Abstract
The genetic abnormalities involved in the pathogenesis of gallbladder carcinoma (GBC) remain unclear. Microsatellite instability (MSI) has been described in many carcinomas, but little is known about the significance of mismatch repair in gallbladder carcinogenesis. Additionally, methylation status of long interspersed element-1 (LINE-1), a surrogate marker of global DNA methylation, has defined distinct subsets of other cancer types but has not been explored in GBC. Immunohistochemical expression of MSH2, MSH6, MLH1, and PMS2 and LINE-1 mRNA in situ hybridization was evaluated in 67 primary and 15 metastatic GBCs from 77 patients. Amplification of human epidermal growth factor receptor 2 (HER2) was evaluated by fluorescence in situ hybridization. Genotyping for 24 genes involved in carcinogenesis was performed using a multiplex PCR-based platform. MSI was present in 6 of 77 GBCs (7.8 %). Loss of MSH2/MSH6 was detected in five cases and loss of MLH1/PMS2 in one case. MSI status was not associated with Lynch syndrome, tumor grade, extracellular mucin, or tumor-infiltrating lymphocytes. There was no significant difference in mean overall survival of patients with and without MSI. Strong LINE-1 staining was identified in none of the GBC with MSI and in 36 of 69 (52 %) of those without MSI (p = 0.005), suggesting that LINE-1 in the former cohort was hypermethylated. All MSI tumors were negative for HER2 amplification, and TP53 and NRAS mutations were only found in GBC without MSI. MSI was identified in a minority of GBC cases. The strong correlation between global DNA methylation as measured by LINE-1 and loss of mismatch repair proteins suggests that methylation may account for the loss of these proteins. These hypermethylated tumors appear to represent a genetically unique cohort of gallbladder neoplasms, and the data suggests that demethylating agents may have a therapeutic value in this class of tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder carcinoma (GBC) is the most common malignancy affecting the biliary tract. The incidence varies worldwide with the greatest numbers of cases occurring in India and Chile. GBC is often diagnosed at advanced stages when surgical resection is no longer an option. Thus, although relatively rare in the USA, GBC remains a highly aggressive cancer with limited therapeutic options and a poor prognosis [1].
Despite advances in molecular pathology, the molecular pathogenesis of GBC remains poorly defined. Mutations in KRAS have been identified in gallbladder dysplasia and carcinoma in the setting of abnormal junction of the pancreatic and bile ducts [2–4]. Activation of the mitogen-activated protein kinase or the phosphoinositide 3-kinase signaling pathways via mutation in one of many involved genes appears to be important in the pathogenesis of GBC associated with cholelithiasis [4–13]. However, the frequency with which these mutations occur and the relationship between these mutations in GBC remain to be clarified.
There is much variation in the literature regarding the frequency with which microsatellite instability (MSI) is present in GBC. Using either polymerase chain reaction (PCR)-based techniques or immunohistochemistry (IHC) for mismatch repair (MMR) proteins, prior studies have reported MSI in a subset of GBC, ranging from 0 to 40 % of cases [6–8, 14–23]. Of note, a higher prevalence of MSI has been reported in GBC cases from patients with abnormal junction of the pancreatic and bile ducts [24]. Few studies have also noted MSI within dysplastic lesions of the gallbladder, suggesting that MSI occurs early in gallbladder carcinogenesis [17, 18]. It is important to note, however, that these investigations evaluated populations with a high prevalence of GBC; no studies have evaluated the frequency of MSI in the North American populations.
Some prior studies have evaluated clinical and histopathologic features of tumors with and without MSI. Roa et al. [18] found no difference in tumor grade, tumor stage, and survival in patients with tumors with and without MSI. Only one small study evaluated the morphologic features of GBC with MSI and found that one of two cases showed evidence of mucinous differentiation on histologic evaluation; both tumors with MSI occurred in patients with no history of Lynch syndrome [7]. Additionally, despite reports that MSI and mutations in select genes, including KRAS and BRAF, were mutually exclusive, the relationship between microsatellite status and the mutational profile of GBC remains unclear [6–8, 15, 20, 21].
