observational study was approved by the Ethical Review Board at Nagoya Medical Center (approval number 2013-682) which waived the need for informed consent.
A total of 871 breast cancer patients (911 breasts with cancer) were identified by a search of a database of pathological reports for 1509 breast disease patients, who were histopathologically diagnosed either by biopsy or surgery in Nagoya Medical Center from January 2005 to December 2010. Of these patients, 63 (7.2 %) patients (75 breasts with cancer) were observed with concomitant SA (46, 12, and 5 patients had unilateral breast cancer, bilateral breast cancer with bilateral SA, and bilateral breast cancer with unilateral SA, respectively). Six patients had bilateral synchronous and 11 had metachronous (median time interval: 38, range: 11 to 148 months) breast cancers (including the first cancer occurring before the study period). Synchronous bilateral breast cancer was defined as the detection of another cancer arising in the contralateral breast within 2 months after diagnosis of the initial breast cancer [13].
The pathological diagnoses were made by four pathologists (SI, SM, MH, and AI) and were reviewed by an experienced breast pathologist (SM). The resected specimen was serially sectioned into 5- or 7-mm-thick slices, and all blocks were processed selectively around the tumor and portions with gross abnormalities identified by the naked eye and examined microscopically. SA equal to or larger than 5 mm were analyzed for the present study, and SA smaller than 5 mm was excluded. When necessary, myoepithelial cell markers such as calponin and p63 were used to differentiate SA from invasive cancer. All invasive cancers were associated with in situ component, indicating that they were primary breast cancers rather than metastases. Similarly, before the diagnosis of bilateral and multicentric breast cancers, the in situ component of each breast cancer was identified. In addition, multicentric breast cancers in which two or more tumors had formed separately from one another in different areas of the breast, and multifocal breast cancers were excluded in this study.
Based on the topographical relationship between cancer and SA, breast cancers with SA (SA-Bc) were classified into the following: type A, in which the cancer area was completely surrounded by the SA; type B, in which the cancer area partially overlapped the SA; and type C, in which the cancer area was located adjacent, but separate from the SA (Figs. 1, 2, and 3). In some cases, either the cancer in type A extended outside of the SA, or the cancer in type C extended into the SA, resulting in the appearance of type B. Therefore, breast cancer characteristics of type A and C cancers were used for comparison to explore possible differences due to the topographical relationship between SA and breast cancer. We evaluated the following clinicopathological variables: manner of detection of breast cancer, mammographic findings of SA-Bc, age, presence or absence of bilateral or multicentric breast cancer, histological type, diameter of tumor invasion, histological grade (HG), and expression of hormone receptors and human epidermal growth factor receptor 2 (HER2).
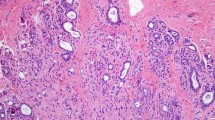
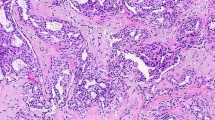
Topographical relationships between breast cancer and sclerosing adenosis: type A: cancer is entirely surrounded by sclerosing adenosis; type B, cancer involves sclerosing adenosis at least focally but is not confined to the sclerosing adenosis area; and type C, cancer is separate from the sclerosing adenosis area
Type A: the breast parenchyma is widely occupied by a small ductular proliferation of sclerosing adenosis. About 80 % of the ductules are involved in noninvasive ductal carcinoma, and the remaining 20 %, on the left side, are uninvolved. The cancerous lesion is confined to the area of sclerosing adenosis (the boundary of the sclerosing adenosis traced by black line and DCIS by red line) (hematoxylin-eosin stain; original magnification × 10)
Type B: high-grade noninvasive ductal carcinoma with sclerosing adenosis on the left side, but it also extends outside of the sclerosing adenosis to the right side (the boundary of the sclerosing adenosis traced by black line and DCIS by red line) (hematoxylin-eosin stain; original magnification × 40)
Negative staining for E-cadherin was confirmed for a diagnosis of invasive lobular carcinoma. HG was determined according to the modified Bloom-Richardson grading system [14]. Expression of estrogen receptors (ER) was determined by immunohistochemical (IHC) staining with Dako monoclonal mouse anti-human ER 1D5. Receptor expression was interpreted as positive when the percentage of positive cells was equal to or more than 1 %, according to the 2010 ASCO/CAP Guideline Recommendations [15]. HER2 expression was evaluated by IHC staining using a Dako Hercep Test Kit, and a tumor was considered HER2 positive if it was scored 3+ or 2+ with a HER2- fluorescence in situ hybridization (FISH) using a HER2/chromosome enumeration probe 17 (CEP17) ratio ≥2.2 [16].
Chi-squared, Fisher’s exact, and Student’s t tests were performed using Statcel 3 software (OMS publisher, Saitama, Japan). A P value of less than 0.05 was considered significant.
Results
SA-Bc in 75 breasts were classified into type A (n = 22, 29 %), type B (n = 26, 35 %), and type C (n = 27, 36 %) SA-Bc. Of 17 patients with bilateral breast cancer with SA, 7 patients were observed with the same type of breast cancer in both breasts (2, 3, and 2 patients had type A, B, and C breast cancers in both breasts, respectively). Contralateral breast cancers of six type A cancers were two type A, a type B, a type C, and two cancers without concomitant SA.
The manner of detection of SA-Bc (n = 75) and non-SA-Bc (n = 836) was compared in 911 breasts of 871 patients. The frequency of detection of SA-Bc was 53 % (n = 40) by MG, which was significantly higher [P < 0.001 (χ 2 test)] than in those with non-SA-Bc (32 %, n = 265). Meanwhile, the frequency of detection of SA-Bc by self-detection (mass) was significantly lower than in those with non-SA-Bc [31 % (n = 23) vs. 64 % (n = 534), respectively, P < 0.001 (χ 2 test)].
The MG findings of SA-Bc in 75 breasts were evaluated between the three topographical types of cancer. Of all breasts with SA-Bc, MG findings showed 64 % (n = 48) had architectural distortion and 45 % (n = 34) had microcalcifications. Type A had a significantly higher frequency of architectural distortion compared to type C [86 % (19 of 22) vs. 33 % (9 of 27), respectively, P < 0.001 (Fisher’s exact probability test)]. Microcalcifications tended to be more frequent in type C compared to type A [63 % (17 of 27) vs. 36 % (8 of 22), respectively, P = 0.064 (χ 2 test)].
Table 1 compares the clinical findings of SA-Bc and non-SA-Bc in 871 breast cancer patients. The mean age of patients with SA-Bc at the initial surgery was 49.9 years (range 27–74 years) and was significantly lower than that of those with non-SA-Bc (57.3 years, range 24–92 years, P < 0.001). The frequency of bilateral breast cancer in patients with SA-Bc was 27 %, which was significantly higher than that (6 %) in those with non-SA-Bc (P < 0.001). The frequency of multicentric breast cancer was also significantly higher in patients with SA-Bc than in those with non-SA-Bc (24 vs. 3 %, respectively, P < 0.001), and the frequency of bilateral or multicentric breast cancer was also significantly higher in patients with SA-Bc (44 vs. 9 %, respectively, P < 0.001). Table 2 compares the pathological findings in breasts with SA-Bc or non-SA-Bc. The invasive lobular carcinoma (ILC) and ductal carcinoma not otherwise specified (NOS) frequency in SA-Bc was 7 and 89 %, respectively, with no significant difference between SA-Bc and non-SA-Bc. The percentage of noninvasive cancer in patients with SA-Bc was significantly higher than in patients with non-SA-Bc (51 vs. 18 %, respectively, P < 0.001). The frequency of low-HG breast cancer was significantly higher in SA-Bc (Sa-BC 35 % vs. non-SA-Bc 24 %, P = 0.034), and that of ER-positive breast cancer tended to be a little lower (SA-Bc 70 % vs. non-SA-Bc 77 %, P = 0.21). The frequencies of HER2-positive breast cancer were almost the same (SA-Bc 19 % vs. non-SA-Bc 17 %).