In order to better characterize the role of MSI in gallbladder carcinogenesis, we evaluated protein expression of MMR genes by IHC in primary and metastatic tumors and assessed the prevalence of Lynch syndrome in the cohort of patients with MSI. We also correlated histologic features with MSI and the relationship of MSI status with the mutational profile, as analyzed by a multiplex PCR-based platform. In addition, we correlated GBC MSI status with expression of long interspersed nuclear element-1 (LINE-1), a surrogate marker of global methylation status. LINE-1 is a retrotransposon that accounts for approximately 18 % of the human genome [25]. Lack of methylation of LINE-1 is believed to account for much of the genomic hypomethylation observed in human cancer, such that it serves as a surrogate marker for global DNA methylation status [26, 27].
Materials and methods
Case selection
The study was approved by the Massachusetts General Hospital (Boston, MA) Institutional Review Board. Cases from 1988 to 2012 were retrospectively selected from a search of the surgical pathology files. A total of 80 cholecystectomy specimens were evaluated, of which 67 contained GBC (4 with concurrent high-grade dysplasia (HGD) and 1 from a patient with subsequent metastatic GBC), 8 contained HGD only, and 7 contained pyloric gland adenomas (PGAs; 2 with HGD and 1 from a patient with concurrent GBC). Additionally, 15 cases of metastatic GBC (including 4 from patients whose primary lesion was also studied here) were collected. Overall, specimens from 92 patients were evaluated in this study. Clinical data, including patient sex and age at the time of diagnosis, history of cigarette smoking, clinical stage of disease at the time of diagnosis, and survival information, was collected from each patient.
Tissue microarray construction
All cases were fixed in 10 % buffered formalin and embedded in paraffin by standard procedures. Selected tissue blocks were obtained for tissue microarray (TMA) construction. For each representative lesion, a 0.3-cm in diameter core, as outlined on review of the corresponding hematoxylin and eosin stained slide, was removed from the tissue blocks, for a total of 101 cores (67 primary GBCs, 15 metastatic GBCs, 12 HGDs, and 7 PGAs) and used to construct the TMA. Each TMA also included sections of liver parenchyma, including biliary epithelium, as controls.
Histologic evaluation
Hematoxylin and eosin stained sections of the primary and metastatic GBC cases were reviewed to assess the histologic features, specifically tumor grade, the presence of extracellular mucin, and tumor-infiltrating lymphocytes (TILs). Tumor grade was defined as well differentiated, moderately differentiated, or poorly differentiated based upon the percentage of the solid component of the tumor (<25, 25–75, or >75 %, respectively). The presence of TILs was defined as ≥5 intraepithelial lymphocytes per high power field.
Immunohistochemical analysis
Protein expression of four MMR proteins, MSH2, MSH6, MLH1, and PMS2, was evaluated by immunohistochemical staining of 5-μm-thick sections cut from the paraffin-embedded TMAs. The immunohistochemical stains were performed using the Leica Bond III auto-stainer (Leica Biosystems, Buffalo Grove, IL), according to the manufacturer’s protocol. After deparaffinization, monoclonal antibodies against hMLH1 (Leica Biosystems), hMSH2 (Leica Biosystems), hMSH6 (Leica Biosystems), and hPMS2 (Becton Dickinson Biosciences, San Jose, CA) were applied to the tissue sections. Slides were counter-stained with hematoxylin. The presence of MSI was defined as complete loss of nuclear staining for one or more of the four MMR proteins. Adjacent normal mucosa, stromal cells, and inflammatory cells with intact nuclear staining served as internal positive controls. The stained slides were reviewed by two authors (APM and VD), and cases were scored as either positive or negative. In cases with loss of protein expression, whole sections stained with antibody corresponding to the particular protein with lost expression were studied to confirm this finding.
Molecular analysis
Twenty-three gallbladders (21 with GBC, 1 with HGD, and 1 with a PGA) were genotyped for hot spot mutations in 24 cancer genes with SNaPshot, a previously described mutation assay (Applied Biosciences; Table 1). In brief, DNA was isolated from formalin-fixed, paraffin-embedded tissues and subjected to a multiplexed PCR and single-base extension reaction platform in order to generate fluorescently labeled signals. These SNaPshot products were then analyzed via capillary electrophoresis in order to detect the presence of selected hot spot mutations [28].
Fluorescence in situ hybridization analysis
Fluorescence in situ hybridization (FISH) was performed on deparaffinized, protease-treated, 5-μm-thick sections cut from the TMA to evaluate copy number changes in the human epidermal growth factor receptor 2 (HER2) gene. Dual-colored FISH with a probe specific to the HER2 gene and a copy number control probe that recognizes centromere 17 (CEP17) were hybridized according to the manufacturer’s protocol (Abbott Molecular). A case was considered positive for low-level HER2 amplification if the HER2/CEP17 signal ratio was 1.8–2.2 and for high-level HER2 amplification if the HER2/CEP17 signal was greater than 2.2. The cases were evaluated for HER2 overexpression by two authors.
LINE-1 in situ hybridization analysis
In situ hybridization (ISH) using an RNA probe aligned to open reading frame 1 of LINE-1 was performed using the QuantiGene® ViewRNA technology (Affymetrix, Santa Clara, CA). ISH staining was scored as follows: weak—the intensity of the stain was similar to the background lymphocytes and stroma; and strong—the intensity of the stain was greater than the signal in stromal cells and lymphocytes.
Statistical analysis
Statistical analysis was done using the SPSS (model 21) for Macintosh. The chi-square test and unpaired Student’s t test were used for categorical data and continuous variables, respectively. Survival was compared using the Kaplan-Meier analysis and log rank test. Statistical significance was set at p = 0.05.
Results
MSI immunohistochemistry
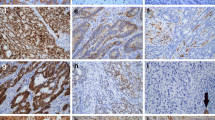
By IHC, MSI was present in specimens from 6 of 77 (7.8 %) patients with GBC (Fig. 1 and Table 2). Concurrent loss of expression of MSH2 and MSH6 was seen in five cases. The loss of mismatch proteins identified initially on the tissue microarray was confirmed by evaluating whole sections. Isolated loss of PMS2 occurred in one case; the metastatic lesion corresponding to this primary GBC showed loss of both PMS2 and MLH1. Neither HGD (without concomitant carcinoma) nor PGA had loss of expression of MMR proteins. Notably, all adjacent areas of normal gallbladder mucosa showed positive staining for all MMR proteins.
Poorly differentiated adenocarcinoma (a) showing loss of MSH2 (b) and MSH6 (not shown) with preserved expression of MLH1 (c) and PMS2 (not shown). Poorly differentiated adenocarcinoma (d) showing loss of MLH1 (e) and PMS2 (not shown) with preserved expression of MSH6 (f) and MSH2 (not shown). Adenocarcinoma (g) showing loss of MSH2 (h) and MSH6 (not shown) with preserved expression of MLH1 (not shown) and PMS2 (i)
Clinical features
Among the patients with GBC with and without MSI, 66.7 and 64.8 % were female, respectively. The mean age of the patients with MSI was 70.8 years while that for patients with microsatellite stable (MSS) GBC was 67.7 years. Four of 5 (80.0 %) patients with MSI and 21 of 51 (41.2 %) patients with MSS GBC were non-smokers (p = 0.15); smoking history was not available for the remainder of patients. There was no significant difference in the stage at initial presentation of patients with and without MSI (Table 3). Of note, none of the patients with MSI had a personal history of malignancy. There was no significant difference in estimated mean overall survival between patients with and without MSI (34.0 ± 19.0 and 34.0 ± 8.0 months, respectively; p = 0.93).
Morphologic evaluation
All studied GBC cases were adenocarcinomas (Table 4). There was no association between MSI status and tumor grade (p = 0.60). Furthermore, there was no correlation between MSI status and the presence of extracellular mucin or TILs (p = 0.664 and p = 0.640, respectively; Table 4).
Correlation of MSI status with molecular data
Mutation status
SNaPshot testing revealed a mutation in two of the six (33.3 %) GBC cases with MSI: one case harbored a mutation in KRAS and the other a mutation in PIK3CA. The remaining four GBC cases with MSI (66.7 %) were wild type for hot spot mutations in 24 genes evaluated by SNaPshot. Of the 15 cases of MSS GBC that were analyzed, 4 (26.7 %) showed mutations in one of the following genes: TP53, NRAS, KRAS, or PIK3CA; the remaining 11 MSS GBC cases that were tested revealed no mutations. Overall, there was no significant difference in the number of mutations identified in GBCs with and without MSI. A PIK3CA mutation and a CTNNB1 (β-catenin) mutation were identified in one HGD and one PGA, respectively.
HER2 status
None of the GBC with MSI had overexpression of HER2 by FISH. Conversely, 4 of 71 (5.6 %) MSS GBC cases had HER2 amplification—2 cases had low-level amplification and 2 cases had high-level amplification. All HGD and PGA lacked HER2 amplification.