Table 3 compares the clinical findings by topographical type. The mean age at surgery tended to be lower (but not significantly) in patients with type C compared to type A SA-Bc (type A, 52.7 years, range 28–73; type C, 48.2 years, range 36–74, P = 0.12). No significant difference in the frequency of bilateral breast cancer was observed between type A and C breasts (36 vs. 37 %, respectively, P = 0.96). The frequency of multicentric breast cancer was slightly lower in type A than in type C breasts, with no significant difference (18 vs. 26 %, respectively, P = 0.38). Table 4 compares the pathological findings by topographical type of cancer. The frequency of noninvasive cancer tended to be higher in type A than in type C cancer (68 vs. 41 %, respectively, P = 0.056), that of ER-positive cancer tended to be higher in type A than in type C cancer (86 vs. 62 %, respectively, P = 0.054), and that of HER2-positive cancer tended to be a little lower in type A than in type C cancer (18 vs. 29 %, respectively, P = 0.35), with no significant differences. No significant differences were observed in the HG, presence or absence of apocrine changes, or in presence of complex sclerosing lesion.
Discussion
In summary, SA-Bc was present in 7.2 % of all breast cancers, and the mean age of SA-Bc patients at time of initial surgery was significantly younger than that of their non-SA-Bc counterparts. Bilateral and multicentric cancers were observed at very high frequencies, regardless of the topographical type. Pathologically, noninvasive and low-HG cancers were significantly more frequent (P < 0.001 and P = 0.034, respectively), and ER-positive cancers were a little less frequent (P = 0.21) in SA-Bc breasts. Type A cancers tended to be more frequently noninvasive (P = 0.056), while type C cancers tended to be less frequently ER-positive (P = 0.054).
As noted in previous studies, SA is not a rare condition and is found in 3.1 % of female breasts at autopsy and in 12.2–12.5 and 5.3–7.0 % of biopsies from noncancerous and cancerous breasts, respectively [17]. In the present study, the frequency of SA-Bc was similar to those previously reported, although use of MG has led to the detection of many cases of SA-Bc. SA-Bc was more frequently detected asymptomatically, often showing architectural distortion or microcalcifications on MG, and so may be more frequently detected as noninvasive cancer compared to non-SA-Bc. The frequency of architectural distortion on MG was significantly higher in type A than in type C cancer. Günhan-Bilgen et al. reviewed mammographic findings in 43 histopathologically proven SA cases; focal architectural distortion was found in three (6.9 %) patients [18]. Taşkin et al. analyzed retrospectively mammographic findings of 41 lesions in which SA was the main histopathological diagnosis by breast biopsy; architectural distortion was found in three (7 %) lesions [19]. Results of previous studies have indicated that cancer involving SA is more often associated with architectural distortion. Yoshida et al. reported that architectural distortion was found in 15 (54 %) cases in a study of 24 patients with 28 cases of ductal carcinoma in situ (DCIS) with SA [11], and Ogura et al. reported 13 (46 %) cases with architectural distortion in a study of 27 patients with 28 cases of cancer with SA [12]. The architectural distortion may become apparent as cancer develops and grows into the sclerotic stroma due to the SA.
Since the SA also had a tendency of bilaterality, breast cancer patients with SA in the contralateral breast were reviewed to identify whether it was accompanying previously undiscovered SA. SA was observed in all three contralateral breasts that were biopsied for abnormalities on mammography or ultrasonography in the 46 patients with unilateral SA-Bc. Of the 17 bilateral breast cancer patients with SA, 12 patients were observed with bilateral concomitant SA (metachronous, 9 of 11; synchronous, 3 of 6). Thus, of 20 contralateral breasts that were biopsied or resected in 63 breast cancer patients with SA, 15 (75 %) breasts were observed with concomitant SA.
According to an earlier review by our group of carcinoma in situ arising from SA, the mean age of patients with DCIS or lobular carcinoma in situ (LCIS) arising from SA is 42.6 years (n = 28), which is markedly younger than that of the average breast cancer patient [20]. In a study of 126 patients with sclerosing lesions, Sloane et al. also found that 17 patients had breast cancer within the sclerosing lesions, and the cancer was common (82.4 %) in those younger than 60 years of age, suggesting that SA-Bc commonly develops in younger people [21]. Our findings were consistent with this point.