Correlation of MSI status with LINE-1 expression
LINE-1 ISH was evaluated in 6 cases with MSI and 69 MSS cases. None of the six patients with MSI showed strong LINE-1 staining. In contrast, 36 (52.2 %) cases without MSI showed strong LINE-1 staining (p = 0.004; Fig. 2).
Discussion
To date, this is the only study from the USA and the largest study overall to evaluate the role of MSI in GBC. We identified MSI in 7.8 % of GBCs. Prior studies report much variation in the prevalence of MSI within GBC [6–8, 14–23]. Many of these studies evaluated small numbers of patients, and several of these were from regions of the world with a high prevalence of GBC. The variation in the frequency of MSI in GBC may thus represent differences in the underlying pathogenesis of carcinoma in the gallbladder.
Molecular testing for MSI relies on the identification of changes in length of a panel of microsatellites by PCR-based techniques within a tumor as compared to normal tissue. The Bethesda guidelines define microsatellite-high (MSI-H) as MSI within two or more of five microsatellite loci and microsatellite-low (MSI-L) as MSI within one microsatellite locus. Chang et al. [14] studied 32 cases of GBC and 11 cases of gallbladder dysplasia and found MSI by PCR-based testing in only 1 GBC. Kim et al. [6] identified MSI by PCR-based techniques in only one of five cases of gallbladder dysplasia; notably, the carcinoma adjacent to this dysplasia showed no MSI and all 15 GBC cases and 3 adenomas studied showed no MSI. Both of these Korean studies conclude that MSI plays a very limited role in gallbladder carcinogenesis. In contrast, Yanagisawa et al. [17] found MSI in 7 of 17 (41 %; 1 MSI-H and 6 MSI-L) patients with GBC and MSI-L in 9 of 30 (41 %) patients with chronic cholecystitis, suggesting that MSI plays an early role in the pathogenesis of GBC.
Immunohistochemistry is now the most commonly used (and widely considered the gold standard) modality to determine MSI status. Unlike IHC, PCR-based techniques are both labor-intensive and technically demanding. Furthermore, unlike with PCR-based methods, evaluation of MSI status by IHC reveals the particular MMR protein that is defective. Prior studies of colon and endometrial carcinoma have demonstrated that IHC is as sensitive as PCR-based analysis in the detection of MSI in tumors [29–32]. Roa et al. [18] studied a series of 59 cases of GBC and found that 10 % of tumors were MSI-H when evaluated with a PCR-based assay and negative for staining of at least one MMR protein by IHC. In this study, we used IHC analysis to evaluate MSI status in gallbladder tumors and found MSI in 7.8 % of GBC cases, supporting earlier studies that suggest that MSI plays an important role in the pathogenesis of a subgroup of GBC. Interestingly, one GBC with MSI in this study showed isolated loss of PMS2 in the primary tumor and loss of both MLH1 and PMS2 in the metastasis. Prior studies in colorectal carcinoma have demonstrated genetic diversity among primary and metastatic tumors, including discordant expression of MMR proteins [33, 34]. We speculate that the preservation of MLH1 in the primary tumor may be related to a somatic mutation in MLH1 resulting in degradation of the PMS2 protein. However, the abnormal MLH1 protein may nevertheless be recognized on immunohistochemistry. Methylation of MLH1 may constitute the second hit, resulting in loss of MLH1 in the metastatic tumor.
Clinical testing of tumors for defective MMR protein function and MSI status has two potential endpoints. First, it allows for the identification of patients with Lynch syndrome who are at increased risk for developing other malignancies of the gastrointestinal tract, such as colorectal, gastric, and small intestinal, and malignancies of other organs, most commonly endometrial carcinoma. These patients benefit from surveillance to detect Lynch-syndrome-associated malignancies. Furthermore, their family members may benefit from genetic testing to determine mutation status. In colonic carcinomas, the loss of MSH2 function is generally associated with a germline mutation in MSH2 and Lynch syndrome. In endometrial carcinoma, the observations are similar to that seen in colonic carcinoma, such that gene mutation analysis is recommended when loss of expression of MSH2 and MSH6 is observed. However, while loss of expression of MSH2 represents the most common cause of MSI in GBC in this study, none of the studied patients had clinical evidence of Lynch syndrome (i.e., a personal or family history of Lynch-syndrome-associated malignancy). Therefore, MSI in GBC does not seem to be a manifestation of Lynch syndrome. Second, the identification of tumors with MSI is important as these tumors may show distinct clinical and pathological features and distinctly different sensitivity to chemotherapy [35–44]. This is the first study to evaluate the clinical and morphologic features of gallbladder tumors in the context of MSI. In other organ systems, tumors with MSI exhibit particular clinical and histologic features. For example, in colonic carcinoma, MSI more commonly occurs in younger patients with right-sided tumors as compared to left-sided tumors. Histologic evaluation of colonic tumors with MSI is characterized by poor differentiation, signet ring and medullary growth patterns, mucinous differentiation, tumor-infiltrating lymphocytes, a dense, Crohn’s-like inflammatory host response, and the absence of “dirty necrosis” [45, 46]. Similarly, the presence of TILs and peritumoral lymphocytes appears to be a sensitive indicator of MSI in endometrial carcinoma [47]. However, in this study, we found no association between tumor grade, the presence of extracellular mucin or TIL, and MSI status. As this was a retrospective study of GBC from the last three decades, we were unable to assess the impact of MSI on response to chemotherapy.
In colorectal carcinoma, tumors with MSI have been associated with a less aggressive clinical course and an improved survival rate [38, 48]; the association of MSI and survival in endometrial carcinoma remains controversial. Some studies show a negative relationship between the presence of MSI and prognostic factors, including higher histological grade, depth of invasion, and the presence of lymphovascular invasion [49–51], while others have shown a positive correlation between the presence of MSI and prognostic factors [52] or survival [53]. Still, other studies have found no significant correlation [54]. In our study, similar to the findings in a prior study of MSI in GBC [18], MSI status had no impact on survival, although the sample size for the MSI group was small.
Prior studies of GBC have attempted to correlate the presence of MSI with the presence of mutations in select genes [6–8, 15, 20, 21]. However, this is the first study to explore the relationship of MSI status and genotype, as evaluated on a large scale with a multiplex PCR-based platform. Amplification of HER2 and mutations in NRAS and TP53 were identified only in MSS GBC. However, mutations in PIK3CA and KRAS were identified in both groups of patients with GBC. In colorectal carcinoma, there is a well-characterized relationship between MSI status and mutational phenotype. Colorectal tumors with MSI have a “mutator phenotype” as they develop mutations in tumor suppressor genes that contain microsatellites, including transforming growth factor beta receptor type II, insulin-like growth factor receptor type II, and BAX. Additionally, mutations in BRAF are seen in 40–50 % of sporadic MSI cases [55–58] but are not seen in colorectal carcinoma associated with Lynch syndrome, such that the presence of a BRAF mutation virtually excludes Lynch syndrome in colorectal carcinomas with MSI. In this analysis, there was no significant correlation between mutational status and GBC. Of note, recent whole exome and targeted sequencing approaches identified mutations in a higher proportion of GBCs [59, 60]. Our targeted approach yielded mutations in a lower proportion of genes as well as a lower incidence of mutations in particular genes, such as p53. It is possible that genetic variations observed in these studies reflects geographic variation as our study, unlike the others, evaluated only tumors from patients in the USA. Additionally, we evaluated a limited number of hot spot mutations in a limited number of genes by SNaPshot and FISH analysis that were performed such that other mutations of possible relevance were not interrogated. A whole exome sequencing effort would be required to thoroughly address the issue of geographic difference in mutation profile of GBCs.
The methylation status of LINE-1, which makes up approximately 18 % of the human genome, has been shown to be a useful marker of global DNA methylation status [25–27], such that decreased LINE-1 expression correlates with the global DNA hypermethylation. This analysis is generally performed using traditional methylation platforms. However, novel ISH platforms allow for the robust evaluation of mRNA in paraffin-embedded tissue. In this study, taking advantage of the advances in chromogenic ISH technology, we assessed LINE-1 RNA ISH as a marker of global methylation status. This ISH platform offers significant advantages over the currently available methylation assays by allowing the distinction of tumor cells and stromal cells; traditional methylation assays reflect the methylation status of the tumor cells as well as stromal cells and lymphocytes, unless microdissection techniques have been employed.