SA is considered a low risk factor for breast cancer, and the relative risk of SA without atypia is 1.7–1.97 [6–8]. Although it is well established that breast cancer occasionally arises from SA, no conclusion has been reached with regard to the role of SA in oncogenesis [8, 9, 22–25]. Although type A cancers tended to be noninvasive, and type C cancers tended to be less frequently ER-positive, no significant differences associated with the location of SA were observed in the pathological features of breast cancer.
HER2 overexpression is seen more frequently in DCIS, and in particular, in high-grade DCIS [26, 27]. In our cases, HER2 overexpression was observed in 10 (38 %) of the 26 noninvasive cancer (DCIS) cases (excluding three unknown cases and nine cases of HER2: 2+ and FISH: unknown). Of the ten DCIS cases with HER2 overexpression, eight DCIS cases were high-grade and six were comedo type. In type A, the frequency of noninvasive cancer was higher but that of HER2-positive cancers was lower, because noninvasive cancers of type A were frequently low-grade (47 %, 7/15).
Our study revealed that bilateral breast cancer commonly occurs in patients with SA-Bc regardless of the topographic type, suggesting that the presence of SA in a breast harboring cancer predicts the development of multiple breast cancers. In a study of 24 patients with DCIS with SA, Yoshida et al. found that as many as nine patients (38 %) had bilateral breast cancer [11]. Ogura et al. reported that, of 27 cancer patients with SA, 5 patients (19 %) had bilateral breast cancer [12]. Both of these studies examined breast cancers involving SA at least focally. We widened the scope of patients to include SA-Bc, in which cancer was located adjacent but away from SA. The frequency of bilateral breast cancer in all breast cancer patients was 7 %, which was comparable to that (7–8 %) in the Japanese Cancer Registry [28]. In contrast, the frequency of bilateral breast cancer in all patients with SA-Bc was as high as 27 % (P < 0.001), regardless of the topographical type. The frequency of bilateral breast cancer in patients with invasive lobular carcinoma has been noted to be 6–47 % [5], which was comparable to that of bilateral breast cancer in patients with SA-Bc. Previous studies indicated that the majority of breast carcinomas developing in adenosis are of the lobular type [29, 30]. However, ILC was observed in five breasts (7 %) in this study, and the combined frequency of ILC and lobular neoplasia seen in ductal carcinoma (NOS) (ten breasts) was 20 %; therefore, it is unlikely that the high frequency of the lobular type explains the higher frequency of bilateral breast cancer in our series. In a retrospective analysis of patients with primary breast cancer (117 and 7400 patients had synchronous bilateral breast cancer and unilateral breast cancer, respectively), Chen et al. concluded that patients with the presence of SA in the affected breast, including carcinomas arising from or involved with SA, may be at a high risk for developing synchronous bilateral breast cancer (hazard ratio, 11.8; 95 % confidence interval, 5.3 to 26.3; P < 0.001) [31].
In addition, multiple ipsilateral breast cancers were observed in 24 % of patients (P < 0.001). In a study of 60 mastectomy cases with DCIS, Faverly et al. [32] reported that only 8 % of DCIS have a multifocal distribution with gaps greater than 10 mm irrespective of histologic type. In a review of cases with noninvasive cancer in adenosis, meanwhile, Oberman et al. reported that 61.1 % (11/18) of the cases had noninvasive cancer in the breast separate from areas of noninvasive cancer arising in adenosis [30]. Our findings were in good agreement with these studies. Thus, we found that, regardless of the topographical relationship between cancer and SA, the presence of SA in the cancer-bearing breast was associated with a high frequency of contralateral or multicentric breast cancer, with synchronous or metachronous bilateral and multicentric cancers accounting for a high percentage (44 %) of patients (P < 0.001).
SA-Bc is commonly detected as a low-HG, noninvasive cancer, and is generally associated with a favorable prognosis. However, because many of the affected patients are relatively young and expected to live for a long time, they are at high risk of multiple recurrences unless treated properly, which is meaningful in the determination of treatment strategies and postoperative follow-up of patients.
Here, we have not directly identified whether SA is a risk factor of breast cancer multicentricity or not. Because it is a possibility, a large multi-institutional prospective study is needed to confirm these findings. In addition, long-term follow-up of patients is necessary to elucidate the pattern of recurrence of SA-Bc in the ipsilateral breast and that of the development of contralateral breast cancer and develop a method for the early diagnosis of multicentric breast cancer.