We found a correlation between LINE-1 expression within tumor cells and MSI status: no cases of GBC with MSI had strong LINE-1 RNA expression suggesting that these cases showed a global hypermethylator phenotype. The strong correlation between LINE-1 reactivity and MSI status suggest that the mechanism underlying the loss of mismatch repair proteins is methylation. However, the possibility that somatic mutations are involved in the silencing of mismatch repair genes [61] cannot be entirely excluded. This data is consistent with the observation made in colorectal carcinoma that hypermethylation of genes implicated in the MSI pathway correlates with global hypermethylation status [62]. Thus, hypermethylated GBCs may represent a distinct pathway in gallbladder carcinogenesis: a paradigm that parallels colorectal carcinoma [63–65]. Additionally, in line with prior studies that there is decreased DNA methylation in smokers as compared to non-smokers [66–68], we found that patients with MSI-positive tumors were more likely to be non-smokers. Overall, this data provides additional insight regarding the hypermethylated pathways of gallbladder carcinogenesis and may have implications for guiding therapy. As it is now widely accepted that epigenetic dysregulation, including alteration of DNA methylation profiles, is involved in cancer development and progression, therapeutic approaches utilizing demethylating agents, including 5-azacytidine and decitabine, have been extensively studied in many tumor types and are currently FDA-approved for clinical use in the treatment of myelodysplastic syndromes. Our findings open up the prospect of investigating demethylating agents for the therapy of this class of gallbladder carcinomas.
References
Konstantinidis IT, Deshpande V, Genevay M et al (2009) Trends in presentation and survival for gallbladder cancer during a period of more than 4 decades: a single-institution experience. Arch Surg 144:441–447
Hanada K, Tsuchida A, Iwao T et al (1999) Gene mutations of K-ras in gallbladder mucosae and gallbladder carcinoma with anomalous junction of the pancreaticobiliary duct. Am J Gastroenterol 94:1638–1642
Shimotake T, Aoi S, Tomiyama H et al (2003) DPC-4 (Smad-4) and K-ras gene mutations in biliary tract epithelium in children with anomalous pancreaticobiliary ductal union. J Pediatr Surg 38:694–697
Hezel AF, Deshpande V, Zhu AX (2010) Genetics of biliary tract cancers and emerging targeted therapies. J Clin Oncol 28:3531–3540
Watanabe H, Date K, Itoi T et al (1999) Histologic and genetic changes in malignant transformation of gallbladder adenoma. Ann Oncol 10:136–139
Kim YT, Kim J, Jang YH et al (2001) Genetic alterations in gallbladder adenoma, dysplasia, and carcinoma. Cancer Lett 169:59–68
Rashid A, Ueki T, Gao YT et al (2002) K-ras mutations, p53 overexpression, and microsatellite instability in biliary tract cancers: a population based study in China. Clin Cancer Res 8:3156–3163
Saetta AA, Papanastasiou P, Michalopoulos NV et al (2004) Mutational analysis of BRAF in gallbladder carcinomas in association with K-ras and p53 mutations and microsatellite instability. Virchows Arch 445:179–182
Nakazawa K, Dobashi Y, Suzuki S et al (2005) Amplication and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. J Pathol 206:356–365
Leone F, Cavalloni G, Pignochino Y et al (2006) Somatic mutations of epidermal growth factor receptor in bile duct and gallbladder carcinoma. Clin Cancer Res 12:1680–1685
Eckel F, Schmid RM (2007) Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902
Riener MO, Bawohl M, Clavien PA et al (2008) Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosom Cancer 47:363–367
Deshpande V, Nduaguba A, Zimmerman SM et al (2001) Mutational profiling reveals PIK3CA mutations in gallbladder carcinoma. BMC Cancer 11:60
Chang HJ, Kim SW, Kim YT et al (1999) Loss of heterozygosity in dysplasia and carcinoma of the gallbladder. Mod Pathol 12:763–769
Yoshida T, Sugai T, Habano W et al (2000) Microsatellite instability in gallbladder carcinoma: two independent genetic pathways of gallbladder carcinogenesis. J Gastroenterol 35:768–774
Sessa F, Furlan D, Genasetti A et al (2003) Microsatellite instability and p53 expression in gallbladder carcinomas. Diagn Mol Pathol 12:96–102
Yanagisawa N, Mikami T, Yamashita K et al (2003) Microsatellite instability in chronic cholecystitis is indicative of an early stage in gallbladder carcinogenesis. Am J Clin Pathol 120:413–417
Roa JC, Roa I, Correa P et al (2005) Microsatellite instability in preneoplastic and neoplastic lesions of the gallbladder. J Gastroenterol 40:79–86
Saetta AA (2006) K-ras, p53 mutations, and microsatellite instability (MSI) in gallbladder carcinoma. J Surg Oncol 93:644–649
Saetta AA, Gigelou F, Papanastasiou PI et al (2006) High-level microsatellite instability is not involved in gallbladder carcinogenesis. Exp Mol Pathol 80:67–71
Nagahashi M, Ajioka Y, Lang I et al (2008) Genetic changes in p53, K-ras, and microsatellite instability in gallbladder carcinoma in high-incidence areas of Japan and Hungary. World J Gastroenterol 14:70–75
Mishra PK, Jatawa SK, Raghuram GV et al (2009) Correlation of aberrant expression of p53, Rad50, and cyclin-E proteins with microsatellite instability in gallbladder adenocarcinomas. Genet Mol Res 8:1202–1210
Maurya SK, Tewari M, Mishra RR et al (2012) Genetic aberrations in gallbladder cancer. Surg Oncol 21:37–43
Nagai M, Watanabe M, Iwase T, Yamao K, Isaji S (2002) Clinical and genetic analysis of noncancerous and cancerous biliary epithelium in patients with pancreaticobiliary maljunction. World J Surg 26:91–98
Rodic N, Burns KH (2013) Long interspersed element-1 (LINE-1): passenger or driver in human neoplasms? PLoS Genet 9:e1003402
Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP (2004) A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res 32:e38
Irahara N, Nosho K, Baba Y et al (2010) Precision of pyrosequencing assay to measure LINE-1 methylation in colon cancer, normal colonic mucosa, and peripheral blood cells. J Mol Diagn 12:177–183
Dias-Santagata D, Akhavanfard S, David SS et al (2010) Rapid targeted mutational analysis of human tumors: a clinical platform to guide personalized cancer medicine. EMBO Mol Med 2:146–158
Lindor NM, Burgat LJ, Leontovich O et al (2002) Immunohistochemistry versus microsatellite instability testing in phenotyping colorectal tumors. J Clin Oncol 20:1043–1048
Modica I, Soslow RA, Black D et al (2007) Utility of immunohistochemistry in predicting microsatellite instability in endometrial carcinoma. Am J Surg Pathol 31:744–751
Shia J (2008) Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome. Part 1: the utility of immunohistochemistry. J Mol Diagn 10:293–300
Shia J, Tang LH, Vakiani E et al (2009) Immunohistochemistry as first-line screening for detecting colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer synrome: a 2-antibody panel may be as predictive as a 4-antibody panel. Am J Surg Pathol 29:96–104
Ishimaru G, Adachi J, Shiseke M, Yamaguchi N, Muto T, Yokota J (1995) Microsatellite instability in primary and metastatic colorectal cancers. Int J Cancer 64:153–157
Messick CA, Sanchez J, DeJulius KL, Church JM, Kalady MF (2009) Genetic and molecular diversity of colon cancer hepatic metastases. Surgery 146:227–231
Fallik D, Borrini F, Boige V et al (2003) Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res 63:5738–5744
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349:247–257
de Vos tot Nederveen Cappel WH, Meulenbeld HJ, Kleibeuker JH et al (2004) Survival after adjuvant 5-FU treatment for stage III colon cancer in hereditary nonpolyposis colorectal cancer. Int J Cancer 109:468–471
Popat S, Houlston RS (2005) A systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 23:609–618
Storojeva I, Boulay JL, Heinimann K et al (2005) Prognostic and predictive relevance of microsatellite instability in colorectal cancer. Oncol Rep 14:241–249
Jover R, Zapater P, Castells A et al (2006) Mismatch repair status in the prediction of benefit from adjuvant fluorouracil chemotherapy in colorectal cancer. Gut 55:848–855
Lanza G, Gafa R, Santini A et al (2006) Immunohistochemical test for MLH1 and MSH2 expression predicts clinical outcome in stage II and III colorectal cancer patients. J Clin Oncol 24:2359–2367
Kim GP, Colangelo LH, Wieand HS et al (2007) Prognostic and predictive roles of high-degree microsatellite instability in colon cancer: a National Cancer Institute-National Surgical Adjuvant Breast and Bowel Project Collaborative Study. J Clin Oncol 25:767–772
Bertagnolli MM, Niedzwiecki D, Comptom CC et al (2009) Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, fluorouracil, and leucovorin in stage III colon cancer: Cancer and Leukemia Group B Protocol 89803. J Clin Oncol 27:1814–1821
De Guetz G, Schischmanoff O, Nicolas P et al (2009) Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systemic review with meta-analysis. Eur J Cancer 45:1890–1896