References
Preece PE (1989) Sclerosing adenosis. World J Surg 13:721–725
Franquet T, de Miguel C, Cozcolluela R, Donoso L (1993) Spiculated lesions of the breast: mammographic-pathologic correlation. Radiographics 13:841–852
Sekine K, Tsunoda-Shimizu H, Kikuchi M, Saida Y, Kawasaki T, Suzuki K (2007) DCIS showing architectural distortion on the screening mammogram—comparison of mammographic and pathological findings. Breast Cancer 14:281–284
Pojchamarnwiputh S, Muttarak M, Na-Chiangmai W, Chaiwun B (2007) Benign breast lesions mimicking carcinoma at mammography. Singap Med J 48:958–968
Rosen PP (2009) Adenosis, invasive lobular carcinoma. In: Pine JW, McGough J (eds) Breast pathology, 3rd edn. Lippincott Williams and Wilkins, Philadelphia, pp 161–175, 690–720
Jensen RA, Page DL, Dupont WD, Rogers LW (1989) Invasive breast cancer risk in women with sclerosing adenosis. Cancer 64:1977–1983
Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, Vierkant RA, Maloney SD, Pankratz VS, Hillman DW, Suman VJ, Johnson J, Blake C, Tlsty T, Vachon CM, Melton LJ 3rd, Visscher DW (2005) Benign breast disease and the risk of breast cancer. N Engl J Med 353:229–237
Visscher DW, Nassar A, Degnim AC, Frost MH, Vierkant RA, Frank RD, Tarabishy Y, Radisky DC, Hartmann LC (2014) Sclerosing adenosis and risk of breast cancer. Breast Cancer Res Treat 144:205–212
Moritani S, Ichihara S, Hasegawa M, Endo T, Oiwa M, Shiraiwa M, Nishida C, Morita T, Sato Y, Hayashi T, Kato A, Aoyama H, Yoshikawa K (2011) Topographical, morphological and immunohistochemical characteristics of carcinoma in situ of the breast involving sclerosing adenosis. Two distinct topographical patterns and histological types of carcinoma in situ. Histopathology 58:835–846
Oiwa M, Endo T, Shiraiwa M, Nishida C, Morita T, Sato Y, Hayashi T, Kato A, Ichihara S, Moritani S, Hasegawa M, Shinohara N (2011) A clinical and histopathological study on breast carcinoma with sclerosing adenosis (in Japanese with English abstract). J Jpn Assoc Breast Cancer Screen 20:196–203
Yoshida A, Hayashi N, Akiyama F, Yamauchi H, Uruno T, Kikuchi M, Yagata H, Tsugawa K, Suzuki K, Nakamura S, Tsunoda H (2012) Ductal carcinoma in Situ that involves sclerosing adenosis: high frequency of bilateral breast cancer occurrence. Clin Breast Cancer 12:398–403
Ogura K, Horii R, Oosako T, Iwase T, Akiyama F (2013) A clinico-pathological study on cancer in sclerosing adenosis. Breast Cancer. Published online 14 Feb. doi: 10.1007/s12282-013-0450-x
Nichol AM, Yerushalmi R, Tyldesley S, Lesperance M, Bajdik CD, Speers C, Gelmon KA, Olivotto IA (2011) A case-match study comparing unilateral with synchronous bilateral breast cancer outcomes. J Clin Oncol 29:4763–4768
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19:403–410
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC (2010) American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 28:2784–2795
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American society of clinical oncology/college of American pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25:118–145
Tavassoli FA (1999) General considerations, Benign lesions. In: Medina MP, Greenfield S (eds) Pathology of the breast, 2nd edn. Appleton and Lange, Stamford, pp 32–34, 130-133
Günhan-Bilgen I, Memiş A, Ustün EE, Ozdemir N, Erhan Y (2002) Sclerosing adenosis: mammographic and ultrasonographic findings with clinical and histopathological correlation. Eur J Radiol 44(3):232–238
Taşkin F, Köseoğlu K, Unsal A, Erkuş M, Ozbaş S, Karaman C (2011) Sclerosing adenosis of the breast: radiologic appearance and efficiency of core needle biopsy. Diagn Interv Radiol 17:311–316