Greenson JK, Bonner JD, Ben-Yzhak O et al (2003) Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumor and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol 27(5):563–570
Shia J, Ellis NA, Paty PB et al (2003) Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol 27:1407–1417
Shia J, Black D, Hummer AJ et al (2008) Routinely assessed morphological features correlate with microsatellite instability status in endometrial cancer. Hum Pathol 39:116–125
Lawes DA, SenGupta S, Boulos PB (2003) The clinical importance and prognostic implications of microsatellite instability in sporadic cancer. Eur J Surg Oncol 29:201–212
Jung An H, Kim KI, Kim JY et al (2007) Microsatellite instability in endometrioid type endometrial adenocarcinoma is associated with poor prognostic indicators. Am J Surg Pathol 31:846–853
Garg K, Leitao M, Kauff N et al (2009) Selection of endometrial carcinomas for DNA mismatch repair protein immunohistochemistry using patient age and tumor morphology enhances detection of mismatch repair abnormalities. Am J Surg Pathol 33:925–933
Garg K, Shih K, Barakat R et al (2009) Endometrial carcinomas in woman age 40 years and younger: tumors associated with loss of DNA mismatch repair proteins comprise a distinct clinicopathologic subset. Am J Surg Pathol 33:1869–1877
Basil B, Goodfellow PJ, Rader JS et al (2000) Clinical significance of microsatellite instability in endometrial carcinoma. Cancer 89:1758–1764
Black D, Soslow RA, Levine DA et al (2006) Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol 24:1745–1753
Zighelboim I, Goodfellow PJ, Gao F et al (2007) Microsatellite instability and epigenetic inactivation of MLH1 and outcome of patients with endometrial carcinomas of the endometrioid type. J Clin Oncol 25:2042–2048
Domingo E, Laiho P, Ollikainen M et al (2004) BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet 41:664–668
Domingo E, Niessen RC, Oliveira C et al (2005) BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene 24:3995–3998
Oliveira C, Velho S, Domingo E et al (2005) Concomitant RASSF1A hypermethylation and KRAS/BRAF mutations occur preferentially in MSI sporadic colorectal cancer. Oncogene 24:7630–7634
Loughrey MB, Waring PM, Tan A et al (2007) Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer 6:301–310
Jiao Y, Pawlik TM, Anders RA et al (2013) Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A, and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet 45:1470–1473
Li M, Zhang Z, Li X et al (2014) Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet 46:872–876
Mensenkamp AR, Vogelaar IP, van Zelst-Stams WAG et al (2014) Somatic mutations in MLH1 and MSH2 are a frequent cause of mismatch-repair deficiency in Lynch syndrome-like tumors. Gatroenterology 146:623–646
Matsuzaki K, Deng G, Tanaka H, Kakar S, Miura S, Kim YS (2005) The relationship between global methylation level, loss of heterozygosity, and microsatellite instability in sporadic colorectal cancer. Clin Cancer Res 11:8564–8569
Estecio MRH, Gharibyan V, Shen L et al (2007) LINE-1 hypomethylation in cancer is highly variable and inversely correlated with microsatellite instability. PLoS ONE 2:e399
Ogino S, Kawasaki T, Nosho K et al (2008) LINE-1 hypomethylation is inversely associated with microsatellite instability and CpG island methylator phenotype in colorectal cancer. Int J Cancer 122:2767–2773
Pavicic W, Joensuu EI, Nieminen T, Peltomaki P (2012) LINE-1 hypomethylation in familial and sporadic cancer. J Mol Med 90:827–835
Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H (2011) Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 88:450–457
Shenker NS, Polidoro S, Van Veldhoven K et al (2012) Epigenome-wide association study in the European Prospective Investigation into Cancer and Nutrition (EPIC-Turin) identified novel genetic loci associated with smoking. Hum Mol Genet 22:843–851
Lee KWK, Pausova Z (2013) Cigarette smoking and DNA methylation. Front Genet 4:132
Acknowledgments/Conflicts of interest
This work was supported by the Burroughs Wellcome Trust (DTT), K12CA087723-11A1 (DTT), and Affymetrix, Inc. (DTT, VD).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moy, A.P., Shahid, M., Ferrone, C.R. et al. Microsatellite instability in gallbladder carcinoma. Virchows Arch 466, 393–402 (2015). https://doi.org/10.1007/s00428-015-1720-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-015-1720-0