Ichihara S, Aoyama H (1994) Incidental carcinoma of the breast arising in sclerosing adenosis. Pathol Int 44:722–726
Sloane JP, Mayers MM (1993) Carcinoma and atypical hyperplasia in radial scars and complex sclerosing lesions: importance of lesion size and patient age. Histopathology 23:225–231
Shoker BS, Jarvis C, Clarke RB, Anderson E, Munro C, Davies MP, Sibson DR, Sloane JP (2000) Abnormal regulation of the oestrogen receptor in benign breast lesions. J Clin Pathol 53:778–783
Selim AG, El-Ayat G, Wells CA (2002) Expression of c-erbB2, p53, Bcl-2, Bax, c-myc and Ki-67 in apocrine metaplasia and apocrine change within sclerosing adenosis of the breast. Virchows Arch 441:449–455
Celis JE, Moreira JM, Gromova I, Cabezón T, Gromov P, Shen T, Timmermans V, Rank F (2007) Characterization of breast precancerous lesions and myoepithelial hyperplasia in sclerosing adenosis with apocrine metaplasia. Mol Oncol 1:97–119
Drosos Y, Kouloukoussa M, Østvold AC, Grundt K, Goutas N, Vlachodimitropoulos D, Havaki S, Kollia P, Kittas C, Marinos E, Aleporou-Marinou V (2009) NUCKS overexpression in breast cancer. Cancer Cell Int. doi:10.1186/1475-2867-9-19
Latta EK, Tjan S, Parkes RK, O’Malley FP (2002) The role of HER2/neu overexpression/amplification in the progression of ductal carcinoma in situ to invasive carcinoma of the breast. Mod Pathol 15:1318–1325
van Bockstal M, Lambein K, Denys H, Braems G, Nuyts A, van den Broecke R, Cocquyt V, de Wever O, Libbrecht L (2014) Histopathological characterization of ductal carcinoma in situ (DCIS) of the breast according to HER2 amplification status and molecular subtype. Virchows Arch 465:275–289
The Japanese Breast Cancer Society (2010) Investigative report on registration of breast cancer patients in Japan 2010. http://www.crsu.org/breast_registration/analyses/2010/Report_2010.pdf (in Japanese). Accessed 22 Jun 2014
Eusebi V, Collina G, Bussolati G (1989) Carcinoma in situ in sclerosing adenosis of the breast: an immnocytochemical study. Semin Diagn Pathol 6:146–152
Oberman HA, Markey BA (1991) Noninvasive carcinoma of the breast presenting in adenosis. Mod Pathol 4:31–35
Chen JJ, Wang Y, Xue JY, Chen Y, Chen YL, Xiao Q, Yang WT, Shao ZM, Wu J (2014) A clinicopathological study of early-stage synchronous bilateral breast cancer: a retrospective evaluation and prospective validation of potential risk factors. PLoS One. doi:10.1371/journal.pone.0095185
Faverly DR, Burgers L, Bult P, Holland R (1994) Three dimensional imaging of mammary ductal carcinoma in situ: clinical implications. Semin Diagn Pathol 11:193–198
Acknowledgments
We thank Isao Okazaki, Takeshi Sakakura, Yuka Yonekura, Mina Yamashita, Sayaka Ogasawara, Noriko Ichikawa, Kaori Ishiki, and Mayumi Kataoka, of the Pathology Department, Nagoya Medical Center, for their excellent technical assistance. We also thank Tetsuo Kuroishi, Department of Advanced Diagnosis, Nagoya Medical Center, and Norimitsu Shinohara, Department of Radiological Technology, Gifu University of Medical Science, for their help in statistical analyses. We especially thank Toshiki I. Saito, Department of Regenerative Medicine, Nagoya Medical Center, for his helpful discussions.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oiwa, M., Endo, T., Ichihara, S. et al. Sclerosing adenosis as a predictor of breast cancer bilaterality and multicentricity. Virchows Arch 467, 71–78 (2015). https://doi.org/10.1007/s00428-015-1769-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1769-9